Approaching Ventricular Tachycardia Ablation in 2024: An Update on Mapping and Ablation Strategies, Timing, and Future Directions
Abstract
1. History
2. Surface ECG and VT Origin
3. Mapping
3.1. Activation Mapping
3.2. Pace Mapping
- (1)
- High correspondence (matching) between the paced and clinical QRS is required [14].
- (2)
- (3)
- Intramural circuits can limit the ability of pace mapping to identify critical isthmuses, as part of the circuit is not mappable [12].
- (4)
- An abrupt change in the QRS morphology may not necessarily pinpoint the isthmus but could represent a remote bystander [12].
- (5)
- Attention should be given to the paced rate and pacing amplitude, as the morphology of the paced QRS can also be influenced by the paced rate, and high pacing amplitudes may result in far-field captures [12].
- (6)
- Stimulation is usually bipolar and, as such, it is less accurate in identifying the critical isthmus.
3.3. Entrainment Mapping
3.4. Substrate Mapping
4. Imaging
4.1. Cardiac Magnetic Resonance
4.2. Multi-Detector Computed Tomography
5. Endocardial and Epicardial Approaches
6. Deferred vs. Early Ablation
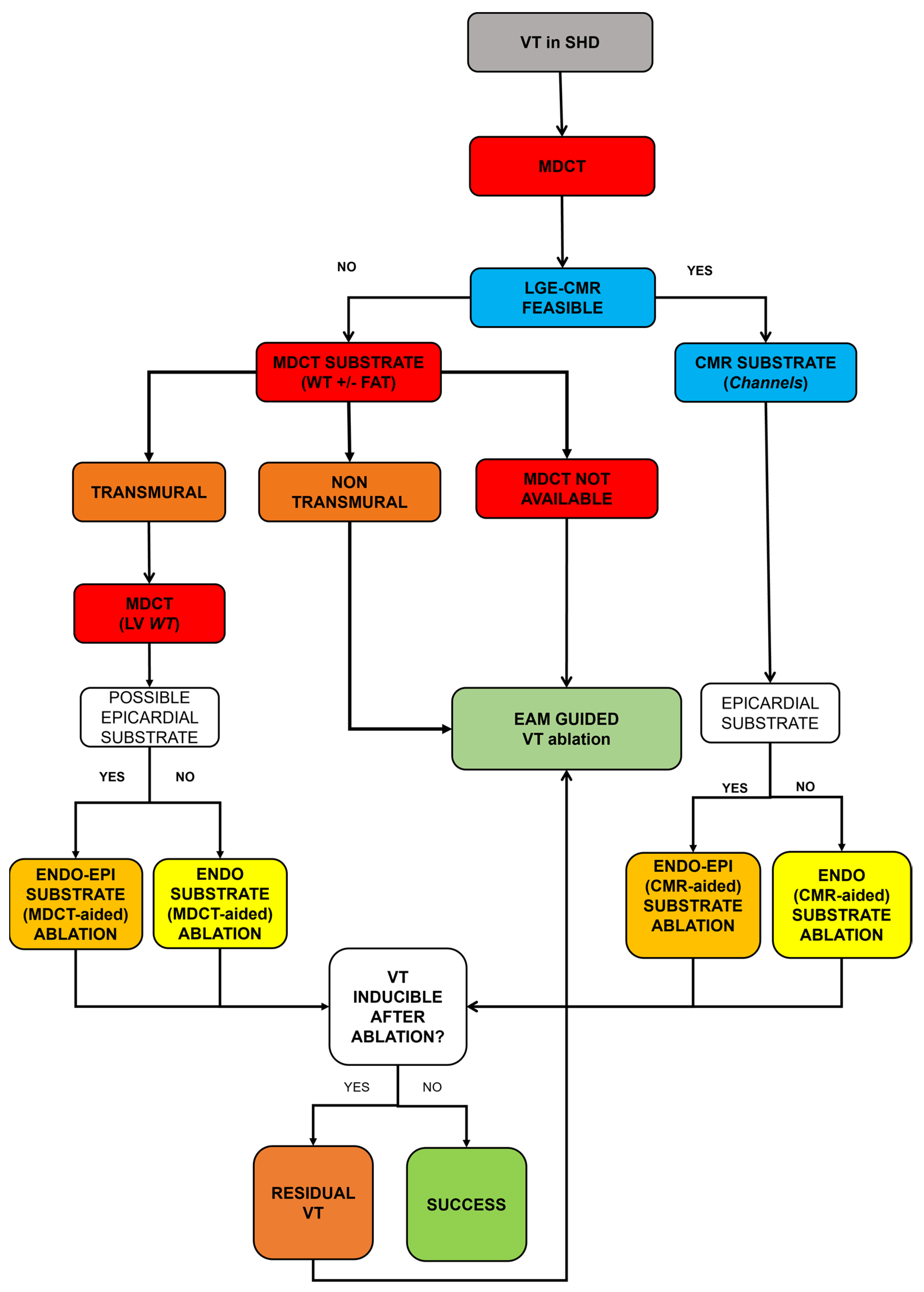
7. Procedural VT Ablation Workflow
8. Complication
9. Future Perspectives in VT Ablation
Funding
Conflicts of Interest
References
- Couch, O.A. Cardiac Aneurysm with Ventricular Tachycardia and Subsequent Excision of Aneurysm. Circulation 1959, 20, 251–253. [Google Scholar] [CrossRef]
- Buxton, A.E.; Locke, A.H.; Miller, J.M.; D’Avila, A.; Marchlinski, F.E. Thirty years of catheter ablation for ventricular tachycardia. Heart Rhythm 2021, 18, 1033–1034. [Google Scholar] [CrossRef] [PubMed]
- Josephson, M.E.; Harken, A.H.; Horowitz, L.N. Endocardial excision: A new surgical technique for the treatment of recurrent ventricular tachycardia. Circulation 1979, 60, 1430–1439. [Google Scholar] [CrossRef]
- Josephson, M.E.; Horowitz, L.N.; Farshidi, A.; Spear, J.F.; Kastor, J.A.; Moore, E.N. Recurrent sustained ventricular tachycardia. 2. Endocardial mapping. Circulation 1978, 57, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Hartzler, G.O. Electrode catheter ablation of refractory focal ventricular tachycardia. J. Am. Coll. Cardiol. 1983, 2, 1107–1113. [Google Scholar] [CrossRef]
- Sosa, E.; Scanavacca, M.; d’Avila, A.; Pilleggi, F. A new technique to perform epicardial mapping in the electrophysiology laboratory. J. Cardiovasc. Electrophysiol. 1996, 7, 531–536. [Google Scholar] [CrossRef]
- Gepstein, L.; Hayam, G.; Ben-Haim, S.A. A novel method for nonfluoroscopic catheter-based electroanatomical mapping of the heart. In vitro and in vivo accuracy results. Circulation 1997, 95, 1611–1622. [Google Scholar] [CrossRef] [PubMed]
- Spurrell, R.A.; Sowton, E.; Deuchar, D.C. Ventricular tachycardia in 4 patients evaluated by programmed electrical stimulation of heart and treated in 2 patients by surgical division of anterior radiation of left bundle-branch. Br. Heart J. 1973, 35, 1014–1025. [Google Scholar] [CrossRef][Green Version]
- Enriquez, A.; Baranchuk, A.; Briceno, D.; Saenz, L.; Garcia, F. How to use the 12-lead ECG to predict the site of origin of idiopathic ventricular arrhythmias. Heart Rhythm 2019, 16, 1538–1544. [Google Scholar] [CrossRef]
- Yamada, T. Twelve-lead electrocardiographic localization of idiopathic premature ventricular contraction origins. J. Cardiovasc. Electrophysiol. 2019, 30, 2603–2617. [Google Scholar] [CrossRef]
- Enriquez, A.; Muser, D.; Markman, T.M.; Garcia, F. Mapping and Ablation of Premature Ventricular Complexes: State of the Art. JACC Clin. Electrophysiol. 2024, 10, 1206–1222. [Google Scholar] [CrossRef]
- Guenancia, C.; Supple, G.; Sellal, J.M.; Magnin-Poull, I.; Benali, K.; Hammache, N.; Echivard, M.; Marchlinski, F.; de Chillou, C. How to use pace mapping for ventricular tachycardia ablation in postinfarct patients. J. Cardiovasc. Electrophysiol. 2022, 33, 1801–1809. [Google Scholar] [CrossRef]
- Killu, A.M.; Mulpuru, S.K.; Asirvatham, S.J. Mapping and Ablation Procedures for the Treatment of Ventricular Tachycardia. Expert Rev. Cardiovasc. Ther. 2016, 14, 1071–1087. [Google Scholar] [CrossRef] [PubMed]
- Dixit, S.; Callans, D.J. Mapping for ventricular tachycardia. Card. Electrophysiol. Rev. 2002, 6, 436–441. [Google Scholar] [CrossRef] [PubMed]
