Ablation Strategies for Persistent Atrial Fibrillation: Beyond the Pulmonary Veins
Abstract
:1. Introduction
2. Ablation Strategies
2.1. Radiofrequency Ablation and Cryoablation
2.2. Pulsed-Field Ablation
2.3. Hybrid Ablation
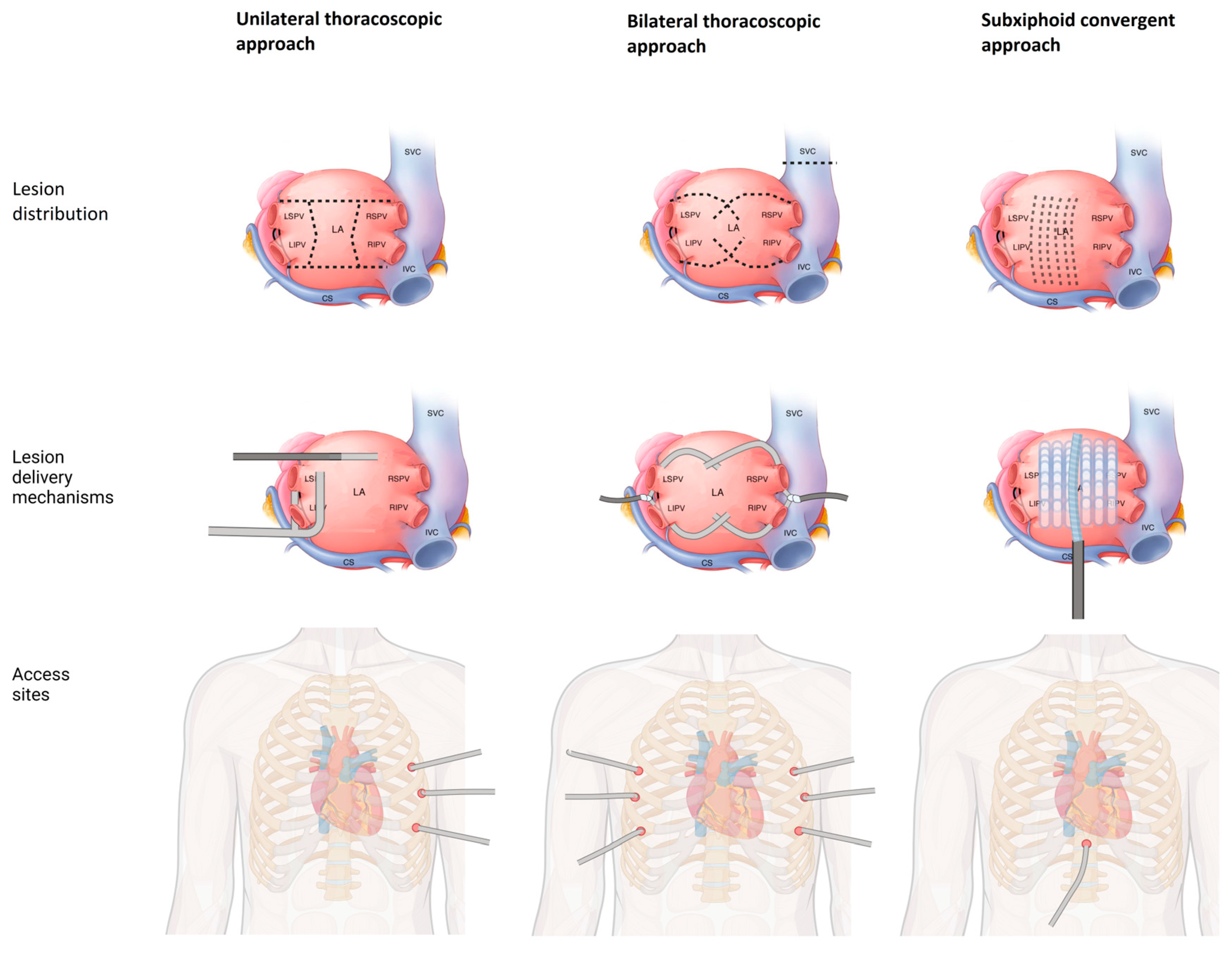
2.3.1. Thoracoscopic Ablation
2.3.2. Hybrid Convergent Ablation
3. Non-Pulmonary Vein Triggers (NPVTs)
3.1. Left Atrial Posterior Wall
3.2. Left Atrial Appendage (LAA)
3.3. Superior Vena Cava (SVC)
3.4. CFAEs, Rotors, and Dispersion
3.5. Vein of Marshall Ethanol Ablation
3.6. Low-Voltage Areas
3.7. Crista Terminalis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oral, H.; Scharf, C.; Chugh, A.; Hall, B.; Cheung, P.; Good, E.; Veerareddy, S.; Pelosi, F., Jr.; Morady, F. Catheter ablation for paroxysmal atrial fibrillation: Segmental pulmonary vein ostial ablation versus left atrial ablation. Circulation 2003, 108, 2355–2360. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Jiang, C.-Y.; Betts, T.R.; Chen, J.; Deisenhofer, I.; Mantovan, R.; Macle, L.; Morillo, C.A.; Haverkamp, W.; Weerasooriya, R.; et al. Approaches to catheter ablation for persistent atrial fibrillation. N. Engl. J. Med. 2015, 372, 1812–1822. [Google Scholar] [CrossRef]
- Ouyang, F.; Tilz, R.; Chun, J.; Schmidt, B.; Wissner, E.; Zerm, T.; Neven, K.; Köktürk, B.; Konstantinidou, M.; Metzner, A.; et al. Long-term results of catheter ablation in paroxysmal atrial fibrillation: Lessons from a 5-year follow-up. Circulation 2010, 122, 2368–2377. [Google Scholar] [CrossRef] [PubMed]
- Brooks, A.G.; Stiles, M.K.; Laborderie, J.; Lau, D.H.; Kuklik, P.; Shipp, N.J.; Hsu, L.F.; Sanders, P. Outcomes of long-standing persistent atrial fibrillation ablation: A systematic review. Heart Rhythm 2010, 7, 835–846. [Google Scholar] [CrossRef]
- Tilz, R.R.; Rillig, A.; Thum, A.M.; Arya, A.; Wohlmuth, P.; Metzner, A.; Mathew, S.; Yoshiga, Y.; Wissner, E.; Kuck, K.H.; et al. Catheter ablation of long-standing persistent atrial fibrillation: 5-year outcomes of the Hamburg Sequential Ablation Strategy. J. Am. Coll. Cardiol. 2012, 60, 1921–1929. [Google Scholar] [CrossRef]
- Marchlinski, F.; Tschabrunn, C.M.; Santangeli, P.; Kubala, M. Clarifying the Definition of Non–Pulmonary Vein Triggers of Atrial Fibrillation. JACC Clin. Electrophysiol. 2019, 5, 1328–1330. [Google Scholar] [CrossRef] [PubMed]
- Brahier, M.S.; Friedman, D.J.; Bahnson, T.D.; Piccini, J.P. Repeat catheter ablation for atrial fibrillation. Heart Rhythm 2024, 21, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Barakat, A.F.; Wazni, O.M.; Saliba, W.I.; Yzeiraj, E.; Amuthan, R.; Abdur Rehman, K.; Tarakji, K.G.; Bassiouny, M.; Baranowski, B.; Tchou, P.; et al. Repeat ablation or medical management alone for recurrent arrhythmias after ablation of persistent atrial fibrillation. J. Cardiovasc. Electrophysiol. 2018, 29, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Joglar, J.A.; Chung, M.K.; Armbruster, A.L.; Benjamin, E.J.; Chyou, J.Y.; Cronin, E.M.; Deswal, A.; Eckhardt, L.L.; Goldberger, Z.D.; Gopinathannair, R.; et al. 2023 ACC/AHA/ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2024, 149, e1–e156. [Google Scholar] [CrossRef] [PubMed]
- Turagam, M.K.; Musikantow, D.; Whang, W.; Koruth, J.S.; Miller, M.A.; Langan, M.-N.; Sofi, A.; Choudry, S.; Dukkipati, S.R.; Reddy, V.Y. Assessment of Catheter Ablation or Antiarrhythmic Drugs for First-line Therapy of Atrial Fibrillation: A Meta-analysis of Randomized Clinical Trials. JAMA Cardiol. 2021, 6, 697–705. [Google Scholar] [CrossRef]
- Nuzzi, V.; Raafs, A.; Manca, P.; Henkens, M.T.H.M.; Gregorio, C.; Boscutti, A.; Verdonschot, J.; Hazebroek, M.; Knackstedt, C.; Merlo, M.; et al. Left Atrial Reverse Remodeling in Dilated Cardiomyopathy. J. Am. Soc. Echocardiogr. 2023, 36, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Hachem, A.H.; Marine, J.E.; Tahboub, H.A.; Kamdar, S.; Kanjwal, S.; Soni, R.; Kanjwal, K. Radiofrequency Ablation versus Cryoablation in the Treatment of Paroxysmal Atrial Fibrillation: A Meta-Analysis. Cardiol. Res. Pract. 2018, 2018, 6276241. [Google Scholar] [CrossRef] [PubMed]
- Su, W.W.; Reddy, V.Y.; Bhasin, K.; Champagne, J.; Sangrigoli, R.M.; Braegelmann, K.M.; Kueffer, F.J.; Novak, P.; Gupta, S.K.; Yamane, T.; et al. Cryoballoon ablation of pulmonary veins for persistent atrial fibrillation: Results from the multicenter STOP Persistent AF trial. Heart Rhythm 2020, 17, 1841–1847. [Google Scholar] [CrossRef] [PubMed]
- Straube, F.; Pongratz, J.; Kosmalla, A.; Brueck, B.; Riess, L.; Hartl, S.; Tesche, C.; Ebersberger, U.; Wankerl, M.; Dorwarth, U.; et al. Cryoballoon Ablation Strategy in Persistent Atrial Fibrillation. Front. Cardiovasc. Med. 2021, 8, 758408. [Google Scholar] [CrossRef]
- Sawhney, V.; Schilling, R.J.; Providencia, R.; Cadd, M.; Perera, D.; Chatha, S.; Mercer, B.; Finlay, M.; Halimi, F.; Pavin, D.; et al. Cryoablation for persistent and longstanding persistent atrial fibrillation: Results from a multicentre European registry. Europace 2020, 22, 375–381. [Google Scholar] [CrossRef]
- Mililis, P.; Kariki, O.; Saplaouras, A.; Bazoukis, G.; Dragasis, S.; Patsiotis, I.G.; Batsouli, A.; Vlachos, K.; Letsas, K.P.; Efremidis, M. Radiofrequency versus cryoballoon catheter ablation in patients with persistent atrial fibrillation: A randomized trial. J. Cardiovasc. Electrophysiol. 2023, 34, 1523–1528. [Google Scholar] [CrossRef]
- Baimbetov, A.K.; Bizhanov, K.A.; Jukenova, A.M.; Aubakirova, A.T.; Ualiyeva, A.Y.; Sagatov, I.Y. Comparative Effectiveness and Safety of Cryoablation Versus Radiofrequency Ablation Treatments for Persistent Atrial Fibrillation. Am. J. Cardiol. 2022, 184, 22–30. [Google Scholar] [CrossRef]
- Kim, J.A.; Chelu, M.G. Comparison of cryoballoon and radiofrequency ablation for persistent atrial fibrillation: A systematic review and meta-analysis. J. Interv. Card. Electrophysiol. 2023, 66, 585–595. [Google Scholar] [CrossRef]
