Esophageal Dysmotility in Multiple System Atrophy: A Retrospective Cross-Sectional Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Ethics
2.2. Methodology
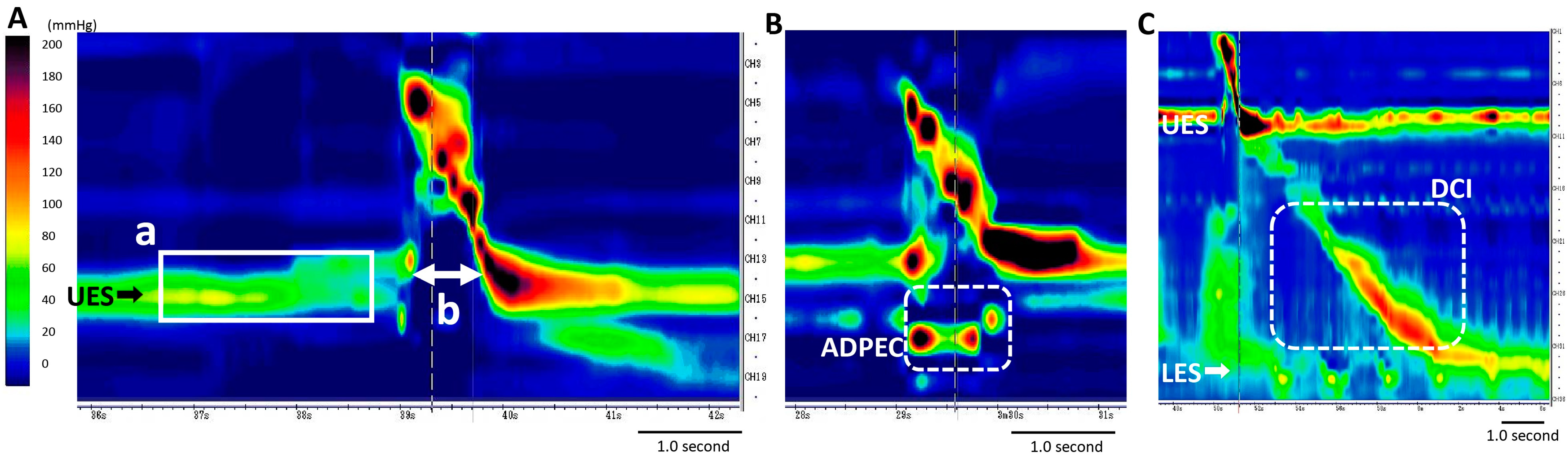
2.3. HRM Study
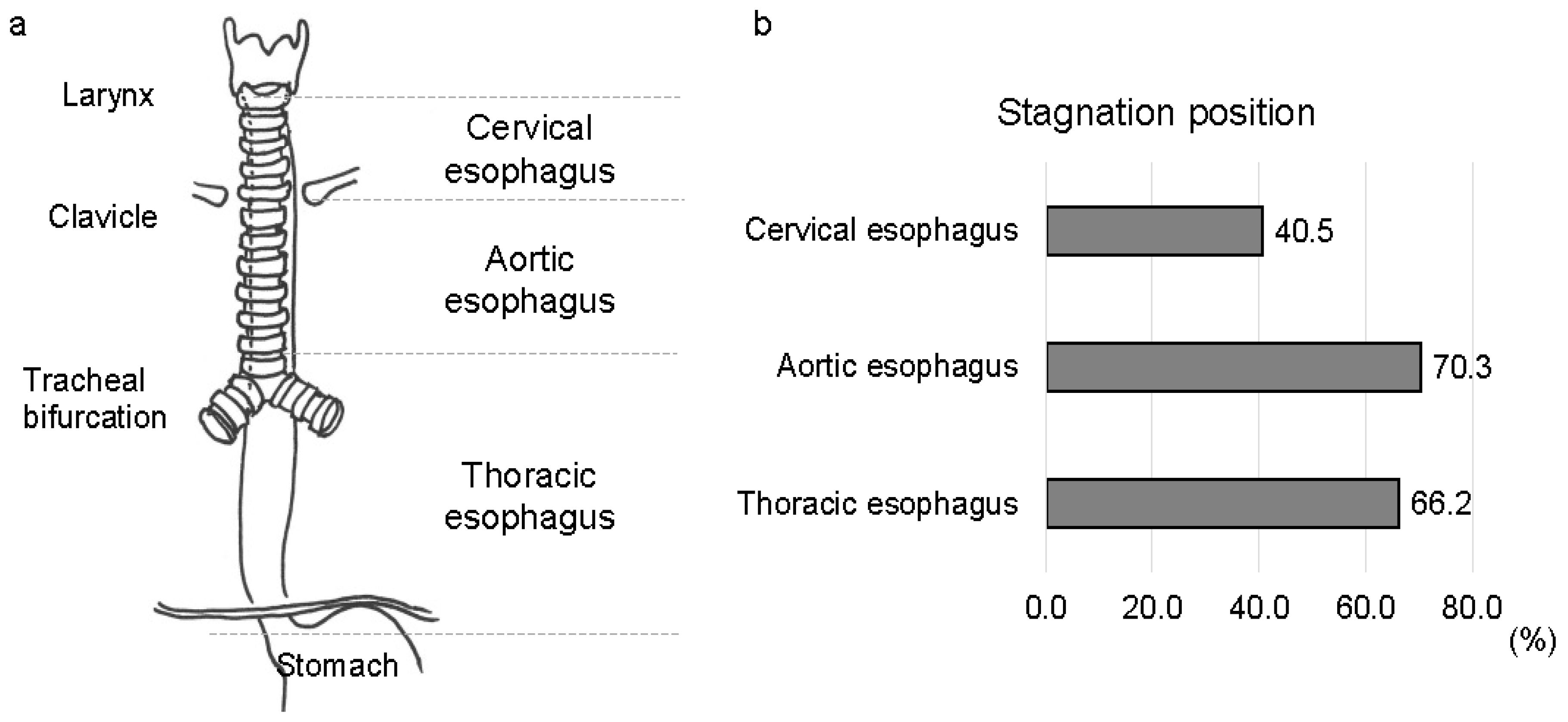
2.4. Videofluorographic Study
2.5. Statistical Analyses
3. Results
3.1. Demographic Data and Characteristics of Patients
3.2. Esophageal Involvement in Patients with MSA
3.3. Clinical Risk Factors for ED in Patients with MSA
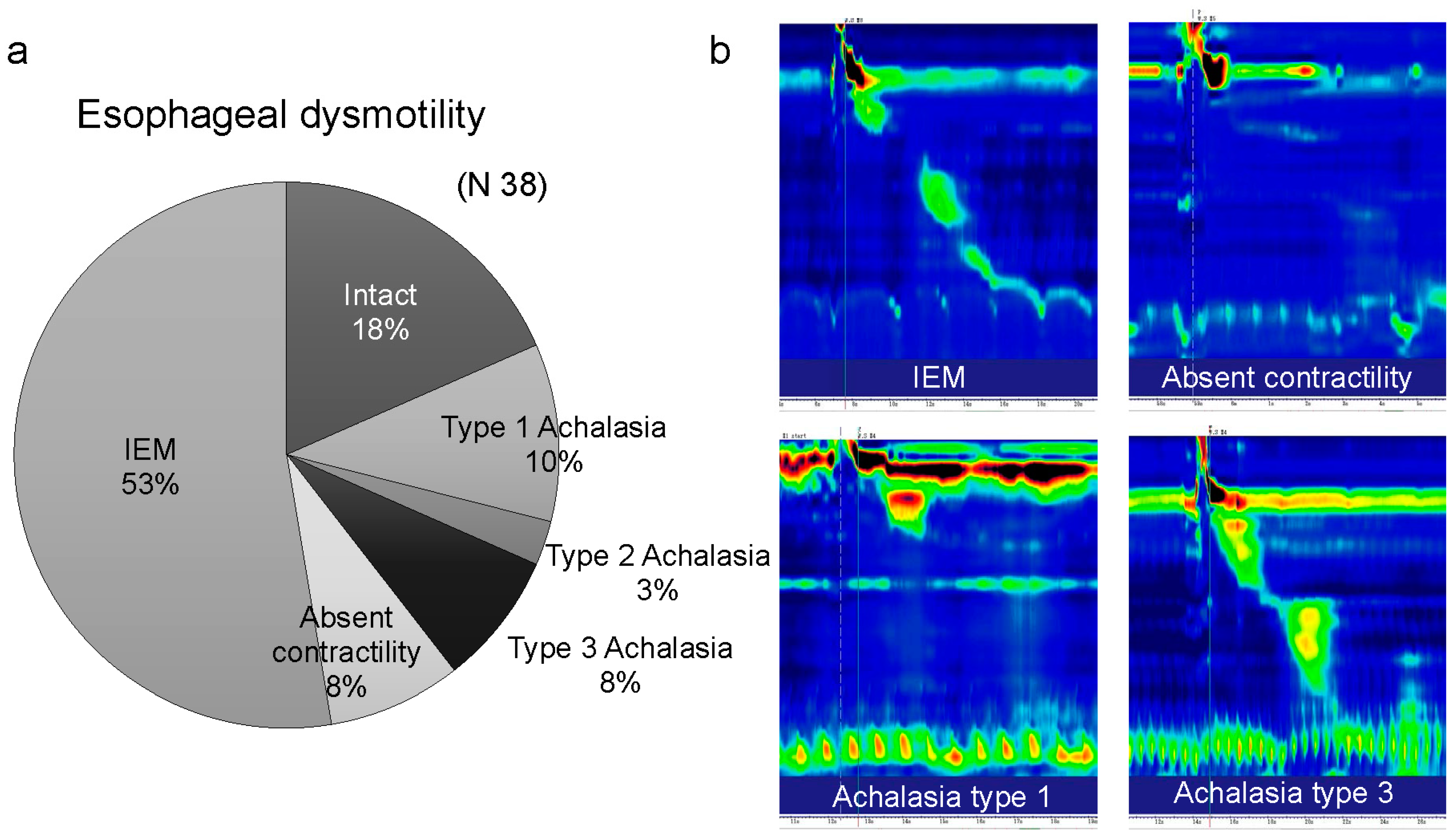
3.4. Various Esophageal Motility Disorders in Patients with MSA
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MSA | multiple system atrophy |
| ED | esophageal dysmotility |
| HRM | high-resolution manometry |
| UES | upper esophageal sphincter |
| MSA-P | multiple system atrophy parkinsonian variant |
| MSA-C | multiple system atrophy cerebellar variant |
| ADPEC | abnormal deglutitive proximal esophageal contraction |
| pHRM | pharyngeal high-resolution manometry |
| VFE | videofluorographic esophagram |
| IES | intraesophageal stasis |
| IER | intraesophageal reflux |
| eHRM | esophageal high-resolution manometry |
| VFMI | vocal fold motion impairment |
| PAS | penetration–aspiration scale |
| FOIS | functional oral intake scale |
| UMSARS | unified multiple system atrophy rating scale |
| GER | gastroesophageal reflux |
| VFSS | videofluoroscopic swallowing study |
| IQR | interquartile range |
References
- Gilman, S.; Wenning, G.; Low, P.A.; Brooks, D.; Mathias, C.J.; Trojanowski, J.Q.; Wood, N.; Colosimo, C.; Durr, A.; Fowler, C.J.; et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology 2008, 71, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Fanciulli, A.; Wenning, G.K. Multiple-system atrophy. N. Engl. J. Med. 2015, 372, 249–263. [Google Scholar] [CrossRef]
- Poewe, W.; Stankovic, I.; Halliday, G.; Meissner, W.G.; Wenning, G.K.; Pellecchia, M.T.; Seppi, K.; Palma, A.; Kaufmann, H. Multiple system atrophy. Nat. Rev. Dis. Primers 2022, 8, 56. [Google Scholar] [CrossRef] [PubMed]
- Flabeau, O.; Meissner, W.G.; Tison, F. Multiple system atrophy: Current and future approaches to management. Ther. Adv. Neurol. Disord. 2010, 3, 249–263. [Google Scholar] [CrossRef]