- Anter, E.; Tschabrunn, C.M.; Buxton, A.E.; Josephson, M.E. High-Resolution Mapping of Postinfarction Reentrant Ventricular Tachycardia: Electrophysiological Characterization of the Circuit. Circulation 2016, 134, 314–327. [Google Scholar] [CrossRef]
- de Chillou, C.; Groben, L.; Magnin-Poull, I.; Andronache, M.; Abbas, M.M.; Zhang, N.; Abdelaal, A.; Ammar, S.; Sellal, J.-M.; Schwartz, J.; et al. Localizing the critical isthmus of postinfarct ventricular tachycardia: The value of pace-mapping during sinus rhythm. Heart Rhythm 2014, 11, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.H.; Stevenson, W.G. Activation times in and adjacent to reentry circuits during entrainment: Implications for mapping ventricular tachycardia. Am. Heart J. 1994, 127 Pt 1, 833–842. [Google Scholar] [CrossRef]
- Zeppenfeld, K. Ventricular Tachycardia Ablation in Nonischemic Cardiomyopathy. JACC Clin. Electrophysiol. 2018, 4, 1123–1140. [Google Scholar] [CrossRef]
- Vassallo, J.A.; Cassidy, D.M.; Kindwall, K.E.; Marchlinski, F.E.; Josephson, M.E. Nonuniform recovery of excitability in the left ventricle. Circulation 1988, 78, 1365–1372. [Google Scholar] [CrossRef]
- Josephson, M.E.; Anter, E. Substrate Mapping for Ventricular Tachycardia: Assumptions and Misconceptions. JACC Clin. Electrophysiol. 2015, 1, 341–352. [Google Scholar] [CrossRef]
- Anter, E.; Kleber, A.G.; Rottmann, M.; Leshem, E.; Barkagan, M.; Tschabrunn, C.M.; Contreras-Valdes, F.M.; Buxton, A.E. Infarct-Related Ventricular Tachycardia: Redefining the Electrophysiological Substrate of the Isthmus during Sinus Rhythm. JACC Clin. Electrophysiol. 2018, 4, 1033–1048. [Google Scholar] [CrossRef] [PubMed]
- Jaïs, P.; Maury, P.; Khairy, P.; Sacher, F.; Nault, I.; Komatsu, Y.; Hocini, M.; Forclaz, A.; Jadidi, A.S.; Weerasooryia, R.; et al. Elimination of local abnormal ventricular activities: A new end point for substrate modification in patients with scar-related ventricular tachycardia. Circulation 2012, 125, 2184–2196. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, S.; Cochet, H.; Sacher, F.; Mahida, S.; Berte, B.; Hooks, D.; Sellal, J.-M.; Al Jefairi, N.; Frontera, A.; Komatsu, Y.; et al. Impact of New Technologies and Approaches for Post-Myocardial Infarction Ventricular Tachycardia Ablation during Long-Term Follow-Up. Circ. Arrhythmia Electrophysiol. 2016, 9, e003901. [Google Scholar] [CrossRef] [PubMed]
- Vergara, P.; Trevisi, N.; Ricco, A.; Petracca, F.; Baratto, F.; Cireddu, M.; Bisceglia, C.; Maccabelli, G.; DELLA Bella, P. Late potentials abolition as an additional technique for reduction of arrhythmia recurrence in scar related ventricular tachycardia ablation. J. Cardiovasc. Electrophysiol. 2012, 23, 621–627. [Google Scholar] [CrossRef]
- Radinovic, A.; Peretto, G.; Sgarito, G.; Cauti, F.M.; Castro, A.; Narducci, M.L.; Mantovan, R.; Scaglione, M.; Solimene, F.; Scopinaro, A.; et al. Matching Ablation Endpoints to Long-Term Outcome: The Prospective Multicenter Italian Ventricular Tachycardia Ablation Registry. JACC Clin. Electrophysiol. 2023, 9, 836–847. [Google Scholar] [CrossRef] [PubMed]
- Irie, T.; Yu, R.; Bradfield, J.S.; Vaseghi, M.; Buch, E.F.; Ajijola, O.; Macias, C.; Fujimura, O.; Mandapati, R.; Boyle, N.G.; et al. Relationship between sinus rhythm late activation zones and critical sites for scar-related ventricular tachycardia: Systematic analysis of isochronal late activation mapping. Circ. Arrhythmia Electrophysiol. 2015, 8, 390–399. [Google Scholar] [CrossRef]
- Aziz, Z.; Shatz, D.; Raiman, M.; A Upadhyay, G.; Beaser, A.D.; Besser, S.A.; Shatz, N.A.; Fu, Z.; Jiang, R.; Nishimura, T.; et al. Targeted Ablation of Ventricular Tachycardia Guided by Wavefront Discontinuities during Sinus Rhythm: A New Functional Substrate Mapping Strategy. Circulation 2019, 140, 1383–1397. [Google Scholar] [CrossRef] [PubMed]