- Iyengar, S.K.; Iyengar, S.; Srivathsan, K. The promise of pulsed field ablation and the challenges ahead. Front. Cardiovasc. Med. 2023, 10, 1235317. [Google Scholar] [CrossRef]
- Koruth, J.; Kuroki, K.; Iwasawa, J.; Enomoto, Y.; Viswanathan, R.; Brose, R.; Buck, E.D.; Speltz, M.; Dukkipati, S.R.; Reddy, V.Y. Preclinical Evaluation of Pulsed Field Ablation: Electrophysiological and Histological Assessment of Thoracic Vein Isolation. Circ. Arrhythm. Electrophysiol. 2019, 12, e007781. [Google Scholar] [CrossRef]
- Metzner, A.; Fiala, M.; Vijgen, J.; Ouss, A.; Gunawardene, M.; Hansen, J.; Kautzner, J.; Schmidt, B.; Duytschaever, M.; Reichlin, T.; et al. Long-term outcomes of the pentaspline pulsed-field ablation catheter for the treatment of paroxysmal atrial fibrillation: Results of the prospective, multicentre FARA-Freedom Study. Europace 2024, 26, euae053. [Google Scholar] [CrossRef] [PubMed]
- Turagam, M.K.; Neuzil, P.; Schmidt, B.; Reichlin, T.; Neven, K.; Metzner, A.; Hansen, J.; Blaauw, Y.; Maury, P.; Arentz, T.; et al. Safety and Effectiveness of Pulsed Field Ablation to Treat Atrial Fibrillation: One-Year Outcomes from the MANIFEST-PF Registry. Circulation 2023, 148, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Haines, D.E.; Boersma, L.V.; Sood, N.; Natale, A.; Marchlinski, F.E.; Calkins, H.; Sanders, P.; Packer, D.L.; Kuck, K.H.; et al. Pulsed Field Ablation for the Treatment of Atrial Fibrillation: PULSED AF Pivotal Trial. Circulation 2023, 147, 1422–1432. [Google Scholar] [CrossRef] [PubMed]
- Gunawardene, M.A.; Frommeyer, G.; Ellermann, C.; Jularic, M.; Leitz, P.; Hartmann, J.; Lange, P.S.; Anwar, O.; Rath, B.; Wahedi, R.; et al. Left Atrial Posterior Wall Isolation with Pulsed Field Ablation in Persistent Atrial Fibrillation. J. Clin. Med. 2023, 12, 6304. [Google Scholar] [CrossRef] [PubMed]
- Kueffer, T.; Stefanova, A.; Madaffari, A.; Seiler, J.; Thalmann, G.; Kozhuharov, N.; Maurhofer, J.; Galuszka, O.; Haeberlin, A.; Noti, F.; et al. Pulmonary vein isolation durability and lesion regression in patients with recurrent arrhythmia after pulsed-field ablation. J. Interv. Card. Electrophysiol. 2024, 67, 503–511. [Google Scholar] [CrossRef]
- Magni, F.T.; Scherr, D.; Manninger, M.; Sohns, C.; Sommer, P.; Hovakimyan, T.; Blaauw, Y.; Mulder, B.A. Electrophysiological findings during re-do procedures after single-shot pulmonary vein isolation for atrial fibrillation with pulsed field ablation. J. Interv. Card. Electrophysiol. 2023, 66, 1729–1737. [Google Scholar] [CrossRef]
- Tohoku, S.; Chun, K.R.J.; Bordignon, S.; Chen, S.; Schaack, D.; Urbanek, L.; Ebrahimi, R.; Hirokami, J.; Bologna, F.; Schmidt, B. Findings from repeat ablation using high-density mapping after pulmonary vein isolation with pulsed field ablation. Europace 2023, 25, 433–440. [Google Scholar] [CrossRef]
- Ruwald, M.H.; Johannessen, A.; Hansen, M.L.; Haugdal, M.; Worck, R.; Hansen, J. Pulsed field ablation in real-world atrial fibrillation patients: Clinical recurrence, operator learning curve and re-do procedural findings. J. Interv. Card. Electrophysiol. 2023, 66, 1837–1848. [Google Scholar] [CrossRef] [PubMed]
- Ruwald, M.H.; Haugdal, M.; Worck, R.; Johannessen, A.; Hansen, M.L.; Sørensen, S.K.; Hansen, J. Characterization of durability and reconnection patterns at time of repeat ablation after single-shot pulsed field pulmonary vein isolation. J. Interv. Card. Electrophysiol. 2024, 67, 379–387. [Google Scholar] [CrossRef]
- Mattison, L.; Verma, A.; Tarakji, K.G.; Reichlin, T.; Hindricks, G.; Sack, K.L.; Önal, B.; Schmidt, M.M.; Miklavčič, D.; Sigg, D.C. Effect of contact force on pulsed field ablation lesions in porcine cardiac tissue. J. Cardiovasc. Electrophysiol. 2023, 34, 693–699. [Google Scholar] [CrossRef]
- Nakagawa, H.; Castellvi, Q.; Neal, R.; Girouard, S.; Laughner, J.; Ikeda, A.; Sugawara, M.; An, Y.; Hussein, A.A.; Nakhla, S.; et al. Effects of Contact Force on Lesion Size during Pulsed Field Catheter Ablation: Histochemical Characterization of Ventricular Lesion Boundaries. Circ. Arrhythm. Electrophysiol. 2024, 17, e012026. [Google Scholar] [CrossRef]
- Howard, B.; Verma, A.; Tzou, W.S.; Mattison, L.; Kos, B.; Miklavčič, D.; Onal, B.; Stewart, M.T.; Sigg, D.C. Effects of Electrode-Tissue Proximity on Cardiac Lesion Formation Using Pulsed Field Ablation. Circ. Arrhythm. Electrophysiol. 2022, 15, e011110. [Google Scholar] [CrossRef]
- Musikantow, D.R.; Neuzil, P.; Petru, J.; Koruth, J.S.; Kralovec, S.; Miller, M.A.; Funasako, M.; Chovanec, M.; Turagam, M.K.; Whang, W.; et al. Pulsed Field Ablation to Treat Atrial Fibrillation: Autonomic Nervous System Effects. JACC Clin. Electrophysiol. 2023, 9, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Musikantow, D.R.; Reddy, V.Y.; Skalsky, I.; Shaburishvili, T.; van Zyl, M.; O’Brien, B.; Coffey, K.; Reilly, J.; Neuzil, P.; Asirvatham, S.; et al. Targeted ablation of epicardial ganglionated plexi during cardiac surgery with pulsed field electroporation (NEURAL AF). J. Interv. Card. Electrophysiol. 2023, 1–8. [Google Scholar] [CrossRef]
- Gerstenfeld, E.P.; Mansour, M.; Whang, W.; Venkateswaran, R.; Harding, J.D.; Ellis, C.R.; Ellenbogen, K.A.; Osorio, J.; DeLurgio, D.B.; Daccarett, M.; et al. Autonomic Effects of Pulsed Field vs Thermal Ablation for Treating Atrial Fibrillation: Subanalysis of ADVENT. JACC Clin. Electrophysiol. 2024, 10, 1634–1644. [Google Scholar] [CrossRef]
- Aldaas, O.M.; Malladi, C.; Han, F.T.; Hoffmayer, K.S.; Krummen, D.; Ho, G.; Raissi, F.; Birgersdotter-Green, U.; Feld, G.K.; Hsu, J.C. Pulsed field ablation versus thermal energy ablation for atrial fibrillation: A systematic review and meta-analysis of procedural efficiency, safety, and efficacy. J. Interv. Card. Electrophysiol. 2024, 67, 639–648. [Google Scholar] [CrossRef]
