Effectiveness of Artificial Intelligence Technologies in Cancer Treatment for Older Adults: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
3. Results
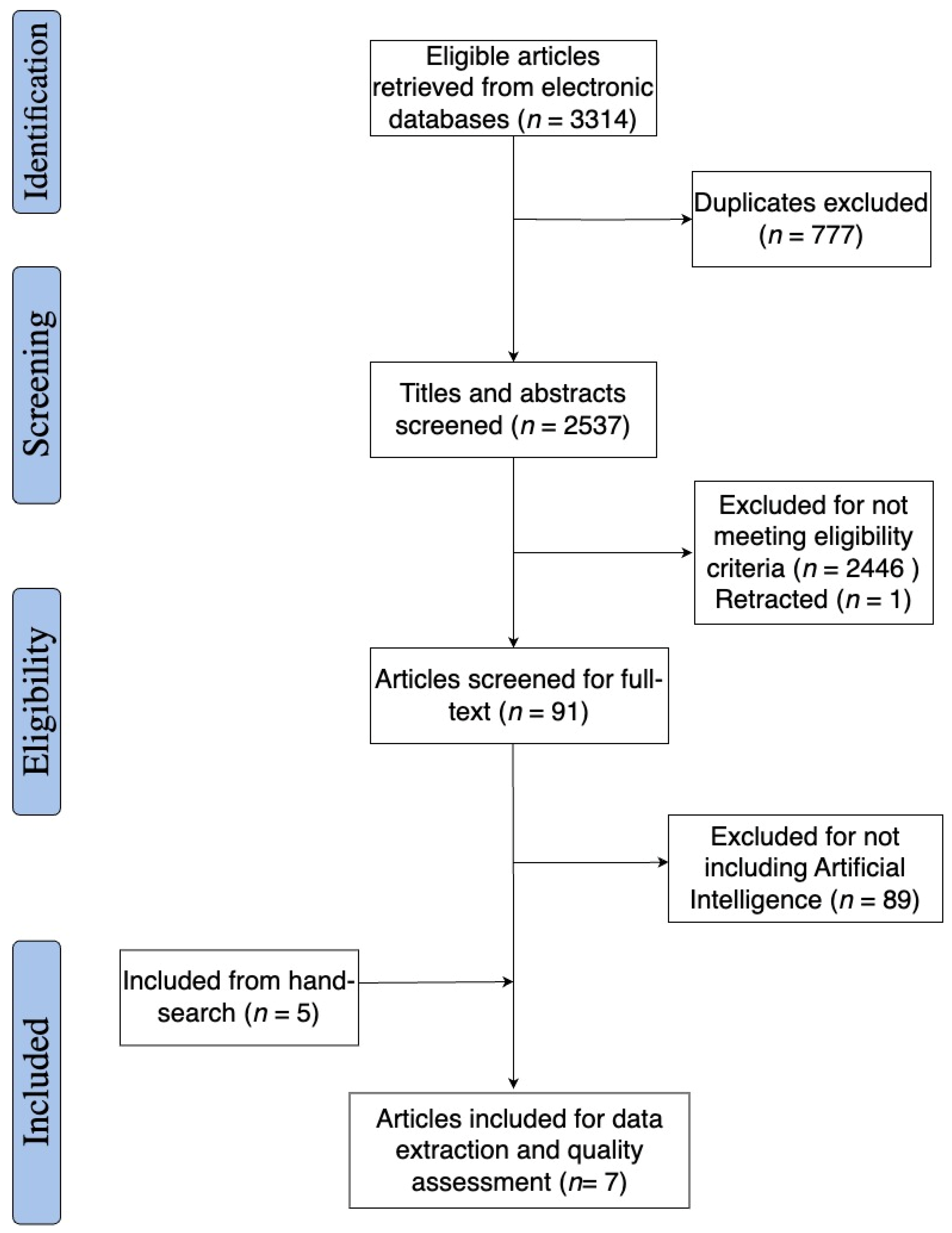
3.1. Study Selection
3.2. Characteristics of Included Studies
3.3. Effectiveness of the AI Interventions in Treatment Planning
3.4. Effectiveness of AI Interventions in Stereotactic Body Radiotherapy
4. Discussion
4.1. Implications of the AI Treatments
4.2. Gaps in Knowledge
4.3. Future Research and Practice
4.4. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Detailed Search Strategy
References
- What Is Cancer?—NCI. Available online: https://www.cancer.gov/about-cancer/understanding/what-is-cancer (accessed on 15 June 2024).
- Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 15 June 2024).
- CDC. Cancer Risk Factors. Available online: https://www.cdc.gov/cancer/risk-factors/index.html (accessed on 15 June 2024).
- Weir, H.K.; Thompson, T.D.; Soman, A.; Møller, B.; Leadbetter, S. The Past, Present, and Future of Cancer Incidence in the United States: 1975 Through 2020. Cancer 2015, 121, 1827–1837. [Google Scholar] [CrossRef]
- Weir, H.K.; Sherman, R.; Yu, M.; Gershman, S.; Hofer, B.M.; Wu, M.; Green, D. Cancer Incidence in Older Adults in the United States: Characteristics, Specificity, and Completeness of the Data. J. Regist. Manag. 2020, 47, 150–160. [Google Scholar]
- Risk Factors: Age—NCI. Available online: https://www.cancer.gov/about-cancer/causes-prevention/risk/age (accessed on 15 June 2024).
- Demographic Turning Points for the United States. Available online: https://www.census.gov/library/publications/2020/demo/p25-1144.html (accessed on 15 June 2024).
- Cancer Deaths—Health, United States. Available online: https://www.cdc.gov/nchs/hus/topics/cancer-deaths.htm (accessed on 15 June 2024).
- Mariotto, A.B.; Enewold, L.; Zhao, J.; Zeruto, C.A.; Yabroff, K.R. Medical Care Costs Associated with Cancer Survivorship in the United States. Cancer Epidemiol. Biomark. Prev. 2020, 29, 1304–1312. [Google Scholar] [CrossRef]
- Deshmukh, A.A.; Zhao, H.; Franzini, L.; Lairson, D.R.; Chiao, E.Y.; Das, P.; Swartz, M.; Giordano, S.H.; Cantor, S.B. Total Lifetime and Cancer-Related Costs for Elderly Patients Diagnosed with Anal Cancer in the United States. Am. J. Clin. Oncol. 2018, 41, 121–127. [Google Scholar] [CrossRef]
- Online Cancer Communities as Informatics Intervention for Social Support: Conceptualization, Characterization, and Impact|Journal of the American Medical Informatics Association|Oxford Academic. Available online: https://academic.oup.com/jamia/article/24/2/451/2631483 (accessed on 15 June 2024).
- Cancer Treatment—Mayo Clinic. Available online: https://www.mayoclinic.org/tests-procedures/cancer-treatment/about/pac-20393344 (accessed on 15 June 2024).
- Types of Cancer Treatments|Memorial Sloan Kettering Cancer Center. Available online: https://www.mskcc.org/cancer-care/diagnosis-treatment/cancer-treatments (accessed on 15 June 2024).
- Debela, D.T.; Muzazu, S.G.; Heraro, K.D.; Ndalama, M.T.; Mesele, B.W.; Haile, D.C.; Kitui, S.K.; Manyazewal, T. New Approaches and Procedures for Cancer Treatment: Current Perspectives. SAGE Open Med. 2021, 9, 20503121211034366. [Google Scholar] [CrossRef] [PubMed]
- Anand, U.; Dey, A.; Chandel, A.K.S.; Sanyal, R.; Mishra, A.; Pandey, D.K.; De Falco, V.; Upadhyay, A.; Kandimalla, R.; Chaudhary, A.; et al. Cancer Chemotherapy and beyond: Current Status, Drug Candidates, Associated Risks and Progress in Targeted Therapeutics. Genes Dis. 2022, 10, 1367–1401. [Google Scholar] [CrossRef] [PubMed]
- Altun, İ.; Sonkaya, A. The Most Common Side Effects Experienced by Patients Were Receiving First Cycle of Chemotherapy. Iran. J. Public Health 2018, 47, 1218–1219. [Google Scholar]
- Majeed, H.; Gupta, V. Adverse Effects of Radiation Therapy. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Possible Problems after Cancer Surgery. Available online: https://www.cancerresearchuk.org/about-cancer/treatment/surgery/long-term-problems (accessed on 15 June 2024).
