Absolute Stenosis Measures of Renal Artery Independently Influence Kidney Perfusion in Contrast-Enhanced Multidetector Computed Tomography
Abstract
1. Introduction
2. Materials and Methods
2.1. Renal Function Assessment
2.2. Contrast-Enhanced Multidetector Computed Tomography
2.2.1. CE-MDCT Protocol
2.2.2. Renal Artery Stenosis Measurement
2.3. Kidney Ultrasound Assessment
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bhalla, V.; Textor, S.C.; Beckman, J.A.; Casanegra, A.I.; Cooper, C.J.; Kim, E.S.H.; Luther, J.M.; Misra, S.; Oderich, G.S. Revascularization for Renovascular Disease: A Scientific Statement from the American Heart Association. Hypertension 2022, 79, E128–E143. [Google Scholar] [CrossRef] [PubMed]
- Hicks, C.W.; Clark, T.W.I.; Cooper, C.J.; de Bhailís, Á.M.; De Carlo, M.; Green, D.; Małyszko, J.; Miglinas, M.; Textor, S.C.; Herzog, C.A.; et al. Atherosclerotic Renovascular Disease: A KDIGO (Kidney Disease: Improving Global Outcomes) Controversies Conference. Am. J. Kidney Dis. 2022, 79, 289–301. [Google Scholar] [CrossRef]
- Aboyans, V.; Ricco, J.B.; Bartelink, M.L.E.L.; Björck, M.; Brodmann, M.; Cohnert, T.; Collet, J.P.; Czerny, M.; De Carlo, M.; Debus, S.; et al. 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in Collaboration with the European Society for Vascular Surgery (ESVS): Document Covering Atherosclerotic Disease of Extracranial Carotid and Vertebral, Mesenteric, Renal, Upper and Lower Extremity Arteries Endorsed by: The European Stroke Organization (ESO)The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery. Eur. Heart J. 2018, 39, 763–816. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.R.; Beckman, J.A.; Dao, T.D.; Misra, S.; Sobieszczyk, P.S.; White, C.J.; Wann, L.S.; Aronow, H.D.; Fazel, R.; Gornik, H.L.; et al. ACC/AHA/SCAI/SIR/SVM 2018 Appropriate Use Criteria for Peripheral Artery Intervention: A Report of the American College of Cardiology Appropriate Use Criteria Task Force, American Heart Association, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, and Society for Vascular Medicine. J. Am. Coll. Cardiol. 2019, 73, 214–237. [Google Scholar] [CrossRef] [PubMed]
- de Leeuw, P.W.; Postma, C.T.; Spiering, W.; Kroon, A.A. Atherosclerotic Renal Artery Stenosis: Should We Intervene Earlier? Curr. Hypertens. Rep. 2018, 20, 35. [Google Scholar] [CrossRef] [PubMed]
- Andersson, M.; Jägervall, K.; Eriksson, P.; Persson, A.; Granerus, G.; Wang, C.; Smedby, Ö. How to Measure Renal Artery Stenosis—A Retrospective Comparison of Morphological Measurement Approaches in Relation to Hemodynamic Significance. BMC Med. Imaging 2015, 15, 42. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.; Castro, A.F.; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A New Equation to Estimate Glomerular Filtration Rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Lubas, A.; Zegadło, A.; Frankowska, E.; Klimkiewicz, J.; Jędrych, E.; Niemczyk, S. Ultrasound Doppler Flow Parameters Are Independently Associated with Renal Cortex Contrast-Enhanced Multidetector Computed Tomography Perfusion and Kidney Function. J. Clin. Med. 2023, 12, 2111. [Google Scholar] [CrossRef]