- Ekanem, E.; Neuzil, P.; Reichlin, T.; Kautzner, J.; van der Voort, P.; Jais, P.; Chierchia, G.B.; Bulava, A.; Blaauw, Y.; Skala, T.; et al. Safety of pulsed field ablation in more than 17,000 patients with atrial fibrillation in the MANIFEST-17K study. Nat. Med. 2024, 30, 2020–2029. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Neuzil, P.; Petru, J.; Funasako, M.; Hala, P.; Kopriva, K.; Koruth, J.S.; Dukkipati, S.R.; Reddy, V.Y. Coronary Artery Spasm during Pulsed Field vs Radiofrequency Catheter Ablation of the Mitral Isthmus. JAMA Cardiol. 2024, 9, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Calvert, P.; Kollias, G.; Pürerfellner, H.; Narasimhan, C.; Osorio, J.; Lip, G.Y.H.; Gupta, D. Silent cerebral lesions following catheter ablation for atrial fibrillation: A state-of-the-art review. Europace 2023, 25, euad151. [Google Scholar] [CrossRef]
- Alkukhun, L.; Sandhu, U.; Hodovan, J.; Zhao, Y.; Chiang, K.; Castellvi, Q.; Stenzel, P.; Woltjer, R.; Li, X.; Barajas, R.F., Jr.; et al. Multi-modality imaging assessment of microbubbles and cerebral emboli in left ventricular pulsed field ablation. J. Interv. Card. Electrophysiol. 2023, 1–7. [Google Scholar] [CrossRef]
- Badertscher, P.; Serban, T.; Isenegger, C.; Krisai, P.; Voellmin, G.; Osswald, S.; Knecht, S.; Sticherling, C.; Kühne, M. Role of 3D electro-anatomical mapping on procedural characteristics and outcomes in pulsed-field ablation for atrial fibrillation. Europace 2024, 26, euae075. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.S.; Hocini, M.; Dubois, R.; Denis, A.; Derval, N.; Zellerhoff, S.; Yamashita, S.; Berte, B.; Mahida, S.; Komatsu, Y.; et al. Complexity and Distribution of Drivers in Relation to Duration of Persistent Atrial Fibrillation. J. Am. Coll. Cardiol. 2017, 69, 1257–1269. [Google Scholar] [CrossRef] [PubMed]
- Platonov, P.G.; Ivanov, V.; Ho, S.Y.; Mitrofanova, L. Left atrial posterior wall thickness in patients with and without atrial fibrillation: Data from 298 consecutive autopsies. J. Cardiovasc. Electrophysiol. 2008, 19, 689–692. [Google Scholar] [CrossRef] [PubMed]
- Schoene, K.; Arya, A.; Grashoff, F.; Knopp, H.; Weber, A.; Lerche, M.; König, S.; Hilbert, S.; Kircher, S.; Bertagnolli, L.; et al. Oesophageal Probe Evaluation in Radiofrequency Ablation of Atrial Fibrillation (OPERA): Results from a prospective randomized trial. Europace 2020, 22, 1487–1494. [Google Scholar] [CrossRef]
- Candemir, B.; Baskovski, E.; Beton, O.; Kozluca, V.; Tan, T.S.; Altın, T.; Tutar, E. Long-Term Results of Pulmonary Vein Isolation Plus Modified Posterior Wall Debulking Utilizing High-Power Short-Duration Strategy: An All-Comers Study in Real World. Anatol. J. Cardiol. 2022, 26, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Thiyagarajah, A.; Kadhim, K.; Lau, D.H.; Emami, M.; Linz, D.; Khokhar, K.; Munawar, D.A.; Mishima, R.; Malik, V.; O’Shea, C.; et al. Feasibility, Safety, and Efficacy of Posterior Wall Isolation during Atrial Fibrillation Ablation: A Systematic Review and Meta-Analysis. Circ. Arrhythm. Electrophysiol. 2019, 12, e007005. [Google Scholar] [CrossRef]
- Kistler, P.M.; Chieng, D.; Sugumar, H.; Ling, L.H.; Segan, L.; Azzopardi, S.; Al-Kaisey, A.; Parameswaran, R.; Anderson, R.D.; Hawson, J.; et al. Effect of Catheter Ablation Using Pulmonary Vein Isolation with vs without Posterior Left Atrial Wall Isolation on Atrial Arrhythmia Recurrence in Patients with Persistent Atrial Fibrillation: The CAPLA Randomized Clinical Trial. JAMA 2023, 329, 127–135. [Google Scholar] [CrossRef]
- Ad, N.; Damiano, R.J., Jr.; Badhwar, V.; Calkins, H.; La Meir, M.; Nitta, T.; Doll, N.; Holmes, S.D.; Weinstein, A.A.; Gillinov, M. Expert consensus guidelines: Examining surgical ablation for atrial fibrillation. J. Thorac. Cardiovasc. Surg. 2017, 153, 1330–1354.E1. [Google Scholar] [CrossRef]
- Tjien, A.T.J.; Akca, F.; Lam, K.; Olsthoorn, J.; Dekker, L.; van der Voort, P.; Verberkmoes, N.; van Brakel, T.J. Concomitant atrial fibrillation ablation in minimally invasive cardiac surgery. Ann. Cardiothorac. Surg. 2024, 13, 91–98. [Google Scholar] [CrossRef]
- Osmancik, P.; Budera, P.; Talavera, D.; Hlavicka, J.; Herman, D.; Holy, J.; Cervinka, P.; Smid, J.; Hanak, P.; Hatala, R.; et al. Five-year outcomes in cardiac surgery patients with atrial fibrillation undergoing concomitant surgical ablation versus no ablation. The long-term follow-up of the PRAGUE-12 Study. Heart Rhythm 2019, 16, 1334–1340. [Google Scholar] [CrossRef]
- Gu, W.; Guo, H.; Lu, C.; Huang, H.; Liu, J.; Liu, J.; Xie, B.; Wu, R.; Chen, J.; Zhuang, J. Surgical ablation for persistent atrial fibrillation in concomitant cardiac surgery: Mid-long-term result. Eur. J. Cardio-Thorac. Surg. 2017, 52, 888–894. [Google Scholar] [CrossRef]
- Eranki, A.; Wilson-Smith, A.; Flynn, C.; Williams, M.; Manganas, C. Mid term freedom from atrial fibrillation following hybrid ablation, a systematic review and meta analysis. J. Cardiothorac. Surg. 2023, 18, 155. [Google Scholar] [CrossRef] [PubMed]
- Van der Heijden, C.A.J.; Aerts, L.; Chaldoupi, S.M.; van Cruchten, C.; Kawczynski, M.; Heuts, S.; Bidar, E.; Luermans, J.; Maesen, B. Hybrid atrial fibrillation ablation. Ann. Cardiothorac. Surg. 2024, 13, 54–70. [Google Scholar] [CrossRef] [PubMed]
- Maesen, B.; Luermans, J.G.L.M.; Bidar, E.; Chaldoupi, S.-M.; Gelsomino, S.; Maessen, J.G.; Pison, L.; Meir, M.L. A hybrid approach to complex arrhythmias. EP Eur. 2021, 23, ii28–ii33. [Google Scholar] [CrossRef] [PubMed]
- van der Heijden, C.A.J.; Weberndörfer, V.; Vroomen, M.; Luermans, J.G.; Chaldoupi, S.M.; Bidar, E.; Vernooy, K.; Maessen, J.G.; Pison, L.; van Kuijk, S.M.J.; et al. Hybrid Ablation Versus Repeated Catheter Ablation in Persistent Atrial Fibrillation: A Randomized Controlled Trial. JACC Clin. Electrophysiol. 2023, 9, 1013–1023. [Google Scholar] [CrossRef]