- Tohme, S.; Simmons, R.L.; Tsung, A. Surgery for Cancer: A Trigger for Metastases. Cancer Res. 2017, 77, 1548–1552. [Google Scholar] [CrossRef]
- Bhatt, V.R. Cancer in Older Adults: Understanding Cause and Effects of Chemotherapy-Related Toxicities. Future Oncol. 2019, 15, 2557–2560. [Google Scholar] [CrossRef]
- Chang, S.; Goldstein, N.E.; Dharmarajan, K.V. Managing an Older Adult with Cancer: Considerations for Radiation Oncologists. BioMed Res. Int. 2017, 2017, 1695101. [Google Scholar] [CrossRef] [PubMed]
- Kowdley, G.C.; Merchant, N.; Richardson, J.P.; Somerville, J.; Gorospe, M.; Cunningham, S.C. Cancer Surgery in the Elderly. Sci. World J. 2012, 2012, 303852. [Google Scholar] [CrossRef]
- Lewis, J.H.; Kilgore, M.L.; Goldman, D.P.; Trimble, E.L.; Kaplan, R.; Montello, M.J.; Housman, M.G.; Escarce, J.J. Participation of Patients 65 Years of Age or Older in Cancer Clinical Trials. J. Clin. Oncol. 2003, 21, 1383–1389. [Google Scholar] [CrossRef] [PubMed]
- Jha, S.; Topol, E.J. Adapting to Artificial Intelligence: Radiologists and Pathologists as Information Specialists. JAMA 2016, 316, 2353–2354. [Google Scholar] [CrossRef]
- Topol, E.J. High-Performance Medicine: The Convergence of Human and Artificial Intelligence. Nat. Med. 2019, 25, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Dave, M.; Patel, N. Artificial Intelligence in Healthcare and Education. Br. Dent. J. 2023, 234, 761–764. [Google Scholar] [CrossRef] [PubMed]
- Amisha; Malik, P.; Pathania, M.; Rathaur, V.K. Overview of Artificial Intelligence in Medicine. J. Fam. Med. Prim. Care 2019, 8, 2328–2331. [Google Scholar] [CrossRef]
- Martínez-Sellés, M.; Marina-Breysse, M. Current and Future Use of Artificial Intelligence in Electrocardiography. J. Cardiovasc. Dev. Dis. 2023, 10, 175. [Google Scholar] [CrossRef]
- Meskó, B.; Görög, M. A Short Guide for Medical Professionals in the Era of Artificial Intelligence. NPJ Digit. Med. 2020, 3, 126. [Google Scholar] [CrossRef]
- AI May Improve Doctor-Patient Interactions for Older Adults with Cancer|Cornell Chronicle. Available online: https://news.cornell.edu/stories/2024/05/ai-may-improve-doctor-patient-interactions-older-adults-cancer (accessed on 15 June 2024).
- O’Connor, A.M.; Wennberg, J.E.; Legare, F.; Llewellyn-Thomas, H.A.; Moulton, B.W.; Sepucha, K.R.; Sodano, A.G.; King, J.S. Toward The ‘Tipping Point’: Decision Aids and Informed Patient Choice. Health Aff. 2007, 26, 716–725. [Google Scholar] [CrossRef]
- Hao, Y.; Liu, Z.; Riter, R.N.; Kalantari, S. Advancing Patient-Centered Shared Decision-Making with AI Systems for Older Adult Cancer Patients. In Proceedings of the CHI Conference on Human Factors in Computing Systems, Oahu, HI, USA, 11–16 May 2024; Association for Computing Machinery: New York, NY, USA, 2024; pp. 1–20. [Google Scholar]
- Hamet, P.; Tremblay, J. Artificial Intelligence in Medicine. Metabolism 2017, 69, S36–S40. [Google Scholar] [CrossRef]
- Cesario, A.; D’Oria, M.; Calvani, R.; Picca, A.; Pietragalla, A.; Lorusso, D.; Daniele, G.; Lohmeyer, F.M.; Boldrini, L.; Valentini, V.; et al. The Role of Artificial Intelligence in Managing Multimorbidity and Cancer. J. Pers. Med. 2021, 11, 314. [Google Scholar] [CrossRef]
- Tinetti, M.E.; Fried, T.R.; Boyd, C.M. Designing Health Care for the Most Common Chronic Condition—Multimorbidity. JAMA J. Am. Med. Assoc. 2012, 307, 2493–2494. [Google Scholar] [CrossRef] [PubMed]
- Definition of Overall Survival Rate—NCI Dictionary of Cancer Terms—NCI. Available online: https://www.cancer.gov/publications/dictionaries/cancer-terms/def/overall-survival-rate (accessed on 16 June 2024).