- Hsia, H.H.; Lin, D.; Sauer, W.H.; Callans, D.J.; Marchlinski, F.E. Anatomic characterization of endocardial substrate for hemodynamically stable reentrant ventricular tachycardia: Identification of endocardial conducting channels. Heart Rhythm 2006, 3, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Marrouche, N.F.; Schweikert, R.A.; Saliba, W.; Wazni, O.; Cummings, J.; Abdul-Karim, A.; Bhargava, M.; Burkhardt, J.D.; Kilicaslan, F.; et al. Relationship between successful ablation sites and the scar border zone defined by substrate mapping for ventricular tachycardia post-myocardial infarction. J. Cardiovasc. Electrophysiol. 2005, 16, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Blauer, J.J.; Swenson, D.; Higuchi, K.; Plank, G.; Ranjan, R.; Marrouche, N.; Macleod, R.S. Sensitivity and specificity of substrate mapping: An in silico framework for the evaluation of electroanatomical substrate mapping strategies. J. Cardiovasc. Electrophysiol. 2014, 25, 774–780. [Google Scholar] [CrossRef]
- Cheung, J.W.; Yeo, I.; Ip, J.E.; Thomas, G.; Liu, C.F.; Markowitz, S.M.; Lerman, B.B.; Kim, L.K. Outcomes, Costs, and 30-Day Readmissions After Catheter Ablation of Myocardial Infarct-Associated Ventricular Tachycardia in the Real World: Nationwide Readmissions Database 2010 to 2015. Circ. Arrhythmia Electrophysiol. 2018, 11, e006754. [Google Scholar] [CrossRef]
- Callans, D.J. Patients with hemodynamically tolerated ventricular tachycardia require implantable cardioverter defibrillators. Circulation 2007, 116, 1196–1203. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jackson, N.; Gizurarson, S.; Viswanathan, K.; King, B.; Massé, S.; Kusha, M.; Porta-Sanchez, A.; Jacob, J.R.; Khan, F.; Das, M.; et al. Decrement Evoked Potential Mapping: Basis of a Mechanistic Strategy for Ventricular Tachycardia Ablation. Circ. Arrhythmia Electrophysiol. 2015, 8, 1433–1442. [Google Scholar] [CrossRef] [PubMed]
- Acosta, J.; Andreu, D.; Penela, D.; Cabrera, M.; Carlosena, A.; Korshunov, V.; Vassanelli, F.; Borras, R.; Martínez, M.; Fernández-Armenta, J.; et al. Elucidation of hidden slow conduction by double ventricular extrastimuli: A method for further arrhythmic substrate identification in ventricular tachycardia ablation procedures. Europace 2018, 20, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Lammers, W.J.; Kirchhof, C.; Bonke, F.I.; Allessie, M.A. Vulnerability of rabbit atrium to reentry by hypoxia. Role of inhomogeneity in conduction and wavelength. Am. J. Physiol. 1992, 262 Pt 2, H47–H55. [Google Scholar] [CrossRef]
- Porta-Sánchez, A.; Jackson, N.; Lukac, P.; Kristiansen, S.B.; Nielsen, J.M.; Gizurarson, S.; Massé, S.; Labos, C.; Viswanathan, K.; King, B.; et al. Multicenter Study of Ischemic Ventricular Tachycardia Ablation with Decrement-Evoked Potential (DEEP) Mapping with Extra Stimulus. JACC Clin. Electrophysiol. 2018, 4, 307–315. [Google Scholar] [CrossRef]
- Berruezo, A.; Penela, D.; Jáuregui, B.; Soto-Iglesias, D. The role of imaging in catheter ablation of ventricular arrhythmias. Pacing Clin. Electrophysiol. 2021, 44, 1115–1125. [Google Scholar] [CrossRef]
- Pistelli, L.; Vetta, G.; Parlavecchio, A.; Crea, P.; Parisi, F.; Magnocavallo, M.; Caminiti, R.; Frea, S.; Vairo, A.; Desalvo, P.; et al. Arrhythmic risk profile in mitral valve prolapse: A systematic review and metanalysis of 1715 patients. J. Cardiovasc. Electrophysiol. 2024, 35, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Gatti, M.; Palmisano, A.; Esposito, A.; Fiore, S.; Monti, C.B.; Andreis, A.; Pistelli, L.; Vergara, P.; Bergamasco, L.; Giustetto, C.; et al. Feature tracking myocardial strain analysis in patients with bileaflet mitral valve prolapse: Relationship with LGE and arrhythmias. Eur. Radiol. 2021, 31, 7273–7282. [Google Scholar] [CrossRef] [PubMed]
- Wijnmaalen, A.P.; van der Geest, R.J.; Taxis, C.F.v.H.v.; Siebelink, H.-M.J.; Kroft, L.J.; Bax, J.J.; Reiber, J.H.; Schalij, M.J.; Zeppenfeld, K. Head-to-head comparison of contrast-enhanced magnetic resonance imaging and electroanatomical voltage mapping to assess post-infarct scar characteristics in patients with ventricular tachycardias: Real-time image integration and reversed registration. Eur. Heart J. 2011, 32, 104–114. [Google Scholar] [CrossRef]
- Piers, S.R.; Tao, Q.; Silva, M.d.R.; Siebelink, H.-M.; Schalij, M.J.; van der Geest, R.J.; Zeppenfeld, K. CMR-based identification of critical isthmus sites of ischemic and nonischemic ventricular tachycardia. JACC Cardiovasc. Imaging 2014, 7, 774–784. [Google Scholar] [CrossRef]