- Zhang, L.; Cao, B.; Zou, Y.; Wei, Q.-Q.; Ou, R.; Liu, W.; Zhao, B.; Yang, J.; Wu, Y.; Shang, H. Causes of death in Chinese patients with multiple system atrophy. Aging Dis. 2018, 9, 102–108. [Google Scholar] [CrossRef]
- Papapetropoulos, S.; Tuchman, A.; Laufer, D.; Papatsoris, A.G.; Papapetropoulos, N.; Mash, D.C. Causes of death in multiple system atrophy. J. Neurol. Neurosurg. Psychiatry 2007, 78, 327–329. [Google Scholar] [CrossRef] [PubMed]
- Davis, P.K.; Remick, D.G.; Parascandola, S.A.; Spangler, S.; Wise, R.K.; Martin, L.F. Intravascular plastic catheters potentiate tumor necrosis factor release and cardiac dysfunction secondary to infection. Curr. Surg. 1989, 46, 486–489. [Google Scholar]
- Taniguchi, H.; Nakayama, H.; Hori, K.; Nishizawa, M.; Inoue, M.; Shimohata, T. Esophageal involvement in multiple system atrophy. Dysphagia 2015, 30, 669–673. [Google Scholar] [CrossRef]
- Ueha, R.; Sato, T.; Goto, T.; Yamauchi, A.; Nativ-Zeltzer, N.; Mitsui, J.; Belafsky, P.C.; Yamasoba, T. Esophageal dysmotility is common in patients with multiple system atrophy. Laryngoscope 2021, 131, 832–838. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Conklin, J.L. Neuromuscular control of esophageal peristalsis. Curr. Gastroenterol. Rep. 1999, 1, 186–197. [Google Scholar] [CrossRef]
- Chen, J.H. Ineffective esophageal motility and the vagus: Current challenges and future prospects. Clin. Exp. Gastroenterol. 2016, 9, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Coon, E.A.; Cutsforth-Gregory, J.K.; Benarroch, E.E. Neuropathology of autonomic dysfunction in synucleinopathies. Mov. Disord. 2018, 33, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Ueha, R.; Goto, T.; Sato, T.; Nativ-Zeltzer, N.; Shen, S.C.; Nito, T.; Belafsky, P.C.; Yamasoba, T. High resolution manofluorographic study in patients with multiple system atrophy: Possible early detection of upper esophageal sphincter and proximal esophageal abnormality. Front. Med. 2018, 5, 286. [Google Scholar] [CrossRef]
- Aspirot, A.; Faure, C. Esophageal dysmotility: Characterization and pathophysiology. Dis. Esophagus 2013, 26, 405–409. [Google Scholar] [CrossRef]