- Scholbach, T.; Dimos, I.; Scholbach, J. A New Method of Color Doppler Perfusion Measurement via Dynamic Sonographic Signal Quantification in Renal Parenchyma. Nephron. Physiol. 2004, 96, p99–p104. [Google Scholar] [CrossRef]
- Lauder, L.; Ewen, S.; Tzafriri, A.R.; Edelman, E.R.; Lüscher, T.F.; Blankestijn, P.J.; Dörr, O.; Schlaich, M.; Sharif, F.; Voskuil, M.; et al. Renal Artery Anatomy Assessed by Quantitative Analysis of Selective Renal Angiography in 1,000 Patients with Hypertension. EuroIntervention 2018, 14, 121–128. [Google Scholar] [CrossRef]
- Spezia, L.; Perandini, S.; Augelli, R.; Puppini, G.; Montemezzi, S. Successful Treatment of Resistant Hypertension by Means of Chronic Renal Artery Occlusion Revascularization in a Fragile Patient. Pol. J. Radiol. 2016, 81, 532. [Google Scholar] [CrossRef][Green Version]
- Potsangbam, G.; Thangjam, G.; Sharma, G.S.K.; Rajkumari, N.; Laishram, S. Successful Treatment of Bilateral Renal Artery Stenosis in a Patient Presenting with Acute Kidney Injury. J. Med. Soc. 2021, 35, 83–85. [Google Scholar] [CrossRef]
- Leertouwer, T.C.; Gussenhoven, E.J.; Bosch, J.L.; Van Jaarsveld, B.C.; Van Dijk, L.C.; Deinum, J.; Manin’t Veld, A.J. Stent Placement for Renal Arterial Stenosis: Where Do We Stand? A Meta-Analysis. Radiology 2000, 216, 78–85. [Google Scholar] [CrossRef]
- Bax, L.; Woittiez, A.J.J.; Kouwenberg, H.J.; Mali, W.P.T.M.; Buskens, E.; Beek, F.J.A.; Braam, B.; Huysmans, F.T.M.; Schultze Kool, L.J.; Rutten, M.J.C.M.; et al. Stent Placement in Patients with Atherosclerotic Renal Artery Stenosis and Impaired Renal Function: A Randomized Trial. Ann. Intern. Med. 2009, 150, 840–848. [Google Scholar] [CrossRef]