- Van Hulsteijn, L.T.; Corssmit, E.P.M.; Coremans, I.E.M.; Smit, J.W.A.; Jansen, J.C.; Dekkers, O.M. Regression and Local Control rates after Radiotherapy for Jugulotympanic Paragangliomas: Systematic Review and Meta-Analysis. Radiother. Oncol. 2013, 106, 161–168. [Google Scholar] [CrossRef]
- Definition of Progression-Free Survival—NCI Dictionary of Cancer Terms—NCI. Available online: https://www.cancer.gov/publications/dictionaries/cancer-terms/def/progression-free-survival (accessed on 16 June 2024).
- Aghamaliyev, U.; Karimbayli, J.; Giessen-Jung, C.; Matthias, I.; Unger, K.; Andrade, D.; Hofmann, F.O.; Weniger, M.; Angele, M.K.; Benedikt Westphalen, C.; et al. ChatGPT’s Gastrointestinal Tumor Board Tango: A Limping Dance Partner? Eur. J. Cancer 2024, 205, 114100. [Google Scholar] [CrossRef] [PubMed]
- Chen, V.J.; Oermann, E.; Vahdat, S.; Rabin, J.; Suy, S.; Yu, X.; Collins, S.P.; Subramaniam, D.; Banovac, F.; Anderson, E.; et al. CyberKnife with Tumor Tracking: An Effective Treatment for High-Risk Surgical Patients with Stage I Non-Small Cell Lung Cancer. Front. Oncol. 2012, 2, 9. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Zhou, B.; Huang, H.; Wang, Y.; Jiang, W.; Wang, J.; Ding, W.; Wang, Z.; Chen, G.; Sun, X. Efficacy and Safety of Stereotactic Radiotherapy on Elderly Patients with Stage I-II Central Non-Small Cell Lung Cancer. Front. Oncol. 2024, 14, 1235630. [Google Scholar] [CrossRef] [PubMed]
- Karam, S.D.; Horne, Z.D.; Hong, R.L.; Baig, N.; Gagnon, G.J.; McRae, D.; Duhamel, D.; Nasr, N.M. Robotic Stereotactic Body Radiation Therapy for Elderly Medically Inoperable Early-Stage Non-Small Cell Lung Cancer. Lung Cancer Targets Ther. 2013, 4, 35–42. [Google Scholar] [CrossRef]
- Kim, M.-S.; Park, H.-Y.; Kho, B.-G.; Park, C.-K.; Oh, I.-J.; Kim, Y.-C.; Kim, S.; Yun, J.-S.; Song, S.-Y.; Na, K.-J.; et al. Artificial Intelligence and Lung Cancer Treatment Decision: Agreement with Recommendation of Multidisciplinary Tumor Board. Transl. Lung Cancer Res. 2020, 9, 507–514. [Google Scholar] [CrossRef]
- Somashekhar, S.P.; Sepúlveda, M.-J.; Puglielli, S.; Norden, A.D.; Shortliffe, E.H.; Kumar, C.R.; Rauthan, A.; Kumar, N.A.; Patil, P.; Rhee, K.; et al. Watson for Oncology and Breast Cancer Treatment Recommendations: Agreement with an Expert Multidisciplinary Tumor Board. Ann. Oncol. 2018, 29, 418–423. [Google Scholar] [CrossRef]
- Wang, Z.; Li, A.-M.; Gao, J.; Li, J.; Li, B.; Lee, P.; Simone, C.B.; Song, Y.; Zhu, X.-X. Clinical Outcomes of CyberKnife Stereotactic Radiosurgery for Elderly Patients with Presumed Primary Stage I Lung Cancer. Transl. Lung Cancer Res. 2017, 6, 6–13. [Google Scholar] [CrossRef]
- Abuelgasim, K.A.; Jazieh, A.R. Quality Measures for Multidisciplinary Tumor Boards and Their Role in Improving Cancer Care. Glob. J. Qual. Saf. Healthc. 2024, 7, 28–33. [Google Scholar] [CrossRef]
- Mano, M.S.; Çitaku, F.T.; Barach, P. Implementing Multidisciplinary Tumor Boards in Oncology: A Narrative Review. Future Oncol. 2022, 18, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Stereotactic Radiotherapy (SRT). Available online: https://www.cancerresearchuk.org/about-cancer/treatment/radiotherapy/external/types/stereotactic-body-radiotherapy-sbrt (accessed on 16 June 2024).