- Bredenoord, A.J.; Fox, M.; Kahrilas, P.J.; Pandolfino, J.E.; Schwizer, W.; Smout, A.J.P.M.; International High Resolution Manometry Working Group. Chicago classification criteria of esophageal motility disorders defined in high resolution esophageal pressure topography. Neurogastroenterol. Motil 2012, 24 (Suppl. S1), 57–65. [Google Scholar] [CrossRef] [PubMed]
- Yadlapati, R.; Kahrilas, P.J.; Fox, M.R.; Bredenoord, A.J.; Prakash Gyawali, C.; Roman, S.; Babaei, A.; Mittal, R.K.; Rommel, N.; Savarino, E.; et al. Esophageal motility disorders on high-resolution manometry: Chicago classification version 4.0©. Neurogastroenterol. Motil. 2021, 33, e14058. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Saito, Y.; Terao, S.; Ando, T.; Kachi, T.; Mukai, E.; Aiba, I.; Abe, Y.; Tamakoshi, A.; Doyu, M.; et al. Progression and prognosis in multiple system atrophy: An analysis of 230 Japanese patients. Brain 2002, 125, 1070–1083. [Google Scholar] [CrossRef]
- Rosenbek, J.C.; Robbins, J.A.; Roecker, E.B.; Coyle, J.L.; Wood, J.L. A penetration-aspiration scale. Dysphagia 1996, 11, 93–98. [Google Scholar] [CrossRef]
- Crary, M.A.; Mann, G.D.C.; Groher, M.E. Initial psychometric assessment of a functional oral intake scale for dysphagia in stroke patients. Arch. Phys. Med. Rehabilit. 2005, 86, 1516–1520. [Google Scholar] [CrossRef]
- Wenning, G.K.; Tison, F.; Seppi, K.; Sampaio, C.; Diem, A.; Yekhlef, F.; Ghorayeb, I.; Ory, F.; Galitzky, M.; Scaravilli, T.; et al. Development and validation of the Unified Multiple System Atrophy Rating Scale (UMSARS). Mov. Disord. 2004, 19, 1391–1402. [Google Scholar] [CrossRef]
- Jou, J.; Radowsky, J.; Gangnon, R.; Sadowski, E.; Kays, S.; Hind, J.; Gaumnitz, E.; Taylor, A.; Robbins, J. Esophageal clearance patterns in normal older adults as documented with videofluoroscopic esophagram. Gastroenterol. Res. Pract. 2009, 2009, 965062. [Google Scholar] [CrossRef] [PubMed]
- Mathews, S.C.; Ciarleglio, M.; Chavez, Y.H.; Clarke, J.O.; Stein, E.; Chander Roland, B. Upper esophageal sphincter abnormalities are strongly predictive of treatment response in patients with achalasia. World J. Clin. Cases 2014, 2, 448–454. [Google Scholar] [CrossRef]