- ASTRAL Investigators; Wheatley, K.; Ives, N.; Gray, R.; Kalra, P.A.; Moss, J.G.; Baigent, C.; Carr, S.; Chalmers, N.; Eadington, D.; et al. Revascularization versus Medical Therapy for Renal-Artery Stenosis. N. Engl. J. Med. 2009, 361, 1953–1962. [Google Scholar] [CrossRef] [PubMed]
- Cooper, C.J.; Murphy, T.P.; Cutlip, D.E.; Jamerson, K.; Henrich, W.; Reid, D.M.; Cohen, D.J.; Matsumoto, A.H.; Steffes, M.; Jaff, M.R.; et al. Stenting and Medical Therapy for Atherosclerotic Renal-Artery Stenosis. N. Engl. J. Med. 2014, 370, 13–22. [Google Scholar] [CrossRef]
- Murphy, T.P.; Cooper, C.J.; Pencina, K.M.; D’Agostino, R.; Massaro, J.; Cutlip, D.E.; Jamerson, K.; Matsumoto, A.H.; Henrich, W.; Shapiro, J.I.; et al. Relationship of Albuminuria and Renal Artery Stent Outcomes. Hypertension 2016, 68, 1145–1152. [Google Scholar] [CrossRef]
- Frąk, W.; Kućmierz, J.; Szlagor, M.; Młynarska, E.; Rysz, J.; Franczyk, B. New Insights into Molecular Mechanisms of Chronic Kidney Disease. Biomedicines 2022, 10, 2846. [Google Scholar] [CrossRef]
- Pappaccogli, M.; Robberechts, T.; Lengelé, J.-P.; Van Der Niepen, P.; Sarafidis, P.; Rabbia, F.; Persu, A. Endovascular Versus Medical Management of Atherosclerotic Renovascular Disease: Update and Emerging Concepts. Hypertension 2023, 80, 1150–1161. [Google Scholar] [CrossRef]
- Crocerossa, F.; Fiori, C.; Capitanio, U.; Minervini, A.; Carbonara, U.; Pandolfo, S.D.; Loizzo, D.; Eun, D.D.; Larcher, A.; Mari, A.; et al. Estimated Glomerular Filtration Rate Decline at 1 Year After Minimally Invasive Partial Nephrectomy: A Multimodel Comparison of Predictors. Eur. Urol. Open Sci. 2022, 38, 52–59. [Google Scholar] [CrossRef]