- Berruezo, A.; Ortiz-Pérez, J.T. Unraveling the Scar with Cardiac Magnetic Resonance. Circ. Cardiovasc. Imaging 2017, 10, e006907. [Google Scholar] [CrossRef] [PubMed]
- Cronin, E.M.; Bogun, F.M.; Maury, P.; Peichl, P.; Chen, M.; Namboodiri, N.; Aguinaga, L.; Leite, L.R.; Al-Khatib, S.M.; Anter, E.; et al. 2019 HRS/EHRA/APHRS/LAHRS expert consensus statement on catheter ablation of ventricular arrhythmias. EP Europace 2019, 21, 1143–1144. [Google Scholar] [CrossRef]
- Zghaib, T.; Ipek, E.G.; Hansford, R.; Ashikaga, H.; Berger, R.D.; Marine, J.E.; Spragg, D.D.; Tandri, H.; Zimmerman, S.L.; Halperin, H.; et al. Standard Ablation Versus Magnetic Resonance Imaging-Guided Ablation in the Treatment of Ventricular Tachycardia. Circ. Arrhythmia Electrophysiol. 2018, 11, e005973. [Google Scholar] [CrossRef]
- Soto-Iglesias, D.; Penela, D.; Jáuregui, B.; Acosta, J.; Fernández-Armenta, J.; Linhart, M.; Zucchelli, G.; Syrovnev, V.; Zaraket, F.; Terés, C.; et al. Cardiac Magnetic Resonance-Guided Ventricular Tachycardia Substrate Ablation. JACC Clin. Electrophysiol. 2020, 6, 436–447. [Google Scholar] [CrossRef] [PubMed]
- Lilli, A.; Parollo, M.; Mazzocchetti, L.; De Sensi, F.; Rossi, A.; Notarstefano, P.; Santoro, A.; Aquaro, G.D.; Cresti, A.; Lapira, F.; et al. Ventricular tachycardia ablation guided or aided by scar characterization with cardiac magnetic resonance: Rationale and design of VOYAGE study. BMC Cardiovasc. Disord. 2022, 22, 169. [Google Scholar] [CrossRef]
- Zaman, A.; Zhao, S.; Kron, J.; Abbate, A.; Tomdio, A.; Hundley, W.G.; Jordan, J.H. Role of Cardiac MRI Imaging of Focal and Diffuse Inflammation and Fibrosis in Cardiomyopathy Patients Who Have Pacemakers/ICD Devices. Curr. Cardiol. Rep. 2022, 24, 1529–1536. [Google Scholar] [CrossRef] [PubMed]
- Bhuva, A.N.; Treibel, T.A.; Seraphim, A.; Scully, P.; Knott, K.D.; Augusto, J.B.; Torlasco, C.; Menacho, K.; Lau, C.; Patel, K.; et al. Measurement of T1 Mapping in Patients with Cardiac Devices: Off-Resonance Error Extends Beyond Visual Artifact but Can Be Quantified and Corrected. Front. Cardiovasc. Med. 2021, 8, 631366. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kawaji, K.; Goyal, N.; Nazir, N.T.; Beaser, A.; O’Keefe-Baker, V.; Addetia, K.; Tung, R.; Hu, P.; Mor-Avi, V.; et al. Feasibility of Cardiac Magnetic Resonance Wideband Protocol in Patients with Implantable Cardioverter Defibrillators and Its Utility for Defining Scar. Am. J. Cardiol. 2019, 123, 1329–1335. [Google Scholar] [CrossRef]
- van Huls van Taxis, C.F.; Wijnmaalen, A.P.; Piers, S.R.; van der Geest, R.J.; Schalij, M.J.; Zeppenfeld, K. Real-time integration of MDCT-derived coronary anatomy and epicardial fat: Impact on epicardial electroanatomic mapping and ablation for ventricular arrhythmias. JACC Cardiovasc. Imaging 2013, 6, 42–52. [Google Scholar] [CrossRef]
- Yamashita, S.; Sacher, F.; Mahida, S.; Berte, B.; Lim, H.S.; Komatsu, Y.; Amraoui, S.; Denis, A.; Derval, N.; Laurent, F.; et al. Role of high-resolution image integration to visualize left phrenic nerve and coronary arteries during epicardial ventricular tachycardia ablation. Circ. Arrhythmia Electrophysiol. 2015, 8, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, Y.; Cochet, H.; Jadidi, A.; Sacher, F.; Shah, A.; Derval, N.; Scherr, D.; Pascale, P.; Roten, L.; Denis, A.; et al. Regional myocardial wall thinning at multidetector computed tomography correlates to arrhythmogenic substrate in postinfarction ventricular tachycardia: Assessment of structural and electrical substrate. Circ. Arrhythmia Electrophysiol. 2013, 6, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Parollo, M.; Mazzocchetti, L.; Di Cori, A.; Segreti, L.; De Lucia, R.; Grifoni, G.; Barletta, V.; Faggioni, L.; Aquaro, G.D.; Neri, E.; et al. Lipomatous metaplasia as the most reliable computed tomography predictor for functional substrate localization in scar-related ventricular tachycardia. Heart Rhythm 2023, 20, 1593–1594. [Google Scholar] [CrossRef]
- Esposito, A.; Palmisano, A.; Antunes, S.; Maccabelli, G.; Colantoni, C.; Rancoita, P.M.V.; Baratto, F.; Di Serio, C.; Rizzo, G.; De Cobelli, F.; et al. Cardiac CT with Delayed Enhancement in the Characterization of Ventricular Tachycardia Structural Substrate: Relationship between CT-Segmented Scar and Electro-Anatomic Mapping. J. Am. Coll. Cardiol. Img. 2016, 9, 822–832. [Google Scholar] [CrossRef]