- Park, C.-H.; Lee, Y.-T.; Yi, Y.; Lee, J.-S.; Park, J.H.; Yoon, K.J. Ability of high-resolution manometry to determine feeding method and to predict aspiration pneumonia in patients with dysphagia. Am. J. Gastroenterol. 2017, 112, 1074–1083. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, K.; Ueha, R.; Suzuki, S.; Goto, T.; Sato, T.; Nito, T.; Yamasoba, T. Heightened risk of early vocal fold motion impairment onset and dysphagia in the parkinsonian variant of multiple system atrophy: A comparative study. Clin. Park. Relat. Disord. 2020, 3, 100037. [Google Scholar] [CrossRef] [PubMed]
- Richards, W.G.; Sugarbaker, D.J. Neuronal control of esophageal function. Chest Surg. Clin. N. Am. 1995, 5, 157–171. [Google Scholar]
- Gutschow, C.A.; Leers, J.M.; Schröder, W.; Prenzel, K.L.; Fuchs, H.; Bollschweiler, E.; Bludau, M.; Hölscher, A.H. Effect of aging on esophageal motility in patients with and without GERD. Ger. Med. Sci. 2011, 9, Doc22. [Google Scholar]
- Park, H.W.; Jung, H.Y.; Lee, D.; Kim, D.H.; Jung, K.W.; Chung, J.W.; Choi, K.S.; Choi, K.D.; Song, H.J.; Kim, J.H. A case of multiple system atrophy presenting with esophageal dysphagia. Korean J. Neurogastroenterol. Motil. 2008, 14, 140–144. [Google Scholar]
- Alfonsi, E.; Versino, M.; Merlo, I.M.; Pacchetti, C.; Martignoni, E.; Bertino, G.; Moglia, A.; Tassorelli, C.; Nappi, G. Electrophysiologic patterns of oral-pharyngeal swallowing in parkinsonian syndromes. Neurology 2007, 68, 583–589. [Google Scholar] [CrossRef]
- Higo, R.; Tayama, N.; Watanabe, T.; Niimi, S. Abnormal elevation of resting pressure at the upper esophageal sphincter of Parkinson’s disease patients. Eur. Arch. Otorhinolaryngol. 2001, 258, 552–556. [Google Scholar] [CrossRef]
- Higo, R.; Tayama, N.; Watanabe, T.; Nitou, T.; Takeuchi, S. Vocal fold motion impairment in patients with multiple system atrophy: Evaluation of its relationship with swallowing function. J. Neurol. Neurosurg. Psychiatry 2003, 74, 982–984. [Google Scholar] [CrossRef]
- Shimohata, T.; Aizawa, N.; Nakayama, H.; Taniguchi, H.; Ohshima, Y.; Okumura, H.; Takahashi, T.; Yokoseki, A.; Inoue, M.; Nishizawa, M. Mechanisms and prevention of sudden death in multiple system atrophy. Park. Relat. Disord. 2016, 30, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, T.; Sekiya, K.; Aizawa, N.; Terajima, K.; Nishizawa, M. Laryngeal stridor in multiple system atrophy: Clinicopathological features and causal hypotheses. J. Neurol. Sci. 2016, 361, 243–249. [Google Scholar] [CrossRef]