- Abumoawad, A.; Saad, A.; Ferguson, C.M.; Eirin, A.; Woollard, J.R.; Herrmann, S.M.; Hickson, L.T.J.; Bendel, E.C.; Misra, S.; Glockner, J.; et al. Tissue Hypoxia, Inflammation, and Loss of Glomerular Filtration Rate in Human Atherosclerotic Renovascular Disease. Kidney Int. 2019, 95, 948–957. [Google Scholar] [CrossRef] [PubMed]
- Li, L.P.; Tan, H.; Thacker, J.M.; Li, W.; Zhou, Y.; Kohn, O.; Sprague, S.M.; Prasad, P.V. Evaluation of Renal Blood Flow in Chronic Kidney Disease Using Arterial Spin Labeling Perfusion Magnetic Resonance Imaging. Kidney Int. Rep. 2017, 2, 36–43. [Google Scholar] [CrossRef]
- Li, X.; Li, C.; Liu, H.; Wang, R.; Niu, G.; Yang, J.; Zhang, Y. Revealing the Decrease of Renal Cortical Perfusion in Primary Glomerular Disease and Renal Aging by Arterial Spin Labeling. Iran. J. Radiol. 2020, 17, e96147. [Google Scholar] [CrossRef]
- Liu, D.; Liu, J.; Wen, Z.; Li, Y.; Sun, Z.; Xu, Q.; Fan, Z. 320-Row CT Renal Perfusion Imaging in Patients with Aortic Dissection: A Preliminary Study. PLoS ONE 2017, 12, e0171235. [Google Scholar] [CrossRef]
- Lubas, A.; Ryczek, R.; Maliborski, A.; Dyrla, P.; Niemczyk, L.; Niemczyk, S. Left Ventricular Strain and Relaxation Are Independently Associated with Renal Cortical Perfusion in Hypertensive Patients. Adv. Exp. Med. Biol. 2019, 1133, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lubas, A.; Kade, G.; Ryczek, R.; Banasiak, P.; Dyrla, P.; Szamotulska, K.; Schneditz, D.; Niemczyk, S. Ultrasonic Evaluation of Renal Cortex Arterial Area Enables Differentiation between Hypertensive and Glomerulonephritis-Related Chronic Kidney Disease. Int. Urol. Nephrol. 2017, 49, 1627–1635. [Google Scholar] [CrossRef]