- Tipton, K.N.; Sullivan, N.; Bruening, W.; Inamdar, R.; Launders, J.; Uhl, S.; Schoelles, K.M. Executive Summary. In Stereotactic Body Radiation Therapy [Internet]; Agency for Healthcare Research and Quality (US): Rockville, MD, USA, 2011. [Google Scholar]
- Watanabe, K.; Katsui, K.; Sugiyama, S.; Yoshio, K.; Kuroda, M.; Hiraki, T.; Kiura, K.; Maeda, Y.; Toyooka, S.; Kanazawa, S. Lung Stereotactic Body Radiation Therapy for Elderly Patients Aged ≥ 80 Years with Pathologically Proven Early-Stage Non-Small Cell Lung Cancer: A Retrospective Cohort Study. Radiat. Oncol. Lond. Engl. 2021, 16, 39. [Google Scholar] [CrossRef]
- Shu, Z.; Dong, B.; Shi, L.; Shen, W.; Hang, Q.; Wang, J.; Chen, Y. Stereotactic Body Radiotherapy for Elderly Patients (≥ 75 years) with Early-Stage Non-Small Cell Lung Cancer. J. Cancer Res. Clin. Oncol. 2020, 146, 1263–1271. [Google Scholar] [CrossRef] [PubMed]
- Aoki, S.; Onishi, H.; Karube, M.; Yamamoto, N.; Yamashita, H.; Shioyama, Y.; Matsumoto, Y.; Matsuo, Y.; Miyakawa, A.; Matsushita, H.; et al. Comparative Analysis of Photon Stereotactic Radiotherapy and Carbon-Ion Radiotherapy for Elderly Patients with Stage I Non-Small-Cell Lung Cancer: A Multicenter Retrospective Study. Cancers 2023, 15, 3633. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.; Wang, J.; Zhu, X.; Chen, Y.; Xu, Y.; Shao, K.; Zheng, L.; Ying, H.; Chen, M.; Cao, J. Comparison of the Outcomes of Stereotactic Body Radiotherapy versus Surgical Treatment for Elderly (≥70) Patients with Early-Stage Non-Small Cell Lung Cancer after Propensity Score Matching. Radiat. Oncol. Lond. Engl. 2019, 14, 195. [Google Scholar] [CrossRef]
- Shirini, D.; Schwartz, L.H.; Dercle, L. Artificial Intelligence for Aging Research in Cancer Drug Development. Aging 2023, 15, 12699–12701. [Google Scholar] [CrossRef]
- Huang, S.; Yang, J.; Fong, S.; Zhao, Q. Artificial Intelligence in Cancer Diagnosis and Prognosis: Opportunities and Challenges. Cancer Lett. 2020, 471, 61–71. [Google Scholar] [CrossRef]
- Zhang, S.; Bantum, E.O.; Owen, J.; Bakken, S.; Elhadad, N. Online Cancer Communities as Informatics Intervention for Social Support: Conceptualization, Characterization, and Impact. J. Am. Med. Inform. Assoc. 2017, 24, 451–459. [Google Scholar] [CrossRef]
- Shinde, A.; Deore, G.; Navsariwala, K.P.; Tabassum, H.; Wani, M. We Are All Aging, and Here’s Why. Aging Med. 2022, 5, 211–231. [Google Scholar] [CrossRef]
- Gruetzemacher, R.; Gupta, A.; Paradice, D. 3D Deep Learning for Detecting Pulmonary Nodules in CT Scans. J. Am. Med. Inform. Assoc. JAMIA 2018, 25, 1301–1310. [Google Scholar] [CrossRef]
- Yasaka, K.; Akai, H.; Abe, O.; Kiryu, S. Deep Learning with Convolutional Neural Network for Differentiation of Liver Masses at Dynamic Contrast-Enhanced CT: A Preliminary Study. Radiology 2018, 286, 887–896. [Google Scholar] [CrossRef]
- Liu, F.; Xie, L.; Xia, Y.; Fishman, E.K.; Yuille, A.L. Joint Shape Representation and Classification for Detecting PDAC. arXiv 2019, arXiv:1804.10684. [Google Scholar] [CrossRef]
- Zhou, W.; Zhang, X.; Ding, J.; Deng, L.; Cheng, G.; Wang, X. Improved Breast Lesion Detection in Mammogram Images Using a Deep Neural Network. Diagn. Interv. Radiol. 2023, 29, 588–595. [Google Scholar] [CrossRef]