- Doll, N.; Weimar, T.; Kosior, D.A.; Bulava, A.; Mokracek, A.; Mönnig, G.; Sahu, J.; Hunter, S.; Wijffels, M.; van Putte, B.; et al. Efficacy and safety of hybrid epicardial and endocardial ablation versus endocardial ablation in patients with persistent and longstanding persistent atrial fibrillation: A randomised, controlled trial. eClinicalMedicine 2023, 61, 102052. [Google Scholar] [CrossRef]
- Pannone, L.; Mouram, S.; Della Rocca, D.G.; Sorgente, A.; Monaco, C.; Del Monte, A.; Gauthey, A.; Bisignani, A.; Kronenberger, R.; Paparella, G.; et al. Hybrid atrial fibrillation ablation: Long-term outcomes from a single-centre 10-year experience. Europace 2023, 25, euad114. [Google Scholar] [CrossRef]
- Badhwar, N.; Al-Dosari, G.; Dukes, J.; Lee, R.J. Subxiphoid Hybrid Approach for Epicardial/Endocardial Ablation and LAA Exclusion in Patients with Persistent and Longstanding Atrial Fibrillation. J. Atr. Fibrillation 2018, 11, 2014. [Google Scholar] [CrossRef] [PubMed]
- Larson, J.; Merchant, F.M.; Patel, A.; Ndubisi, N.M.; Patel, A.M.; DeLurgio, D.B.; Lloyd, M.S.; El-Chami, M.F.; Leon, A.R.; Hoskins, M.H.; et al. Outcomes of convergent atrial fibrillation ablation with continuous rhythm monitoring. J. Cardiovasc. Electrophysiol. 2020, 31, 1270–1276. [Google Scholar] [CrossRef]
- DeLurgio, D.B.; Crossen, K.J.; Gill, J.; Blauth, C.; Oza, S.R.; Magnano, A.R.; Mostovych, M.A.; Halkos, M.E.; Tschopp, D.R.; Kerendi, F.; et al. Hybrid Convergent Procedure for the Treatment of Persistent and Long-Standing Persistent Atrial Fibrillation: Results of CONVERGE Clinical Trial. Circ. Arrhythm. Electrophysiol. 2020, 13, e009288. [Google Scholar] [CrossRef]
- Shrestha, S.; Plasseraud, K.M.; Makati, K.; Sood, N.; Killu, A.M.; Contractor, T.; Ahsan, S.; De Lurgio, D.B.; Shults, C.C.; Eldadah, Z.A.; et al. Hybrid Convergent ablation for atrial fibrillation: A systematic review and meta-analysis. Heart Rhythm O2 2022, 3, 396–404. [Google Scholar] [CrossRef]
- Carpenter, A.; Pannell, L.M.K.; Rizvi, S.I.A.; Maciver, K.; Rajakaruna, C.; Ciulli, F.; Duncan, E.R.; Thomas, G.; Barman, P.; Bond, R.; et al. Convergent approach to persistent atrial fibrillation ablation: Long-term single-centre safety and efficacy. Front. Cardiovasc. Med. 2024, 10, 1336801. [Google Scholar] [CrossRef]
- Haldar, S.; Khan, H.R.; Boyalla, V.; Kralj-Hans, I.; Jones, S.; Lord, J.; Onyimadu, O.; Satishkumar, A.; Bahrami, T.; De Souza, A.; et al. Catheter ablation vs. thoracoscopic surgical ablation in long-standing persistent atrial fibrillation: CASA-AF randomized controlled trial. Eur. Heart J. 2020, 41, 4471–4480. [Google Scholar] [CrossRef]
- Boyalla, V.; Haldar, S.; Khan, H.; Kralj-Hans, I.; Banya, W.; Lord, J.; Gnanasekar, A.; Bahrami, T.; Desouza, A.; Clague, J.; et al. LB-469804-02 LONG-TERM OUTCOMES IN LONG STANDING PERSISTENT ATRIAL FIBRILLATION FOLLOWING CATHETER OR THORACOSCOPIC SURGICAL ABLATION USING CONTINUOUS MONITORING: 3-YEAR FOLLOW-UP OF THE CASA-AF RANDOMISED CONTROLLED TRIAL. Heart Rhythm 2024, 21, 1196–1197. [Google Scholar] [CrossRef]
- Benali, K.; Barré, V.; Hermida, A.; Galand, V.; Milhem, A.; Philibert, S.; Boveda, S.; Bars, C.; Anselme, F.; Maille, B.; et al. Recurrences of Atrial Fibrillation Despite Durable Pulmonary Vein Isolation: The PARTY-PVI Study. Circ. Arrhythm. Electrophysiol. 2023, 16, e011354. [Google Scholar] [CrossRef] [PubMed]
- Sugumar, H.; Thomas, S.P.; Prabhu, S.; Voskoboinik, A.; Kistler, P.M. How to perform posterior wall isolation in catheter ablation for atrial fibrillation. J. Cardiovasc. Electrophysiol. 2018, 29, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Cutler, M.J.; Johnson, J.; Abozguia, K.; Rowan, S.; Lewis, W.; Costantini, O.; Natale, A.; Ziv, O. Impact of Voltage Mapping to Guide whether to Perform Ablation of the Posterior Wall in Patients with Persistent Atrial Fibrillation. J. Cardiovasc. Electrophysiol. 2016, 27, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.; Di Biase, L.; Mohanty, P.; Trivedi, C.; Russo, A.D.; Themistoclakis, S.; Casella, M.; Santarelli, P.; Fassini, G.; Santangeli, P.; et al. Proven isolation of the pulmonary vein antrum with or without left atrial posterior wall isolation in patients with persistent atrial fibrillation. Heart Rhythm 2016, 13, 132–140. [Google Scholar] [CrossRef]
- Liang, Z.; Liu, L.; Cheng, L.; Wang, Z.; Zhang, J.; Yang, W.; Wang, Y.; Wu, Y. New method and electrophysiological characteristics of LA posterior wall isolation in persistent atrial fibrillation. Pacing Clin. Electrophysiol. 2021, 44, 1691–1700. [Google Scholar] [CrossRef]
- Kim, J.S.; Shin, S.Y.; Na, J.O.; Choi, C.U.; Kim, S.H.; Kim, J.W.; Kim, E.J.; Rha, S.W.; Park, C.G.; Seo, H.S.; et al. Does isolation of the left atrial posterior wall improve clinical outcomes after radiofrequency catheter ablation for persistent atrial fibrillation?: A prospective randomized clinical trial. Int. J. Cardiol. 2015, 181, 277–283. [Google Scholar] [CrossRef]
- Sternik, L.; Kogan, A.; Luria, D.; Glikson, M.; Malachy, A.; Levin, S.; Raanani, E. Box lesion in the open left atrium for surgical ablation of atrial fibrillation. J. Thorac. Cardiovasc. Surg. 2014, 147, 956–959. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, L.; Hensey, M.; Nolan, W.; Keane, D. Clinical outcome when left atrial posterior wall box isolation is included as a catheter ablation strategy in patients with persistent atrial fibrillation. J. Interv. Card. Electrophysiol. 2015, 44, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Calkins, H.; Hindricks, G.; Cappato, R.; Kim, Y.H.; Saad, E.B.; Aguinaga, L.; Akar, J.G.; Badhwar, V.; Brugada, J.; Camm, J.; et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm 2017, 14, e275–e444. [Google Scholar] [CrossRef] [PubMed]