- Gutowski, M.; Klimkiewicz, J.; Rustecki, B.; Michałowski, A.; Paryż, K.; Lubas, A. Effect of Respiratory Failure on Peripheral and Organ Perfusion Markers in Severe COVID-19: A Prospective Cohort Study. J. Clin. Med. 2024, 13, 469. [Google Scholar] [CrossRef]
- Gloviczki, M.L.; Glockner, J.F.; Lerman, L.O.; McKusick, M.A.; Misra, S.; Grande, J.P.; Textor, S.C. Preserved Oxygenation despite Reduced Blood Flow in Poststenotic Kidneys in Human Atherosclerotic Renal Artery Stenosis. Hypertension 2010, 55, 961–966. [Google Scholar] [CrossRef]

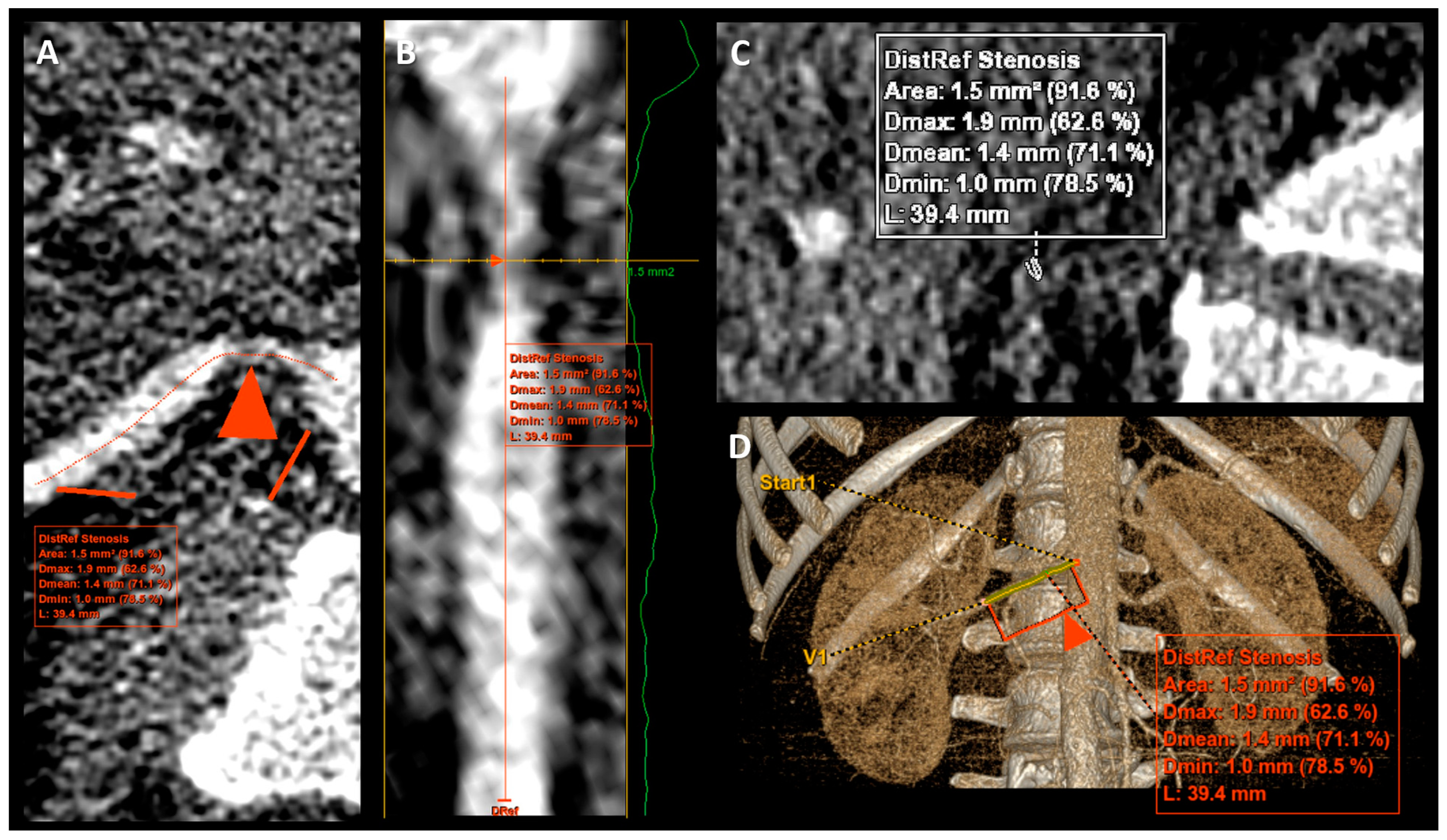
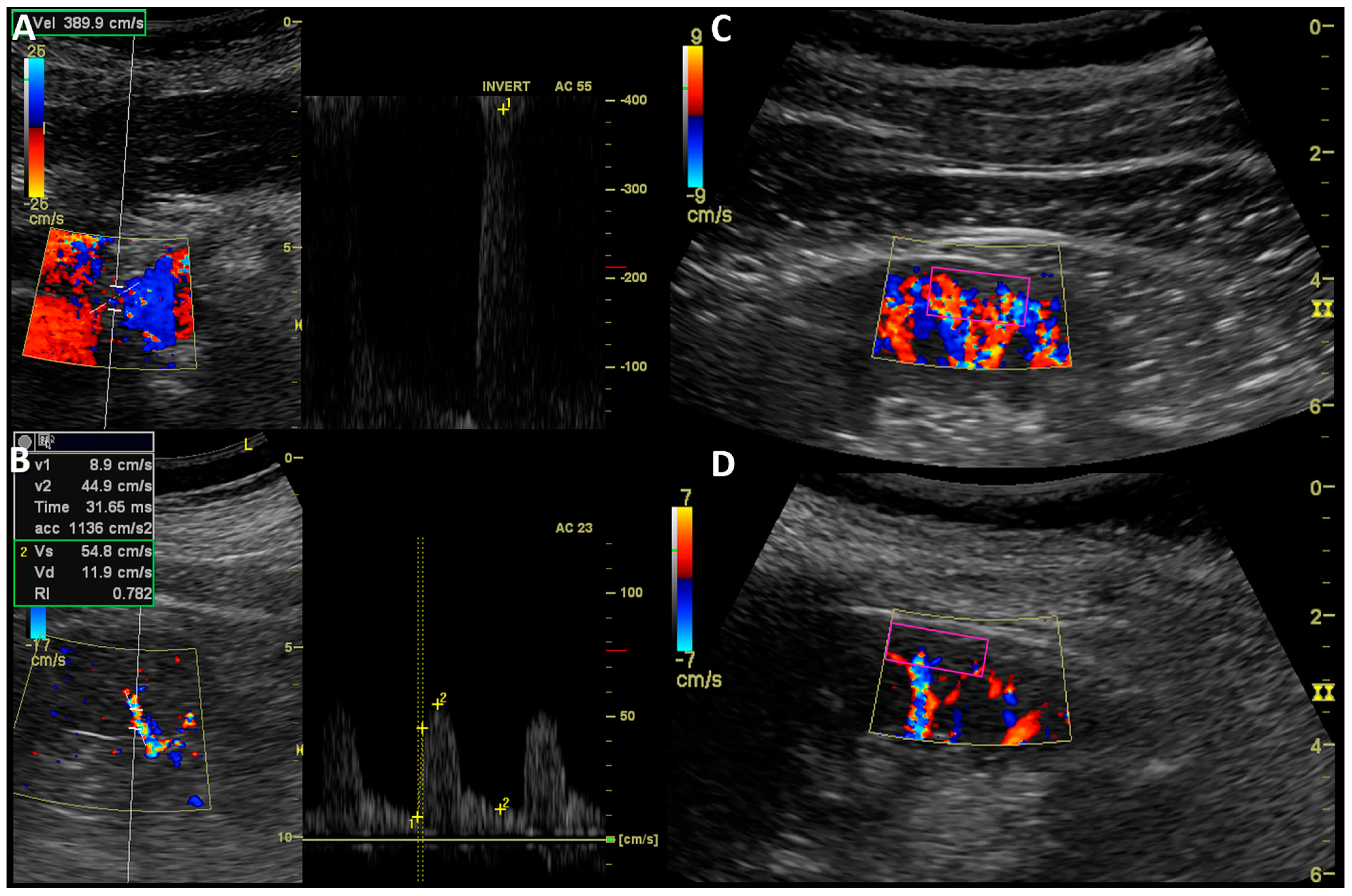
| Parameter | Mean | SD | Median | IQR |
|---|---|---|---|---|
| Age [years] | 61.8 | 15.0 | 65.1 | 18.3 |
| Creatinine [mg/dL] | 1.62 | 0.94 | 1.30 | 0.80 |
| eGFR [mL/min/1.73 m2] | 52.8 | 25.6 | 47.0 | 30.8 |
| Kidney lenght [mm] | 109.4 | 41.8 | 107.5 | 19.5 |
| CSAR [%] | 55.3 | 21.4 | 55.0 | 30.0 |
| MeD [mm] | 3.74 | 1.42 | 3.60 | 1.85 |
| MinD [mm] | 2.81 | 1.23 | 2.80 | 1.20 |
| MaxDR [%] | 41.19 | 18.69 | 37.00 | 22.50 |
| PSV [cm/s] | 199.1 | 95.4 | 185.0 | 129.5 |
| RAR | 2.39 | 1.46 | 2.00 | 1.98 |
| ACC [m/s2] | 6.61 | 4.16 | 6.00 | 4.79 |
| ACT [ms] | 46.6 | 24.7 | 39.7 | 22.0 |
| RI [ratio] | 0.703 | 0.104 | 0.735 | 0.155 |
| CBF [mL/100 g/min] | 190.7 | 64.5 | 191.0 | 86.7 |
| dRCP [mL/s] | 0.357 | 0.398 | 0.280 | 0.345 |
| Parameter | CSAR [%] (p) | MeD [mm] (p) | MinD [mm] (p) | MaxDR [%] (p) |
|---|---|---|---|---|