- Lehman, C.D.; Yala, A.; Schuster, T.; Dontchos, B.; Bahl, M.; Swanson, K.; Barzilay, R. Mammographic Breast Density Assessment Using Deep Learning: Clinical Implementation. Radiology 2019, 290, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Lieman-Sifry, J.; Le, M.; Lau, F.; Sall, S.; Golden, D. FastVentricle: Cardiac Segmentation with ENet. arXiv 2017, arXiv:1704.04296. [Google Scholar] [CrossRef]
- Wood, D.E.; White, J.R.; Georgiadis, A.; Van Emburgh, B.; Parpart-Li, S.; Mitchell, J.; Anagnostou, V.; Niknafs, N.; Karchin, R.; Papp, E.; et al. A Machine Learning Approach for Somatic Mutation Discovery. Sci. Transl. Med. 2018, 10, eaar7939. [Google Scholar] [CrossRef]
- Lin, C.; Jain, S.; Kim, H.; Bar-Joseph, Z. Using Neural Networks for Reducing the Dimensions of Single-Cell RNA-Seq data. Nucleic Acids Res. 2017, 45, e156. [Google Scholar] [CrossRef] [PubMed]
- Angermueller, C.; Lee, H.J.; Reik, W.; Stegle, O. DeepCpG: Accurate Prediction of Single-Cell DNA Methylation States Using Deep Learning. Genome Biol. 2017, 18, 67. [Google Scholar] [CrossRef]
- Van Dijk, D.; Sharma, R.; Nainys, J.; Yim, K.; Kathail, P.; Carr, A.; Burdziak, C.; Moon, K.R.; Chaffer, C.L.; Pattabiraman, D.; et al. Recovering Gene Interactions from Single-Cell Data Using Data Diffusion. Cell 2018, 174, 716–729. [Google Scholar] [CrossRef]
- Caravagna, G.; Giarratano, Y.; Ramazzotti, D.; Tomlinson, I.; Graham, T.A.; Sanguinetti, G.; Sottoriva, A. Detecting Repeated Cancer Evolution from Multi-Region Tumor Sequencing Data. Nat. Methods 2018, 15, 707–714. [Google Scholar] [CrossRef]
- Manak, M.S.; Varsanik, J.S.; Hogan, B.J.; Whitfield, M.J.; Su, W.R.; Joshi, N.; Steinke, N.; Min, A.; Berger, D.; Saphirstein, R.J.; et al. Live-Cell Phenotypic-Biomarker Microfluidic Assay for the Risk Stratification of Cancer Patients via Machine Learning. Nat. Biomed. Eng. 2018, 2, 761–772. [Google Scholar] [CrossRef] [PubMed]
- Stringhini, S.; Carmeli, C.; Jokela, M.; Avendaño, M.; Muennig, P.; Guida, F.; Ricceri, F.; d’Errico, A.; Barros, H.; Bochud, M.; et al. Socioeconomic Status and the 25 × 25 Risk Factors as Determinants of Premature Mortality: A Multicohort Study and Meta-analysis of 1·7 Million Men and Women. Lancet Lond. Engl. 2017, 389, 1229–1237. [Google Scholar] [CrossRef] [PubMed]
- Guan, Z.; Li, H.; Liu, R.; Cai, C.; Liu, Y.; Li, J.; Wang, X.; Huang, S.; Wu, L.; Liu, D.; et al. Artificial Intelligence in Diabetes Management: Advancements, Opportunities, and Challenges. Cell Rep. Med. 2023, 4, 101213. [Google Scholar] [CrossRef]
- Alhalafi, A.; Alqahtani, S.M.; Alqarni, N.A.; Aljuaid, A.T.; Aljaber, G.T.; Alshahrani, L.M.; Mushait, H. Utilizing Artificial Intelligence Among Patients with Diabetes: A Systematic Review and Meta-Analysis. Cureus 2024, 16, e58713. [Google Scholar] [CrossRef]
- Rajpurkar, P.; Irvin, J.; Bagul, A.; Ding, D.; Duan, T.; Mehta, H.; Yang, B.; Zhu, K.; Laird, D.; Ball, R.L.; et al. MURA: Large Dataset for Abnormality Detection in Musculoskeletal Radiographs. arXiv 2018, arXiv:1712.06957. [Google Scholar] [CrossRef]