- Berte, B.; Bogun, F.M.; Santangeli, P.; Tedrow, U.B.; Ene, E.; Takigawa, M.; Garg, L.; Dherange, P.; Cheniti, G.; Dilling-Boer, D.M.; et al. PO-651-03 image-integration during VT ablation results in major procedural shortening: Results from the international MUSIC consortium. Heart Rhythm 2022, 19, S248. [Google Scholar] [CrossRef]
- Tung, R.; Raiman, M.; Liao, H.; Zhan, X.; Chung, F.P.; Nagel, R.; Hu, H.; Jian, J.; Shatz, D.Y.; Besser, S.A.; et al. Simultaneous Endocardial and Epicardial Delineation of 3D Reentrant Ventricular Tachycardia. J. Am. Coll. Cardiol. 2020, 75, 884–897. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Armenta, J.; Berruezo, A.; Andreu, D.; Camara, O.; Silva, E.; Serra, L.; Barbarito, V.; Carotenutto, L.; Evertz, R.; Ortiz-Pérez, J.T.; et al. Three-dimensional architecture of scar and conducting channels based on high resolution ce-CMR: Insights for ventricular tachycardia ablation. Circ. Arrhythmia Electrophysiol. 2013, 6, 528–537. [Google Scholar] [CrossRef]
- Tung, R.; Michowitz, Y.; Yu, R.; Mathuria, N.; Vaseghi, M.; Buch, E.; Bradfield, J.; Fujimura, O.; Gima, J.; Discepolo, W.; et al. Epicardial ablation of ventricular tachycardia: An institutional experience of safety and efficacy. Heart Rhythm 2013, 10, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Di Biase, L.; Santangeli, P.; Burkhardt, D.J.; Bai, R.; Mohanty, P.; Carbucicchio, C.; Russo, A.D.; Casella, M.; Mohanty, S.; Pump, A.; et al. Endo-epicardial homogenization of the scar versus limited substrate ablation for the treatment of electrical storms in patients with ischemic cardiomyopathy. J. Am. Coll. Cardiol. 2012, 60, 132–141. [Google Scholar] [CrossRef]
- Acosta, J.; Fernández-Armenta, J.; Penela, D.; Andreu, D.; Borras, R.; Vassanelli, F.; Korshunov, V.; Perea, R.J.; de Caralt, T.M.; Ortiz, J.T.; et al. Infarct transmurality as a criterion for first-line endo-epicardial substrate-guided ventricular tachycardia ablation in ischemic cardiomyopathy. Heart Rhythm 2016, 13, 85–95. [Google Scholar] [CrossRef]
- Nishimura, T.; Shatz, N.; Weiss, J.P.; Zawaneh, M.; Bai, R.; Beaser, A.D.; Upadhyay, G.A.; Aziz, Z.A.; Nayak, H.M.; Shatz, D.Y.; et al. Identification of Human Ventricular Tachycardia Demarcated by Fixed Lines of Conduction Block in a 3-Dimensional Hyperboloid Circuit. Circulation 2023, 148, 1354–1367. [Google Scholar] [CrossRef] [PubMed]
- Zucchelli, G.; Parollo, M.; Di Cori, A.; Mazzocchetti, L.; Segreti, L.; Grifoni, G.; Torre, M.; Sbragi, S.; De Lucia, R.; Barletta, V.; et al. Feasibility of carbon dioxide insufflation and impact on epicardial approach utilization for ventricular tachycardia ablation in a midvolume referral center. Heart Rhythm 2024, 21, 1032–1039. [Google Scholar] [CrossRef] [PubMed]
- Juliá, J.; Bokhari, F.; Uuetoa, H.; Derejko, P.; Traykov, V.B.; Gwizdala, A.; Sebag, F.A.; Hegbom, F.; Anfinsen, O.-G.; AlQubbany, A.; et al. A New Era in Epicardial Access for the Ablation of Ventricular Arrhythmias: The Epi-Co(2) Registry. JACC Clin. Electrophysiol. 2021, 7, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Sapp, J.L.; Wells, G.A.; Parkash, R.; Stevenson, W.G.; Blier, L.; Sarrazin, J.-F.; Thibault, B.; Rivard, L.; Gula, L.; Leong-Sit, P.; et al. Ventricular Tachycardia Ablation versus Escalation of Antiarrhythmic Drugs. N. Engl. J. Med. 2016, 375, 111–121. [Google Scholar] [CrossRef]
- Della Bella, P.; Baratto, F.; Vergara, P.; Bertocchi, P.; Santamaria, M.; Notarstefano, P.; Calò, L.; Orsida, D.; Tomasi, L.; Piacenti, M.; et al. Does Timing of Ventricular Tachycardia Ablation Affect Prognosis in Patients with an Implantable Cardioverter Defibrillator? Results From the Multicenter Randomized PARTITA Trial. Circulation 2022, 145, 1829–1838. [Google Scholar] [CrossRef]
- Willems, S.; Tilz, R.R.; Steven, D.; Kääb, S.; Wegscheider, K.; Gellér, L.; Meyer, C.; Heeger, C.-H.; Metzner, A.; Sinner, M.F.; et al. Preventive or Deferred Ablation of Ventricular Tachycardia in Patients with Ischemic Cardiomyopathy and Implantable Defibrillator (BERLIN VT): A Multicenter Randomized Trial. Circulation 2020, 141, 1057–1067. [Google Scholar] [CrossRef]