- Isozaki, E.; Naito, A.; Horiguchi, S.; Kawamura, R.; Hayashida, T.; Tanabe, H. Early diagnosis and stage classification of vocal cord abductor paralysis in patients with multiple system atrophy. J. Neurol. Neurosurg. Psychiatry 1996, 60, 399–402. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, K.; Hillman, D.; Eastwood, P. Symptoms of aerophagia are common in patients on continuous positive airway pressure therapy and are related to the presence of nighttime gastroesophageal reflux. J. Clin. Sleep Med. 2013, 9, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, T.; Oodaira, H.; Suzuki, Y.; Hashimoto, R.; Kato, H. Successive application of percutaneous endoscopic gastrostomy with jejunal extension and percutaneous endoscopic jejunostomy in a case of multiple system atrophy. Rinsho Shinkeigaku 2009, 49, 370–373. (In Japanese) [Google Scholar] [CrossRef]
- Taciuc, I.-A.; Dumitru, M.; Vrinceanu, D.; Gherghe, M.; Manole, F.; Marinescu, A.; Serboiu, C.; Neagos, A.; Costache, A. Applications and challenges of neural networks in otolaryngology (Review). Biomed. Rep. 2024, 20, 92. [Google Scholar] [CrossRef]
- Kusano, M.; Shimoyama, Y.; Sugimoto, S.; Kawamura, O.; Maeda, M.; Minashi, K.; Kuribayashi, S.; Higuchi, T.; Zai, H.; Ino, K.; et al. Development and evaluation of FSSG: Frequency scale for the symptoms of GERD. J. Gastroenterol. 2004, 39, 888–891. [Google Scholar] [CrossRef]


| Unified multiple system atrophy rating scale, part IV: global disability scale | |
| Scale 1 | Completely independent. Able to do all chores with minimal difficulty or impairment. Essentially normal. Unaware of any difficulty. |
| Scale 2 | Not completely independent. Needs help with some chores. |
| Scale 3 | More dependent. Help with half of chores. Spends a large part of the day with chores. |
| Scale 4 | Very dependent. Now and then does a few chores alone or begins alone. Much help needed. |
| Scale 5 | Totally dependent and helpless. Bedridden. |
| Penetration–aspiration scale | |
| Score 1 | Material does not enter the airway. |
| Score 2 | Material enters the airway, remains above the vocal folds, and is ejected from the airway. |
| Score 3 | Material enters the airway, remains above the vocal folds, and is not ejected from the airway. |