- Ehteshami Bejnordi, B.; Veta, M.; Johannes van Diest, P.; van Ginneken, B.; Karssemeijer, N.; Litjens, G.; van der Laak, J.A.W.M.; Hermsen, M.; Manson, Q.F.; Balkenhol, M.; et al. Diagnostic Assessment of Deep Learning Algorithms for Detection of Lymph Node Metastases in Women with Breast Cancer. JAMA 2017, 318, 2199–2210. [Google Scholar] [CrossRef] [PubMed]
- Steiner, D.F.; MacDonald, R.; Liu, Y.; Truszkowski, P.; Hipp, J.D.; Gammage, C.; Thng, F.; Peng, L.; Stumpe, M.C. Impact of Deep Learning Assistance on the Histopathologic Review of Lymph Nodes for Metastatic Breast Cancer. Am. J. Surg. Pathol. 2018, 42, 1636–1646. [Google Scholar] [CrossRef] [PubMed]
| Study | Country | AI Used | Age (Percent Female) | Cancer (Stage) | Race and Ethnicity | Study Design |
|---|---|---|---|---|---|---|
| Aghamaliyev et al., 2024 [39] | Germany | ChatGPT 3.5 | NR (44.3%) | Gastrointestinal cancer (NR) | NR | Retrospective |
| Chen et al., 2012 [40] | USA | Cyberknife™ (version unspecified) | Range: 63–87 y (60.0%) | NSCLC (I) | 82.5% Caucasian 17.5% African | Retrospective |
| Ji et al., 2024 [41] | China | Cyberknife™ (version unspecified) | Range: ≥65 y (9.1%) | NSCLC (I, II) | NR | Retrospective |
| Karam et al., 2013 [42] | USA | Cyberknife™ (version unspecified) | Range: 65–90 (39.0%) | NSCLC (I) | NR | Retrospective |
| Kim et al., 2020 [43] | South Korea | IBM WFO (version 18.4) | Median: 71 y (16.1%) | Lung Cancer (I, II, III, IV) | NR | Retrospective |
| Somashekhar et al., 2018 [44] | India | IBM WFO (version 16.4) | Mean: 52 (100.0%) | Breast cancer (I, II, III, IV) | NR | Retrospective |
| Wang et al., 2017 [45] | USA | Cyberknife™ (version unspecified) | Range: ≥75 y (20.0%) | Lung cancer (I) | NR | Retrospective |
| Study | Objectives | Outcomes | Conclusions |
|---|---|---|---|
| Aghamaliyev et al., 2023 [39] | ChatGPT 3.5 was used to create appropriate treatment plans for patients with gastrointestinal cancers. | ChatGPT 3.5 created specific treatment plans 70% of the time and general plans 30% of the time for those older than 80 years. These respective percentages for those younger than 80 years were 83.2% and 16.8%, respectively. | When comparing patients older than 80 years to those younger than 80 years, there was a statistically insignificant difference (p = 0.2) in the percentage of general and specific treatment plans devised for each age group. |
| Chen et al., 2012 [40] | CyberKnife (version unspecified) was used on medically inoperable older adult patients with stage I lung cancer and early stage (NSCLC). | Patients had a 3-year OS rate of 75% and a 3-year locoregional control rate of 91%. | CyberKnife is a reasonable treatment option for patients with clinical stage I NSCLC who are not eligible for segmentectomy or lobectomy. This is evidenced through CyberKnife treatment tumor tracking, which resulted in locoregional control of 70% and overall survival of 80% for high-risk surgical patients with clinical stage I NSCLC. |
| Ji et al., 2024 [41] | Cyberknife SBRT (version unspecified) was used to treat older adult patients with stage I-II central NSCLC. | The 1-year overall survival rate of patients who received these treatments was 86%. The studies reported median progression-free survival lengths of 19 months. | Cyberknife SBRT can safely and effectively control local tumor progression and acceptable radiation toxicity among older patients with centrally located stage I–II NSCLC. |