- Kanitsoraphan, C.; Rattanawong, P.; Techorueangwiwat, C.; Kewcharoen, J.; Mekritthikrai, R.; Prasitlumkum, N.; Shah, P.; El Masry, H. The efficacy of posterior wall isolation in atrial fibrillation ablation: A systematic review and meta-analysis of randomized controlled trials. J. Arrhythm. 2022, 38, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Shim, J.; Park, J.; Yu, H.T.; Kim, T.H.; Park, J.K.; Uhm, J.S.; Kim, J.B.; Joung, B.; Lee, M.H.; et al. The Electrical Isolation of the Left Atrial Posterior Wall in Catheter Ablation of Persistent Atrial Fibrillation. JACC Clin. Electrophysiol. 2019, 5, 1253–1261. [Google Scholar] [CrossRef]
- Turagam, M.K.; Neuzil, P.; Schmidt, B.; Reichlin, T.; Neven, K.; Metzner, A.; Hansen, J.; Blaauw, Y.; Maury, P.; Arentz, T.; et al. Impact of Left Atrial Posterior Wall Ablation during Pulsed-Field Ablation for Persistent Atrial Fibrillation. JACC Clin. Electrophysiol. 2024, 10, 900–912. [Google Scholar] [CrossRef]
- Di Biase, L.; Burkhardt, J.D.; Mohanty, P.; Sanchez, J.; Mohanty, S.; Horton, R.; Gallinghouse, G.J.; Bailey, S.M.; Zagrodzky, J.D.; Santangeli, P.; et al. Left atrial appendage: An underrecognized trigger site of atrial fibrillation. Circulation 2010, 122, 109–118. [Google Scholar] [CrossRef]
- Di Biase, L.; Burkhardt, J.D.; Mohanty, P.; Mohanty, S.; Sanchez, J.E.; Trivedi, C.; Güneş, M.; Gökoğlan, Y.; Gianni, C.; Horton, R.P.; et al. Left Atrial Appendage Isolation in Patients with Longstanding Persistent AF Undergoing Catheter Ablation: BELIEF Trial. J. Am. Coll. Cardiol. 2016, 68, 1929–1940. [Google Scholar] [CrossRef]
- Romanov, A.; Pokushalov, E.; Elesin, D.; Bogachev-Prokophiev, A.; Ponomarev, D.; Losik, D.; Bayramova, S.; Strelnikov, A.; Shabanov, V.; Pidanov, O.; et al. Effect of left atrial appendage excision on procedure outcome in patients with persistent atrial fibrillation undergoing surgical ablation. Heart Rhythm 2016, 13, 1803–1809. [Google Scholar] [CrossRef]
- Lakkireddy, D.R.; Wilber, D.J.; Mittal, S.; Tschopp, D.; Ellis, C.R.; Rasekh, A.; Hounshell, T.; Evonich, R.; Chandhok, S.; Berger, R.D.; et al. Pulmonary Vein Isolation with or without Left Atrial Appendage Ligation in Atrial Fibrillation: The aMAZE Randomized Clinical Trial. JAMA 2024, 331, 1099–1108. [Google Scholar] [CrossRef]
- Romero, J.; Di Biase, L.; Mohanty, S.; Trivedi, C.; Patel, K.; Parides, M.; Alviz, I.; Diaz, J.C.; Natale, V.; Sanchez, J.; et al. Long-Term Outcomes of Left Atrial Appendage Electrical Isolation in Patients with Nonparoxysmal Atrial Fibrillation: A Propensity Score-Matched Analysis. Circ. Arrhythm. Electrophysiol. 2020, 13, e008390. [Google Scholar] [CrossRef]
- Rillig, A.; Tilz, R.R.; Lin, T.; Fink, T.; Heeger, C.H.; Arya, A.; Metzner, A.; Mathew, S.; Wissner, E.; Makimoto, H.; et al. Unexpectedly High Incidence of Stroke and Left Atrial Appendage Thrombus Formation after Electrical Isolation of the Left Atrial Appendage for the Treatment of Atrial Tachyarrhythmias. Circ. Arrhythm. Electrophysiol. 2016, 9, e003461. [Google Scholar] [CrossRef] [PubMed]
- Romero, J.; Gabr, M.; Patel, K.; Briceno, D.; Diaz, J.C.; Alviz, I.; Trivedi, C.; Mohanty, S.; Polanco, D.; Della Rocca, D.G.; et al. Efficacy and safety of left atrial appendage electrical isolation during catheter ablation of atrial fibrillation: An updated meta-analysis. Europace 2021, 23, 226–237. [Google Scholar] [CrossRef]
- Tsai, C.F.; Tai, C.T.; Hsieh, M.H.; Lin, W.S.; Yu, W.C.; Ueng, K.C.; Ding, Y.A.; Chang, M.S.; Chen, S.A. Initiation of atrial fibrillation by ectopic beats originating from the superior vena cava: Electrophysiological characteristics and results of radiofrequency ablation. Circulation 2000, 102, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Tai, C.T.; Chiou, C.W.; Wen, Z.C.; Hsieh, M.H.; Tsai, C.F.; Lin, W.S.; Chen, C.C.; Lin, Y.K.; Yu, W.C.; Ding, Y.A.; et al. Effect of phenylephrine on focal atrial fibrillation originating in the pulmonary veins and superior vena cava. J. Am. Coll. Cardiol. 2000, 36, 788–793. [Google Scholar] [CrossRef]
- Goya, M.; Ouyang, F.; Ernst, S.; Volkmer, M.; Antz, M.; Kuck, K.H. Electroanatomic mapping and catheter ablation of breakthroughs from the right atrium to the superior vena cava in patients with atrial fibrillation. Circulation 2002, 106, 1317–1320. [Google Scholar] [CrossRef] [PubMed]
- Kholová, I.; Kautzner, J. Morphology of atrial myocardial extensions into human caval veins: A postmortem study in patients with and without atrial fibrillation. Circulation 2004, 110, 483–488. [Google Scholar] [CrossRef]
- Santangeli, P.; Marchlinski, F.E. Techniques for the provocation, localization, and ablation of non-pulmonary vein triggers for atrial fibrillation. Heart Rhythm 2017, 14, 1087–1096. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.P.; Sangha, R.S.; Dahal, K.; Krishnamoorthy, P. The role of empiric superior vena cava isolation in atrial fibrillation: A systematic review and meta-analysis of randomized controlled trials. J. Interv. Card. Electrophysiol. 2017, 48, 61–67. [Google Scholar] [CrossRef]
- Li, J.Y.; Jiang, J.B.; Zhong, G.Q.; Ke, H.H.; He, Y. Comparison of Empiric Isolation and Conventional Isolation of Superior Vena Cava in Addition to Pulmonary Vein Isolation on the Outcome of Paroxysmal Atrial Fibrillation Ablation. Int. Heart J. 2017, 58, 500–505. [Google Scholar] [CrossRef]
- Yoshida, K.; Hattori, A.; Tsuneoka, H.; Tsumagari, Y.; Yui, Y.; Kimata, A.; Ito, Y.; Ebine, M.; Uehara, Y.; Koda, N.; et al. Electrophysiological relation between the superior vena cava and right superior pulmonary vein in patients with paroxysmal atrial fibrillation. J. Cardiovasc. Electrophysiol. 2017, 28, 1117–1126. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Zhao, D.; Chen, X.; Shi, L.; Chen, Q.; Zhang, H.; Yu, Y.; Ullah, I.; Kojodjojo, P.; Zhang, F. Role of electroanatomical mapping—Guided superior vena cava isolation in paroxysmal atrial fibrillation patients without provoked superior vena cava triggers: A randomized controlled study. Europace 2024, 26, euae039. [Google Scholar] [CrossRef] [PubMed]