| Age [years] | 0.307 (0.014) | −0.340 (0.006) | −0.133 (0.293) | 0.167 (0.186) |
| Creatinine [mg/dL] | 0.232 (0.066) | −0.116 (0.363) | −0.201 (0.111) | 0.212 (0.093) |
| eGFR [mL/min/1.73 m2] | −0.220 (0.081) | 0.196 (0.120) | 0.246 (0.049) | −0.206 (0.102) |
| Kidney length [mm] | −0.098 (0.444) | 0.233 (0.067) | 0.345 (0.011) | −0.035 (0.785) |
| PSV [cm/s] | 0.252 (0.052) | −0.346 (0.007) | −0.448 (<0.001) | 0.336 (0.009) |
| RAR | 0.215 (0.099) | −0.283 (0.028) | −0.351 (0.006) | 0.305 (0.018) |
| ACC [m/s2] | 0.067 (0.599) | 0.039 (0.760) | 0.084 (0.510) | 0.173 (0.176) |
| ACT [ms] | −0.076 (0.556) | −0.047 (0.712) | −0.113 (0.379) | −0.065 (0.614) |
| RI [ratio] | −0.007 (0.958) | −0.105 (0.414) | −0.027 (0.833) | 0.013 (0.922) |
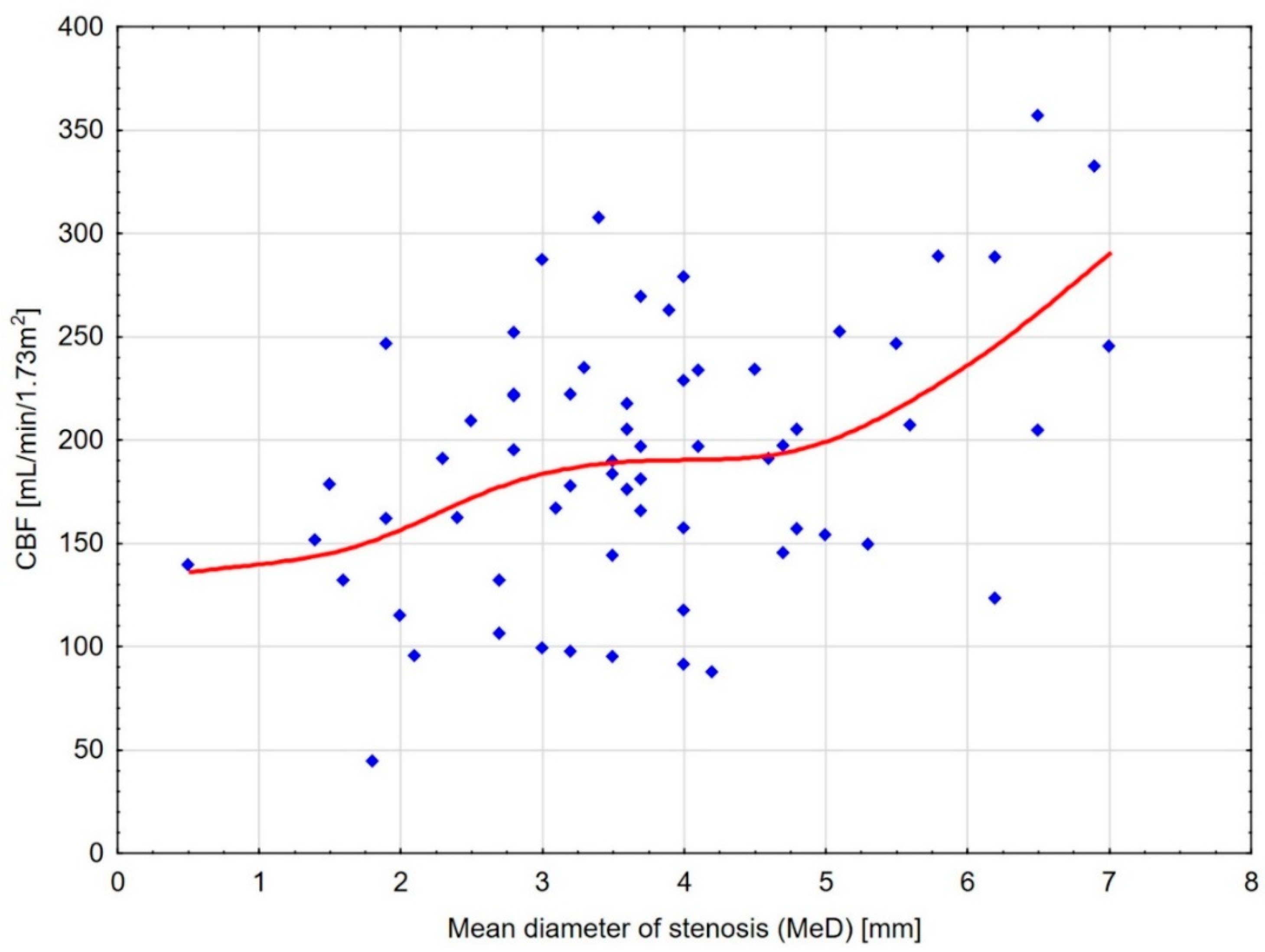
| CBF [mL/100 g/min] | −0.422 (0.003) | 0.344 (0.005) | 0.348 (0.005) | −0.190 (0.133) |
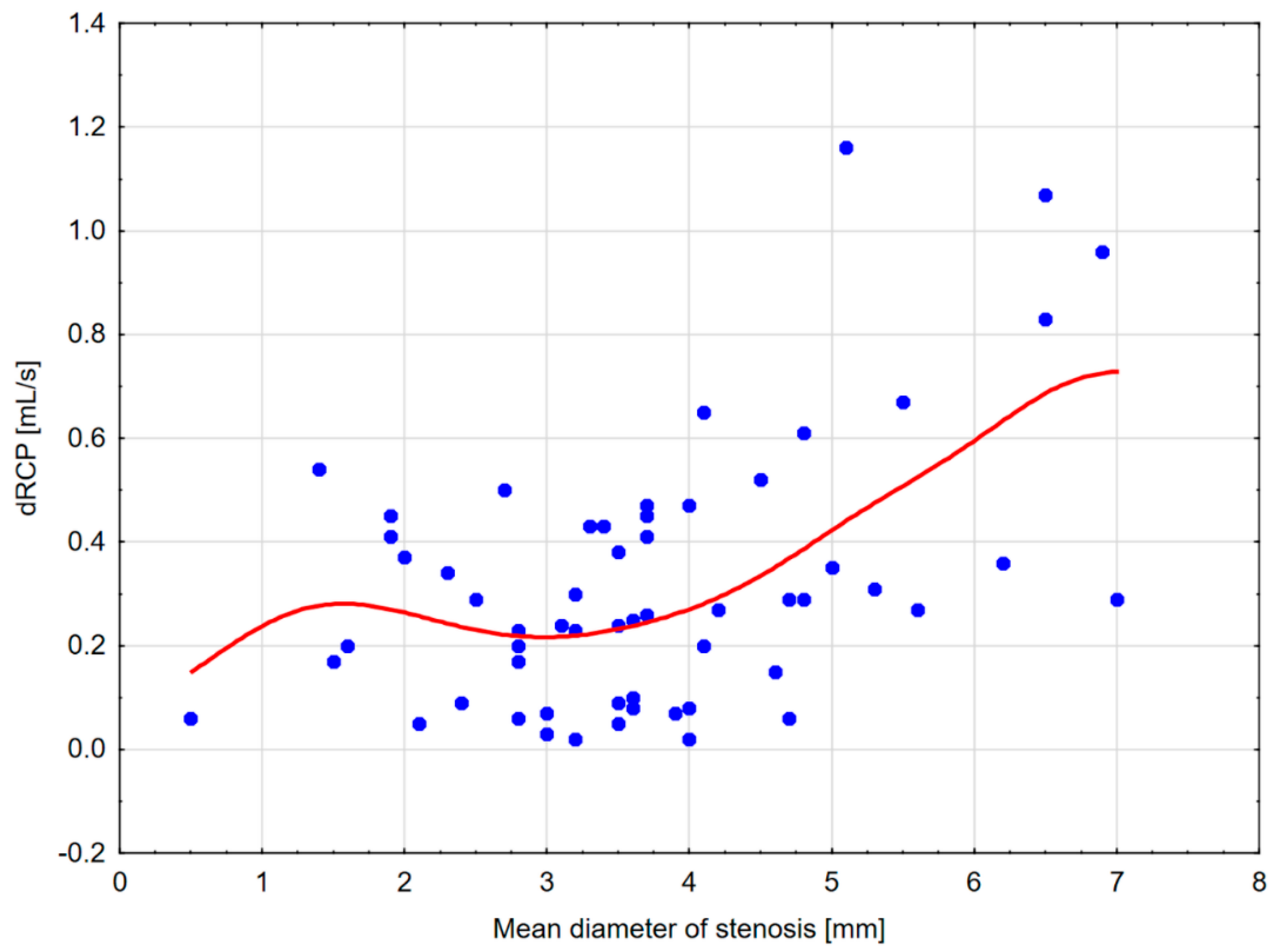
| dRCP [mL/s] | −0.167 (0.201) | 0.357 (0.005) | 0.427 (<0.001) | −0.150 (0.250) |
| CBF Threshold [mL/100 g/min] | MeD Cut-Off [mm] | Sensitivity [%] | Specificity [%] | AUC | p-Value |
|---|---|---|---|---|---|
| 100 | 3.4 | 57.1 | 61.4 | 0.639 | 0.134 |
| 150 | 3.5 | 64.7 | 59.6 | 0.634 | 0.099 |
| 175 | 3.5 | 60.0 | 61.5 | 0.649 | 0.038 |
| 200 | 3.6 | 59.5 | 59.3 | 0.681 | 0.007 |
| 250 | 3.7 | 62.3 | 63.6 | 0.697 | 0.021 |
| 300 | 4.0 | 68.9 | 66.7 | 0.790 | 0.067 |
| Variable | Cut-Off Value | Sensitivity [%] | Specificity [%] | AUC | p-Value |
|---|---|---|---|---|---|
| CSAR [%] | 58.0 | 73.3 | 73.5 | 0.734 | <0.001 |
| MinD [mm] | 2.6 | 90.0 | 88.2 | 0.944 | <0.001 |
| MaxDR [%] | 37.8 | 63.3 | 67.6 | 0.668 | 0.005 |