| Score 4 | Material enters the airway, contacts the vocal folds, and is ejected from the airway. |
| Score 5 | Material enters the airway, contacts the vocal folds, and is not ejected from the airway. |
| Score 6 | Material enters the airway, passes below the vocal folds, and is ejected into the larynx or out of the airway. |
| Score 7 | Material enters the airway, passes below the vocal folds, and is not ejected from the trachea despite effort. |
| Score 8 | Material enters the airway, passes below the vocal folds, and no effort is made to eject. |
| Functional oral intake scale | |
| Level 1 | No oral intake. |
| Level 2 | Tube dependent with minimal/inconsistent oral intake. |
| Level 3 | Tube supplements with consistent oral intake. |
| Level 4 | Total oral intake of a single consistency. |
| Level 5 | Total oral intake of multiple consistencies requiring special preparation. |
| Level 6 | Total oral intake with no special preparation but must avoid specific foods or liquid items. |
| Level 7 | Total oral intake with no restrictions. |
| Grading of intraesophageal stasis of contrast agent | |
| Grade 0 | Absence of intraesophageal stasis. |
| Grade 1 | A minimal amount of contrast agent stagnation or a coating of contrast agent. |
| Grade 2 | Stagnation with contrast agent that completely filled the lumen of the esophagus. |
| Characteristic | |
|---|---|
| Patients-no. | 74 |
| Age-years, median (IQR) | 64 (58–70) |
| Men-no. (%) | 48 (64.9) |
| MSA-C: MSA-P (no.) | 51:23 |
| MSA disease duration-mth. median (IQR) | 48 (33–66) |
| Disease severity level | 3 (2–4) |
| Use of prokinetic agents-no. (%) | 4 (4.1) |
| Vocal fold motion impairment-no. (%) | 39 (52.7) |
| Tracheostomy-no. (%) | 8 (10.8) |
| PAS score. median (IQR) | 2 (1–5) |
| FOIS score. median (IQR) | 7 (5–7) |
| Esophageal Involvement | ||
|---|---|---|
| Abnormal resting UES pressure-no. (%) | 34 (45.9) | |
| Higher UES pressure | 5 (6.8) | |
| Lower UES pressure | 29 (39.2) | |
| Impaired UES opening-no. (%) | 8 (10.8) | |
| ADPEC-no. (%) | 26 (35.1) | |
| Intraesophageal stasis | Grade median (IQR) | 1 (1–1) |
| Grade 0-no. (%) | 9 (12.2) | |
| Grade 1-no. (%) | 48 (64.9) | |
| Grade 2-no. (%) | 17 (23.0) | |
| Intraesophageal reflux-no. (%) | 46 (62.2) | |
| Gastroesophageal reflux-no. (%) | 6 (8.1) | |