- Nademanee, K.; McKenzie, J.; Kosar, E.; Schwab, M.; Sunsaneewitayakul, B.; Vasavakul, T.; Khunnawat, C.; Ngarmukos, T. A new approach for catheter ablation of atrial fibrillation: Mapping of the electrophysiologic substrate. J. Am. Coll. Cardiol. 2004, 43, 2044–2053. [Google Scholar] [CrossRef]
- Chugh, A. Complex Fractionated Atrial Electrograms in Catheter Ablation of Atrial Fibrillation. Circ. Arrhythm. Electrophysiol. 2015, 8, 999–1001. [Google Scholar] [CrossRef]
- Providência, R.; Lambiase, P.D.; Srinivasan, N.; Ganesh Babu, G.; Bronis, K.; Ahsan, S.; Khan, F.Z.; Chow, A.W.; Rowland, E.; Lowe, M.; et al. Is There Still a Role for Complex Fractionated Atrial Electrogram Ablation in Addition to Pulmonary Vein Isolation in Patients with Paroxysmal and Persistent Atrial Fibrillation? Meta-Analysis of 1415 Patients. Circ. Arrhythm. Electrophysiol. 2015, 8, 1017–1029. [Google Scholar] [CrossRef] [PubMed]
- Li, W.J.; Bai, Y.Y.; Zhang, H.Y.; Tang, R.B.; Miao, C.L.; Sang, C.H.; Yin, X.D.; Dong, J.Z.; Ma, C.S. Additional ablation of complex fractionated atrial electrograms after pulmonary vein isolation in patients with atrial fibrillation: A meta-analysis. Circ. Arrhythm. Electrophysiol. 2011, 4, 143–148. [Google Scholar] [CrossRef]
- Lau, D.H.; Maesen, B.; Zeemering, S.; Kuklik, P.; van Hunnik, A.; Lankveld, T.A.; Bidar, E.; Verheule, S.; Nijs, J.; Maessen, J.; et al. Indices of bipolar complex fractionated atrial electrograms correlate poorly with each other and atrial fibrillation substrate complexity. Heart Rhythm 2015, 12, 1415–1423. [Google Scholar] [CrossRef] [PubMed]
- Narayan, S.M.; Krummen, D.E.; Shivkumar, K.; Clopton, P.; Rappel, W.-J.; Miller, J.M. Treatment of Atrial Fibrillation by the Ablation of Localized Sources. J. Am. Coll. Cardiol. 2012, 60, 628–636. [Google Scholar] [CrossRef]
- Miller, J.M.; Kowal, R.C.; Swarup, V.; Daubert, J.P.; Daoud, E.G.; Day, J.D.; Ellenbogen, K.A.; Hummel, J.D.; Baykaner, T.; Krummen, D.E.; et al. Initial independent outcomes from focal impulse and rotor modulation ablation for atrial fibrillation: Multicenter FIRM registry. J. Cardiovasc. Electrophysiol. 2014, 25, 921–929. [Google Scholar] [CrossRef]
- Spitzer, S.G.; Károlyi, L.; Rämmler, C.; Scharfe, F.; Weinmann, T.; Zieschank, M.; Langbein, A. Treatment of Recurrent Nonparoxysmal Atrial Fibrillation Using Focal Impulse and Rotor Mapping (FIRM)-Guided Rotor Ablation: Early Recurrence and Long-Term Outcomes. J. Cardiovasc. Electrophysiol. 2017, 28, 31–38. [Google Scholar] [CrossRef]
- Berntsen, R.F.; Håland, T.F.; Skårdal, R.; Holm, T. Focal impulse and rotor modulation as a stand-alone procedure for the treatment of paroxysmal atrial fibrillation: A within-patient controlled study with implanted cardiac monitoring. Heart Rhythm 2016, 13, 1768–1774. [Google Scholar] [CrossRef]
- Spitzer, S.G.; Miller, J.M.; Sommer, P.; Szili-Torok, T.; Reddy, V.Y.; Nölker, G.; Williams, C.; Sarver, A.; Wilber, D.J. Randomized evaluation of redo ablation procedures of atrial fibrillation with focal impulse and rotor modulation-guided procedures: The REDO-FIRM study. Europace 2023, 25, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Gianni, C.; Mohanty, S.; Di Biase, L.; Metz, T.; Trivedi, C.; Gökoğlan, Y.; Güneş, M.F.; Bai, R.; Al-Ahmad, A.; Burkhardt, J.D.; et al. Acute and early outcomes of focal impulse and rotor modulation (FIRM)-guided rotors-only ablation in patients with nonparoxysmal atrial fibrillation. Heart Rhythm 2016, 13, 830–835. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, S.; Mohanty, P.; Trivedi, C.; Gianni, C.; Della Rocca, D.G.; Di Biase, L.; Natale, A. Long-Term Outcome of Pulmonary Vein Isolation with and without Focal Impulse and Rotor Modulation Mapping: Insights From a Meta-Analysis. Circ. Arrhythm. Electrophysiol. 2018, 11, e005789. [Google Scholar] [CrossRef]
- Xu, C.-H.; Xiong, F.; Jiang, W.-F.; Liu, X.; Liu, T.; Qin, M. Rotor mechanism and its mapping in atrial fibrillation. EP Eur. 2023, 25, 783–792. [Google Scholar] [CrossRef]
- Qin, M.; Lin, R.J.; Wu, S.H.; Liu, X. Extra pulmonary vein driver mapping and ablation in paroxysmal atrial fibrillation by electrogram dispersion analysis. J. Cardiovasc. Electrophysiol. 2019, 30, 164–170. [Google Scholar] [CrossRef]
- Seitz, J.; Bars, C.; Théodore, G.; Beurtheret, S.; Lellouche, N.; Bremondy, M.; Ferracci, A.; Faure, J.; Penaranda, G.; Yamazaki, M.; et al. AF Ablation Guided by Spatiotemporal Electrogram Dispersion without Pulmonary Vein Isolation: A Wholly Patient-Tailored Approach. J. Am. Coll. Cardiol. 2017, 69, 303–321. [Google Scholar] [CrossRef]
- Hu, X.; Jiang, W.; Wu, S.; Xu, K.; Zhang, D.; Zhang, Y.; Liu, X.; Qin, M. Extra-pulmonary vein driver mapping and ablation for persistent atrial fibrillation in obese patients. Europace 2021, 23, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Seitz, J.; Durdez, T.M.; Albenque, J.P.; Pisapia, A.; Gitenay, E.; Durand, C.; Monteau, J.; Moubarak, G.; Théodore, G.; Lepillier, A.; et al. Artificial intelligence software standardizes electrogram-based ablation outcome for persistent atrial fibrillation. J. Cardiovasc. Electrophysiol. 2022, 33, 2250–2260. [Google Scholar] [CrossRef]
- Manongi, N.; Kim, J.; Goldbarg, S. Dispersion electrogram detection with an artificial intelligence software in redo paroxysmal atrial fibrillation ablation. Heart Case Rep. 2023, 9, 948–953. [Google Scholar] [CrossRef]
- Sousonis, V.; Voglimacci-Stephanopoli, Q.; Zeriouh, S.; Boveda, S.; Albenque, J.P. Pulsed field ablation of spatiotemporal electrogram dispersion following pulmonary vein isolation and left atrial linear lesions for persistent atrial fibrillation: A case report. Eur. Heart J.-Case Rep. 2024, 8, ytae085. [Google Scholar] [CrossRef] [PubMed]