| CBF [mL/100 g/min] | 189.8 | 63.3 | 64.7 | 0.653 | 0.027 |
| dRCP [mL/s] | 0.250 | 71.9 | 60.7 | 0.662 | 0.020 |
| eGFR [mL/min/1.73 m2] | 46.3 | 56.7 | 55.9 | 0.567 | 0.359 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lubas, A.; Zegadło, A.; Frankowska, E.; Jędrych, E.; Lubas, T.; Grzywacz, A.; Leśniak, K.; Niemczyk, S. Absolute Stenosis Measures of Renal Artery Independently Influence Kidney Perfusion in Contrast-Enhanced Multidetector Computed Tomography. J. Clin. Med. 2024, 13, 5022. https://doi.org/10.3390/jcm13175022
Lubas A, Zegadło A, Frankowska E, Jędrych E, Lubas T, Grzywacz A, Leśniak K, Niemczyk S. Absolute Stenosis Measures of Renal Artery Independently Influence Kidney Perfusion in Contrast-Enhanced Multidetector Computed Tomography. Journal of Clinical Medicine. 2024; 13(17):5022. https://doi.org/10.3390/jcm13175022
Chicago/Turabian StyleLubas, Arkadiusz, Arkadiusz Zegadło, Emilia Frankowska, Ewelina Jędrych, Tymoteusz Lubas, Anna Grzywacz, Ksymena Leśniak, and Stanisław Niemczyk. 2024. "Absolute Stenosis Measures of Renal Artery Independently Influence Kidney Perfusion in Contrast-Enhanced Multidetector Computed Tomography" Journal of Clinical Medicine 13, no. 17: 5022. https://doi.org/10.3390/jcm13175022
APA StyleLubas, A., Zegadło, A., Frankowska, E., Jędrych, E., Lubas, T., Grzywacz, A., Leśniak, K., & Niemczyk, S. (2024). Absolute Stenosis Measures of Renal Artery Independently Influence Kidney Perfusion in Contrast-Enhanced Multidetector Computed Tomography. Journal of Clinical Medicine, 13(17), 5022. https://doi.org/10.3390/jcm13175022