| Karam et al., 2013 [42] | Cyberknife SBRT (version unspecified) was used to treat older adult patients with inoperable early stage NSCLC. | The 1-year overall survival rate of patients who received these treatments was 70%. The studies reported median progression-free survival lengths of 31 months, while the 1-year local control rate was 80%. | Cyberknife SBRT can safely and effectively treat patients with inoperable early stage NSCLC. The median biologically effective dose, histology, and tumor size are predictors of local control, whereas tumor size and gender predict overall survival. |
| Kim et al., 2020 [43] | The use of WFO (version 18.4) was assessed for clinical treatment for patients with lung cancer. | Between the MTB and WFO in patients with lung cancer, there was an overall concordance rate of 92.4%. | Though concordance varied by stage, the treatment decisions of the WFO exhibited a high degree of agreement with those of the MTB. |
| Somashekhar et al., 2018 [44] | The use of WFO (version 16.4) was assessed for clinical treatment for patients with breast cancer. | Between the MTB and WFO in patients with breast cancer, there was an overall concordance rate of 73–93%. | Though concordance varied by stage, the treatment decisions of the WFO exhibited a high degree of agreement with those of the MTB. |
| Wang et al., 2017 [45] | Cyberknife SBRT (version unspecified) was used to treat older adult patients with presumed primary stage I lung cancer. | The 1-year overall survival rates of patients who received these treatments was 96%. The studies reported median progression-free survival lengths of 48 months, while the 1-year local control rate was 100%. | Cyberknife SBRT can safely and effectively treat patients with presumed primary stage I lung cancer, especially when there is difficulty confirming pathological malignancy. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obimba, D.C.; Esteva, C.; Nzouatcham Tsicheu, E.N.; Wong, R. Effectiveness of Artificial Intelligence Technologies in Cancer Treatment for Older Adults: A Systematic Review. J. Clin. Med. 2024, 13, 4979. https://doi.org/10.3390/jcm13174979
Obimba DC, Esteva C, Nzouatcham Tsicheu EN, Wong R. Effectiveness of Artificial Intelligence Technologies in Cancer Treatment for Older Adults: A Systematic Review. Journal of Clinical Medicine. 2024; 13(17):4979. https://doi.org/10.3390/jcm13174979
Chicago/Turabian StyleObimba, Doris C., Charlene Esteva, Eurika N. Nzouatcham Tsicheu, and Roger Wong. 2024. "Effectiveness of Artificial Intelligence Technologies in Cancer Treatment for Older Adults: A Systematic Review" Journal of Clinical Medicine 13, no. 17: 4979. https://doi.org/10.3390/jcm13174979
APA StyleObimba, D. C., Esteva, C., Nzouatcham Tsicheu, E. N., & Wong, R. (2024). Effectiveness of Artificial Intelligence Technologies in Cancer Treatment for Older Adults: A Systematic Review. Journal of Clinical Medicine, 13(17), 4979. https://doi.org/10.3390/jcm13174979