- Bahlke, F.; Englert, F.; Popa, M.; Bourier, F.; Reents, T.; Lennerz, C.; Kraft, H.; Martinez, A.T.; Kottmaier, M.; Syväri, J.; et al. First clinical data on artificial intelligence-guided catheter ablation in long-standing persistent atrial fibrillation. J. Cardiovasc. Electrophysiol. 2024, 35, 406–414. [Google Scholar] [CrossRef]
- Betancur-Gutierrez, A.; Bartoletti, S.; Voglimacci-Stephanopoli, Q.; Saitta, F.; Benadel, M.; Sousonis, V.; Zeriouh, S.; Mene, R.; Scripcariu, A.; Rodriguez-Silva, J.; et al. Single-centre experience with an electrogram-based artificial intelligence software system to identify drivers in pulsed field ablation of persistent atrial fibrillation. EP Eur. 2024, 26, euae102.177. [Google Scholar] [CrossRef]
- Harmon, D.M.; Sehrawat, O.; Maanja, M.; Wight, J.; Noseworthy, P.A. Artificial Intelligence for the Detection and Treatment of Atrial Fibrillation. Arrhythm. Electrophysiol. Rev. 2023, 12, e12. [Google Scholar] [CrossRef] [PubMed]
- Gilard, M.; Mansourati, J.; Etienne, Y.; Larlet, J.M.; Truong, B.; Boschat, J.; Blanc, J.J. Angiographic anatomy of the coronary sinus and its tributaries. Pacing Clin. Electrophysiol. 1998, 21, 2280–2284. [Google Scholar] [CrossRef] [PubMed]
- Hwang, C.; Chen, P.S. Ligament of Marshall: Why it is important for atrial fibrillation ablation. Heart Rhythm 2009, 6, S35–S40. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.T.; Lai, A.C.; Hwang, C.; Fan, L.T.; Karagueuzian, H.S.; Chen, P.S.; Fishbein, M.C. The ligament of Marshall: A structural analysis in human hearts with implications for atrial arrhythmias. J. Am. Coll. Cardiol. 2000, 36, 1324–1327. [Google Scholar] [CrossRef]
- Haïssaguerre, M.; Jaïs, P.; Shah, D.C.; Takahashi, A.; Hocini, M.; Quiniou, G.; Garrigue, S.; Le Mouroux, A.; Le Métayer, P.; Clémenty, J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N. Engl. J. Med. 1998, 339, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Hsu, L.F.; Jaïs, P.; Keane, D.; Wharton, J.M.; Deisenhofer, I.; Hocini, M.; Shah, D.C.; Sanders, P.; Scavée, C.; Weerasooriya, R.; et al. Atrial fibrillation originating from persistent left superior vena cava. Circulation 2004, 109, 828–832. [Google Scholar] [CrossRef] [PubMed]
- Ulphani, J.S.; Arora, R.; Cain, J.H.; Villuendas, R.; Shen, S.; Gordon, D.; Inderyas, F.; Harvey, L.A.; Morris, A.; Goldberger, J.J.; et al. The ligament of Marshall as a parasympathetic conduit. Am. J. Physiol.-Heart Circ. Physiol. 2007, 293, H1629–H1635. [Google Scholar] [CrossRef]
- Wu, T.J.; Ong, J.J.; Chang, C.M.; Doshi, R.N.; Yashima, M.; Huang, H.L.; Fishbein, M.C.; Ting, C.T.; Karagueuzian, H.S.; Chen, P.S. Pulmonary veins and ligament of Marshall as sources of rapid activations in a canine model of sustained atrial fibrillation. Circulation 2001, 103, 1157–1163. [Google Scholar] [CrossRef] [PubMed]
- Scherlag, B.J.; Yeh, B.K.; Robinson, M.J. Inferior interatrial pathway in the dog. Circ. Res. 1972, 31, 18–35. [Google Scholar] [CrossRef]
- Hwang, C.; Karagueuzian, H.S.; Chen, P.S. Idiopathic paroxysmal atrial fibrillation induced by a focal discharge mechanism in the left superior pulmonary vein: Possible roles of the ligament of Marshall. J. Cardiovasc. Electrophysiol. 1999, 10, 636–648. [Google Scholar] [CrossRef] [PubMed]
- Hwang, C.; Wu, T.-J.; Doshi, R.N.; Peter, C.T.; Chen, P.-S. Vein of Marshall cannulation for the analysis of electrical activity in patients with focal atrial fibrillation. Circulation 2000, 101, 1503–1505. [Google Scholar] [CrossRef] [PubMed]
- Valderrábano, M.; Chen, H.R.; Sidhu, J.; Rao, L.; Ling, Y.; Khoury, D.S. Retrograde ethanol infusion in the vein of Marshall: Regional left atrial ablation, vagal denervation and feasibility in humans. Circ. Arrhythm. Electrophysiol. 2009, 2, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Valderrábano, M.; Peterson, L.E.; Swarup, V.; Schurmann, P.A.; Makkar, A.; Doshi, R.N.; DeLurgio, D.; Athill, C.A.; Ellenbogen, K.A.; Natale, A.; et al. Effect of Catheter Ablation with Vein of Marshall Ethanol Infusion vs Catheter Ablation Alone on Persistent Atrial Fibrillation: The VENUS Randomized Clinical Trial. JAMA 2020, 324, 1620–1628. [Google Scholar] [CrossRef] [PubMed]
- Valderrábano, M. Vein of Marshall ethanol infusion in the treatment of atrial fibrillation: From concept to clinical practice. Heart Rhythm 2021, 18, 1074–1082. [Google Scholar] [CrossRef]
- Yamamoto, T.; Maruyama, M.; Seino, Y.; Shimizu, W. Marshall bundle reentry: A novel type of macroreentrant atrial tachycardia. Heart Rhythm 2014, 11, 1229–1232. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, S.; Shah, A.J.; Haïssaguerre, M. Recurrent perimitral tachycardia using epicardial coronary sinus connection to bypass endocardial conduction block at the mitral isthmus. Circ. Arrhythm. Electrophysiol. 2011, 4, e39–e41. [Google Scholar] [CrossRef]
- Kawaguchi, N.; Okishige, K.; Yamauchi, Y.; Kurabayashi, M.; Nakamura, T.; Keida, T.; Sasano, T.; Hirao, K.; Valderrábano, M. Clinical impact of ethanol infusion into the vein of Marshall on the mitral isthmus area evaluated by atrial electrograms recorded inside the coronary sinus. Heart Rhythm 2019, 16, 1030–1038. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, T.; Pambrun, T.; Vlachos, K.; Goujeau, C.; André, C.; Krisai, P.; Ramirez, F.D.; Kamakura, T.; Takagi, T.; Nakatani, Y.; et al. Impact of Vein of Marshall Ethanol Infusion on Mitral Isthmus Block: Efficacy and Durability. Circ. Arrhythm. Electrophysiol. 2020, 13, e008884. [Google Scholar] [CrossRef] [PubMed]
- Ge, W.L.; Lu, Y.F.; Li, T.; Wang, Y.; Yin, J.; Li, X.R.; Jiang, J.J.; Mi, Y.F.; Tung, T.H.; Yan, S.H. Clinical effect of vein of Marshall ethanol infusion on mitral isthmus ablation. Front. Cardiovasc. Med. 2024, 11, 1253554. [Google Scholar] [CrossRef] [PubMed]