| Resting UES Pressure | UES Opening during Swallow | |||||
| Normal | Abnormal | p Value | Normal | Impaired | p Value | |
| Number (%) | 40 (54.1) | 34 (45.9) | 66 (89.2) | 8 (10.8) | ||
| Age-years, median (IQR) | 64 (55–69) | 65 (60–71) | 0.14 | 64 (58–70) | 62 (55–67) | 0.58 |
| Men-no. (%) | 19 (55.9) | 29 (72.5) | 0.14 | 44 (66.7) | 4 (50.0) | 0.35 |
| MSA-C: MSA-P (no.) | C 20, P 14 | C 31, P 9 | 0.08 | C 47, P 19 | C 4, P 4 | 0.22 |
| Disease severity level | 3 (2–4] | 3 (3–4] | 0.038 * | 3 (2–4) | 4 (4–5) | 0.0013 ** |
| Vocal fold motion impairment-no. (%) | 20 (58.8) | 19 (47.5) | 0.33 | 33 (50.0) | 6 (75.0) | 0.18 |
| PAS score. median (IQR) | 1 (1–3) | 3 (1–7) | 0.034 * | 1 (1–3) | 8 (5–8) | <0.001 *** |
| ADPEC | IES | |||||
| Absent | Present | p Value | Grade 0–1 | Grade 2 | p Value | |
| Number (%) | 48 (64.9) | 26 (35.1) | 57 (77.0) | 17 (23.0) | ||
| Age-years, median (IQR) | 66 (59–73) | 63 (56–65) | 0.047 * | 63 (57–71) | 67 (63–70) | 0.28 |
| Men-no. (%) | 31 (64.6) | 17 (65.4) | 0.95 | 22 (61.4) | 13 (76.5) | 0.25 |
| MSA-C: MSA-P (no.) | C 35, P 15 | C 18, P 8 | 0.97 | C 39, P 18 | C 12, P 5 | 0.87 |
| Disease severity level | 3 (2–4) | 3 (3–4) | 0.0035 ** | 3 (2–4) | 3 (3–4) | 0.32 |
| Vocal fold motion impairment-no. (%) | 20 (41.7) | 19 (73.1) | 0.001 ** | 28 (49.1) | 11 (64.7) | 0.26 |
| PAS score. median (IQR) | 1 (1–5) | 3 (1–6) | 0.30 | 2 (1–5) | 1 (1–6) | 0.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ueha, R.; Koyama, M.; Seto, A.; Sato, T.; Goto, T.; Orimo, K.; Mitsui, J.; Yamasoba, T. Esophageal Dysmotility in Multiple System Atrophy: A Retrospective Cross-Sectional Study. J. Clin. Med. 2024, 13, 5026. https://doi.org/10.3390/jcm13175026
Ueha R, Koyama M, Seto A, Sato T, Goto T, Orimo K, Mitsui J, Yamasoba T. Esophageal Dysmotility in Multiple System Atrophy: A Retrospective Cross-Sectional Study. Journal of Clinical Medicine. 2024; 13(17):5026. https://doi.org/10.3390/jcm13175026
Chicago/Turabian StyleUeha, Rumi, Misaki Koyama, Akiko Seto, Taku Sato, Takao Goto, Kenta Orimo, Jun Mitsui, and Tatsuya Yamasoba. 2024. "Esophageal Dysmotility in Multiple System Atrophy: A Retrospective Cross-Sectional Study" Journal of Clinical Medicine 13, no. 17: 5026. https://doi.org/10.3390/jcm13175026