- Kuck, K.-H.; Schaumann, A.; Eckardt, L.; Willems, S.; Ventura, R.; Delacrétaz, E.; Pitschner, H.-F.; Kautzner, J.; Schumacher, B.; Hansen, P.S. Catheter ablation of stable ventricular tachycardia before defibrillator implantation in patients with coronary heart disease (VTACH): A multicentre randomised controlled trial. Lancet 2010, 375, 31–40. [Google Scholar] [CrossRef]
- Reddy, V.Y.; Reynolds, M.R.; Neuzil, P.; Richardson, A.W.; Taborsky, M.; Jongnarangsin, K.; Kralovec, S.; Sediva, L.; Ruskin, J.N.; Josephson, M.E. Prophylactic catheter ablation for the prevention of defibrillator therapy. N. Engl. J. Med. 2007, 357, 2657–2665. [Google Scholar] [CrossRef]
- Kuck, K.-H.; Tilz, R.R.; Deneke, T.; Hoffmann, B.A.; Ventura, R.; Hansen, P.S.; Zarse, M.; Hohnloser, S.H.; Kautzner, J.; Willems, S. Impact of Substrate Modification by Catheter Ablation on Implantable Cardioverter-Defibrillator Interventions in Patients with Unstable Ventricular Arrhythmias and Coronary Artery Disease: Results From the Multicenter Randomized Controlled SMS (Substrate Modification Study). Circ. Arrhythmia Electrophysiol. 2017, 10, e004422. [Google Scholar] [CrossRef]
- Tung, R.; Xue, Y.; Chen, M.; Jiang, C.; Shatz, D.Y.; Besser, S.A.; Hu, H.; Chung, F.-P.; Nakahara, S.; Kim, Y.-H.; et al. First-Line Catheter Ablation of Monomorphic Ventricular Tachycardia in Cardiomyopathy Concurrent with Defibrillator Implantation: The PAUSE-SCD Randomized Trial. Circulation 2022, 145, 1839–1849. [Google Scholar] [CrossRef]
- Kampaktsis, P.N.; Doulamis, I.P.; Tzani, A.; Cheung, J.W. Preventive versus deferred catheter ablation of myocardial infarct-associated ventricular tachycardia: A meta-analysis. Hear Rhythm O2 2020, 1, 275–282. [Google Scholar] [CrossRef]
- Falasconi, G.; Penela, D.; Soto-Iglesias, D.; Francia, P.; Teres, C.; Viveros, D.; Bellido, A.; Alderete, J.; Meca-Santamaria, J.; Franco, P.; et al. Preventive substrate ablation in chronic post-myocardial infarction patients with high-risk scar characteristics for ventricular arrhythmias: Rationale and design of PREVENT-VT study. J. Interv. Card. Electrophysiol. 2023, 66, 39–47. [Google Scholar] [CrossRef]
- Žižek, D.; Mrak, M.; Jan, M.; Mežnar, A.Z.; Ivanovski, M.; Žlahtič, T.; Kajdič, N.; Antolič, B.; Klemen, L.; Skale, R.; et al. Impact of preventive substrate catheter ablation on implantable cardioverter-defibrillator interventions in patients with ischaemic cardiomyopathy and infarct-related coronary chronic total occlusion. Europace 2024, 26, euae109. [Google Scholar] [CrossRef]
- Di Biase, L.; Burkhardt, J.D.; Lakkireddy, D.; Carbucicchio, C.; Mohanty, S.; Mohanty, P.; Trivedi, C.; Santangeli, P.; Bai, R.; Forleo, G.; et al. Ablation of Stable VTs Versus Substrate Ablation in Ischemic Cardiomyopathy: The VISTA Randomized Multicenter Trial. J. Am. Coll. Cardiol. 2015, 66, 2872–2882. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Armenta, J.; Soto-Iglesias, D.; Silva, E.; Penela, D.; Jáuregui, B.; Linhart, M.; Bisbal, F.; Acosta, J.; Fernandez, M.; Borras, R.; et al. Safety and Outcomes of Ventricular Tachycardia Substrate Ablation during Sinus Rhythm: A Prospective Multicenter Registry. JACC Clin. Electrophysiol. 2020, 6, 1435–1448. [Google Scholar] [CrossRef] [PubMed]
- Briceño, D.F.; Romero, J.; A Villablanca, P.; Londoño, A.; Diaz, J.C.; Maraj, I.; Batul, S.A.; Madan, N.; Patel, J.; Jagannath, A.; et al. Long-term outcomes of different ablation strategies for ventricular tachycardia in patients with structural heart disease: Systematic review and meta-analysis. EP Europace 2018, 20, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Baldinger, S.H.; Romero, J.; Fujii, A.; Mahida, S.N.; Tedrow, U.B.; Stevenson, W.G. Substrate-Based Ablation Versus Ablation Guided by Activation and Entrainment Mapping for Ventricular Tachycardia: A Systematic Review and Meta-Analysis. J. Cardiovasc. Electrophysiol. 2016, 27, 1437–1447. [Google Scholar] [CrossRef]
- Eckardt, L.; Doldi, F.; Anwar, O.; Gessler, N.; Scherschel, K.; Kahle, A.-K.; von Falkenhausen, A.S.; Thaler, R.; Wolfes, J.; Metzner, A.; et al. Major in-hospital complications after catheter ablation of cardiac arrhythmias: Individual case analysis of 43 031 procedures. Europace 2023, 26, euad361. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.Y.; Pearman, C.M.; Bonnett, L.; Adlan, A.; Chin, S.H.; Denham, N.; Modi, S.; Todd, D.; Hall, M.C.S.; Mahida, S. Complication rates following ventricular tachycardia ablation in ischaemic and non-ischaemic cardiomyopathies: A systematic review. J. Interv. Card. Electrophysiol. 2022, 63, 59–67. [Google Scholar] [CrossRef]
- Tan, M.C.; Yeo, Y.H.; Ang, Q.X.; Kiwan, C.; Fatunde, O.; Lee, J.Z.; Tolat, A.; Sorajja, D. Impact of age on hospital outcomes after catheter ablation for ventricular tachycardia. J. Arrhythmia 2024, 40, 317–324. [Google Scholar] [CrossRef]
- Subramanian, M.; Atreya, A.R.; Saggu, D.K.; Yalagudri, S.; Calambur, N. Catheter ablation of ventricular tachycardia: Strategies to improve outcomes. Front. Cardiovasc. Med. 2023, 10, 966634. [Google Scholar] [CrossRef]
- Monaci, S.; Qian, S.; Gillette, K.; Puyol-Antón, E.; Mukherjee, R.; Elliott, M.K.; Whitaker, J.; Rajani, R.; O’neill, M.; A Rinaldi, C.; et al. Non-invasive localization of post-infarct ventricular tachycardia exit sites to guide ablation planning: A computational deep learning platform utilizing the 12-lead electrocardiogram and intracardiac electrograms from implanted devices. EP Eurospace 2023, 25, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.T.; Olson, M.; Zheng, L.; Barham, W.; Moss, J.D.; Sauer, W.H. Effect of Irrigant Characteristics on Lesion Formation After Radiofrequency Energy Delivery Using Ablation Catheters with Actively Cooled Tips. J. Cardiovasc. Electrophysiol. 2015, 26, 792–798. [Google Scholar] [CrossRef]
- Hohmann, S.; Deisher, A.J.; Konishi, H.; Rettmann, M.E.; Suzuki, A.; Merrell, K.W.; Kruse, J.J.; Fitzgerald, S.T.; Newman, L.K.; Parker, K.D.; et al. Catheter-free ablation of infarct scar through proton beam therapy: Tissue effects in a porcine model. Heart Rhythm 2020, 17, 2190–2199. [Google Scholar] [CrossRef]
- Imamura, K.; Deisher, A.J.; Dickow, J.; Rettmann, M.E.; Yasin, O.Z.; Pepin, M.D.; Hohmann, S.; Konishi, H.; Suzuki, A.; Newman, L.K.; et al. Early Impact of Proton Beam Therapy on Electrophysiological Characteristics in a Porcine Model. Circ. Arrhythmia Electrophysiol. 2023, 16, e011179. [Google Scholar] [CrossRef]
- Hohmann, S.; Deisher, A.J.; Suzuki, A.; Konishi, H.; Rettmann, M.E.; Merrell, K.W.; Kruse, J.J.; Newman, L.K.; Parker, K.D.; Monahan, K.H.; et al. Left ventricular function after noninvasive cardiac ablation using proton beam therapy in a porcine model. Heart Rhythm 2019, 16, 1710–1719. [Google Scholar] [CrossRef]
- Stevenson, W.G.; Tedrow, U.B.; Reddy, V.; AbdelWahab, A.; Dukkipati, S.; John, R.M.; Fujii, A.; Schaeffer, B.; Tanigawa, S.; Elsokkari, I.; et al. Infusion Needle Radiofrequency Ablation for Treatment of Refractory Ventricular Arrhythmias. J. Am. Coll. Cardiol. 2019, 73, 1413–1425. [Google Scholar] [CrossRef] [PubMed]
- Tavares, L.; Lador, A.; Fuentes, S.; Da-Wariboko, A.; Blaszyk, K.; Malaczynska-Rajpold, K.; Papiashvili, G.; Korolev, S.; Peichl, P.; Kautzner, J.; et al. Intramural Venous Ethanol Infusion for Refractory Ventricular Arrhythmias: Outcomes of a Multicenter Experience. JACC Clin. Electrophysiol. 2020, 6, 1420–1431. [Google Scholar] [CrossRef]
- Kreidieh, B.; Rodríguez-Mañero, M.; Schurmann, P.; Ibarra-Cortez, S.H.; Dave, A.S.; Valderrábano, M. Retrograde Coronary Venous Ethanol Infusion for Ablation of Refractory Ventricular Tachycardia. Circ. Arrhythmia Electrophysiol. 2016, 9, e004352. [Google Scholar] [CrossRef]
- Flautt, T.; Valderrábano, M. Retrograde Coronary Venous Ethanol Infusion for Ablation of Refractory Left Ventricular Summit Arrhythmias. Card. Electrophysiol. Clin. 2023, 15, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Neven, K.; van Driel, V.; van Wessel, H.; van Es, R.; Doevendans, P.A.; Wittkampf, F. Myocardial lesion size after epicardial electroporation catheter ablation after subxiphoid puncture. Circ. Arrhythmia Electrophysiol. 2014, 7, 728–733. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Essebag, V.; Neuzil, P.; Dyrda, K.; Balt, J.; Dinov, B.; Darma, A.; Arya, A.; Sacher, F.; Reddy, V.Y.; et al. Cryocure-VT: The safety and effectiveness of ultra-low-temperature cryoablation of monomorphic ventricular tachycardia in patients with ischaemic and non-ischaemic cardiomyopathies. Europace 2024, 26, euae076. [Google Scholar] [CrossRef] [PubMed]
- Guandalini, G.S.; Liang, J.J.; Marchlinski, F.E. Ventricular Tachycardia Ablation: Past, Present, and Future Perspectives. JACC Clin. Electrophysiol. 2019, 5, 1363–1383. [Google Scholar] [CrossRef] [PubMed]

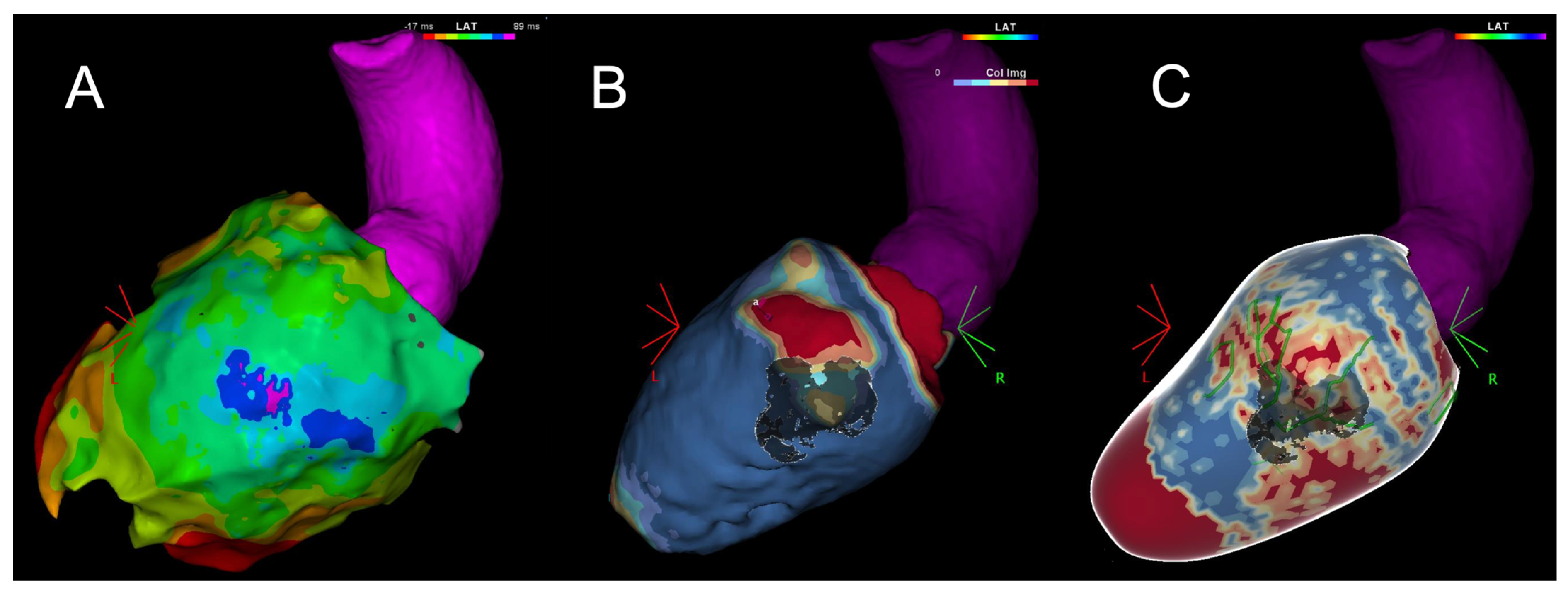
| Mapping Approach | Procedure | Target | Strength | Weakness | Setting |
|---|---|---|---|---|---|
| Activation Mapping | Activation sequence during VT | Electrogram with the earliest activation (30–50 ms) Critical isthmus in reentrant | Directly identify arrhythmia source/circuit Not limited by functional line of block | 70% of VTs are not tolerated | Enhanced automaticity and reentry |
| Pace Mapping | Ventricular pacing during sinus rhythm | Correspondence between paced QRS and clinical VT QRS | Identify either the exit site in focal arrhythmias or the mid-isthmus zone | Qualitative assessment Intramural circuits are not mappable Not specific for isthmus in reentrant VT Influenced by pacing threshold and rate | Enhanced automaticity and reentry |
| Entrainment Mapping | Entrain VT | Isthmus (PPI-TCL < 30 ms; S-QRS interval less than 50% TCL VT) | Identify isthmus | VT may not be tolerated Bystander sites may be confounding Antidromic activation of the circuit | Reentry |
| Substrate Mapping | Characterize arrhythmic substrate | LAVA Late potentials and isochrones Low-voltage zone | P2 distal to proximal during VT | Time consuming Not effective in functional blocks Far-field potentials and unstable contact lead to false results | Enhanced automaticity and reentry |
| Study | Year | Author | Country | FU (Years) | N Patients | Group | LVEF | MI | Not MI | Death (All Causes) | VT Recurrence | Appropriate ICD Shock | BB | Amiodarone | Other AAD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PARTITA [65] | 2012–2021 | Della Bella et al. | Europe | 2 | 47 | Early ablation | 31.9 | 20 | 3 | 0 | 7 | 2 | 23 | 1 | - |
| Control | 32.4 | 18 | 6 | 8 | 12 | 10 | 24 | 4 | - | ||||||
| BERLIN-VT [66] | 2015–2018 | Willems et al. | Europe Russia | 1 | 159 | Early ablation | 41.6 | 83 | - | 6 | 29 | 13 | 58 | 31 | - |
| Control | 41.6 | 72 | - | 2 | 40 | 18 | 59 | 22 | - | ||||||
| VTACH [67] | 2002–2006 | Kuck et al. | Europe | 2 | 107 | Early ablation | 31.9 | 20 | 3 | 0 | 7 | 2 | 23 | 1 | - |
| Control | 34.1 | 55 | - | 4 | 39 | 26 | 41 | 19 | - | ||||||
| SMASH-VT [68] | 2000–2006 | Reddy et al. | USA Czech Republic | 2 | 128 | Early ablation | 31 | 64 | - | 6 | 8 | 6 | 60 | 0 | - |
| Control | 33 | 64 | - | 11 | 21 | 20 | 63 | 0 | - | ||||||
| SMS [69] | 2002–2011 | Kuck et al. | Europe | 2 | 111 | Early ablation | 32 | 54 | - | 9 | 25 | 13 | 49 | 16 | 0 |
| Control | 33 | 57 | - | 11 | 21 | 20 | 63 | 0 | 2 | ||||||
| PAUSE-SCD [70] | 2015–2020 | Tung et al. | USA China | 3 | 121 | Early ablation | 41 | 20 | 40 | 5 | 19 | 6 | 47 | 20 | 3 |
| Control | 40 | 22 | 39 | 4 | 31 | 15 | 53 | 23 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Cori, A.; Pistelli, L.; Parollo, M.; Zaurino, N.; Segreti, L.; Zucchelli, G. Approaching Ventricular Tachycardia Ablation in 2024: An Update on Mapping and Ablation Strategies, Timing, and Future Directions. J. Clin. Med. 2024, 13, 5017. https://doi.org/10.3390/jcm13175017
Di Cori A, Pistelli L, Parollo M, Zaurino N, Segreti L, Zucchelli G. Approaching Ventricular Tachycardia Ablation in 2024: An Update on Mapping and Ablation Strategies, Timing, and Future Directions. Journal of Clinical Medicine. 2024; 13(17):5017. https://doi.org/10.3390/jcm13175017
Chicago/Turabian StyleDi Cori, Andrea, Lorenzo Pistelli, Matteo Parollo, Nicola Zaurino, Luca Segreti, and Giulio Zucchelli. 2024. "Approaching Ventricular Tachycardia Ablation in 2024: An Update on Mapping and Ablation Strategies, Timing, and Future Directions" Journal of Clinical Medicine 13, no. 17: 5017. https://doi.org/10.3390/jcm13175017
APA StyleDi Cori, A., Pistelli, L., Parollo, M., Zaurino, N., Segreti, L., & Zucchelli, G. (2024). Approaching Ventricular Tachycardia Ablation in 2024: An Update on Mapping and Ablation Strategies, Timing, and Future Directions. Journal of Clinical Medicine, 13(17), 5017. https://doi.org/10.3390/jcm13175017