- Pambrun, T.; Denis, A.; Duchateau, J.; Sacher, F.; Hocini, M.; Jaïs, P.; Haïssaguerre, M.; Derval, N. MARSHALL bundles elimination, Pulmonary veins isolation and Lines completion for ANatomical ablation of persistent atrial fibrillation: MARSHALL-PLAN case series. J. Cardiovasc. Electrophysiol. 2019, 30, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Derval, N.; Duchateau, J.; Denis, A.; Ramirez, F.D.; Mahida, S.; André, C.; Krisai, P.; Nakatani, Y.; Kitamura, T.; Takigawa, M.; et al. Marshall bundle elimination, Pulmonary vein isolation, and Line completion for ANatomical ablation of persistent atrial fibrillation (Marshall-PLAN): Prospective, single-center study. Heart Rhythm 2021, 18, 529–537. [Google Scholar] [CrossRef]
- Li, K.; Xu, C.; Zhu, X.; Wang, X.; Ye, P.; Jiang, W.; Wu, S.; Xu, K.; Li, X.; Wang, Y.; et al. Multi-centre, prospective randomized comparison of three different substrate ablation strategies for persistent atrial fibrillation. Europace 2023, 25, euad090. [Google Scholar] [CrossRef] [PubMed]
- Masuda, M.; Asai, M.; Iida, O.; Okamoto, S.; Ishihara, T.; Nanto, K.; Kanda, T.; Tsujimura, T.; Matsuda, Y.; Okuno, S.; et al. Additional Low-Voltage-Area Ablation in Patients with Paroxysmal Atrial Fibrillation: Results of the Randomized Controlled VOLCANO Trial. J. Am. Heart Assoc. 2020, 9, e015927. [Google Scholar] [CrossRef]
- Masuda, M.; Asai, M.; Iida, O.; Okamoto, S.; Ishihara, T.; Nanto, K.; Kanda, T.; Tsujimura, T.; Matsuda, Y.; Hata, Y.; et al. Low-Voltage-Area Ablation in Paroxysmal Atrial Fibrillation—Extended Follow-up Results of the VOLCANO Trial. Circ. J. 2022, 86, 245–252. [Google Scholar] [CrossRef]
- Chen, H.; Li, C.; Han, B.; Xiao, F.; Yi, F.; Wei, Y.; Jiang, C.; Zou, C.; Shi, L.; Ma, W.; et al. Circumferential Pulmonary Vein Isolation with vs without Additional Low-Voltage-Area Ablation in Older Patients with Paroxysmal Atrial Fibrillation: A Randomized Clinical Trial. JAMA Cardiol. 2023, 8, 765–772. [Google Scholar] [CrossRef]
- Huo, Y.; Gaspar, T.; Schönbauer, R.; Wójcik, M.; Fiedler, L.; Roithinger, F.X.; Martinek, M.; Pürerfellner, H.; Kirstein, B.; Richter, U.; et al. Low-Voltage Myocardium-Guided Ablation Trial of Persistent Atrial Fibrillation. NEJM Evid. 2022, 1, EVIDoa2200141. [Google Scholar] [CrossRef]
- Jadidi, A.; Mueller-Edenborn, B.; Eichenlaub, M.; Lehrmann, H.; Allgeier, J.; Trenk, D.; Neumann, F.; Westermann, D.; Arentz, T. Pulmonary vein isolation and additional selective ablation of low voltage areas and atrial late potentials for persistent atrial fibrillation (SOLVE-AF-Study). Europace 2023, 25 (Suppl. S1), euad122.135. [Google Scholar] [CrossRef]
- Cutler, M.J.; Sattayaprasert, P.; Pivato, E.; Jabri, A.; AlMahameed, S.T.; Ziv, O. Low voltage-guided ablation of posterior wall improves 5-year arrhythmia-free survival in persistent atrial fibrillation. J. Cardiovasc. Electrophysiol. 2022, 33, 2475–2484. [Google Scholar] [CrossRef]
- Moustafa, A.; Karim, S.; Kahaly, O.; Elzanaty, A.; Meenakshisundaram, C.; Abi-Saleh, B.; Eltahawy, E.; Chacko, P. Low voltage area guided substrate modification in nonparoxysmal atrial fibrillation: A systematic review and meta-analysis. J. Cardiovasc. Electrophysiol. 2023, 34, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.Y.; Tai, C.T.; Chen, S.A. Treatment of atrial fibrillation by catheter ablation of conduction gaps in the crista terminalis and cavotricuspid isthmus of the right atrium. J. Cardiovasc. Electrophysiol. 2002, 13, 1044–1046. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Murakami, Y.; Okada, T.; Murohara, T. Focal atrial fibrillation associated with multiple breakout sites at the crista terminalis. Pacing Clin. Electrophysiol. 2006, 29, 207–210. [Google Scholar] [CrossRef]
- Fynn, S.P.; Morton, J.B.; Deen, V.R.; Kistler, P.M.; Vohra, J.K.; Sparks, P.B.; Kalman, J.M. Conduction characteristics at the crista terminalis during onset of pulmonary vein atrial fibrillation. J. Cardiovasc. Electrophysiol. 2004, 15, 855–861. [Google Scholar] [CrossRef]
- Morris, G.M.; Segan, L.; Wong, G.; Wynn, G.; Watts, T.; Heck, P.; Walters, T.E.; Nisbet, A.; Sparks, P.; Morton, J.B.; et al. Atrial Tachycardia Arising from the Crista Terminalis, Detailed Electrophysiological Features and Long-Term Ablation Outcomes. JACC Clin. Electrophysiol. 2019, 5, 448–458. [Google Scholar] [CrossRef] [PubMed]
- La Fazia, V.; Pierucci, N.; Gianni, C.; Torlapati, P.G.; Mohanty, S.; Della Rocca, D.G.; Gigante, C.; Bode, W.; Bassiouny, M.; Burkhardt, J.D.; et al. Prevalence of non pulmonary vein triggers and pulmonary pressure change in atrial fibrillation ablation for cardiac amyloidosis patients. EP Eur. 2024, 26, euae102.186. [Google Scholar] [CrossRef]
- Oral, H.; Chugh, A.; Good, E.; Crawford, T.; Sarrazin, J.F.; Kuhne, M.; Chalfoun, N.; Wells, D.; Boonyapisit, W.; Gadeela, N.; et al. Randomized evaluation of right atrial ablation after left atrial ablation of complex fractionated atrial electrograms for long-lasting persistent atrial fibrillation. Circ. Arrhythm. Electrophysiol. 2008, 1, 6–13. [Google Scholar] [CrossRef]
- Dixit, S.; Marchlinski, F.E.; Lin, D.; Callans, D.J.; Bala, R.; Riley, M.P.; Garcia, F.C.; Hutchinson, M.D.; Ratcliffe, S.J.; Cooper, J.M.; et al. Randomized ablation strategies for the treatment of persistent atrial fibrillation: RASTA study. Circ. Arrhythm. Electrophysiol. 2012, 5, 287–294. [Google Scholar] [CrossRef]
- De Greef, Y.; Bogaerts, K.; Sofianos, D.; Buysschaert, I. Impact of Diagnosis-to-Ablation Time on AF Recurrence: Pronounced the First 3 Years, Irrelevant Thereafter. JACC Clin. Electrophysiol. 2023, 9, 2263–2272. [Google Scholar] [CrossRef]
| Ablation Target | Study | Study Population | Study Design | Outcome |
|---|---|---|---|---|
| Left atrial posterior wall (LAPW) | Kistler 2023 (CAPLA trial) | 338 symptomatic patients with PeAF | Multicenter, randomized 1:1 to either PVI with PWI (n = 170) or PVI alone (n = 168) | Addition of PWI to PVI did not significantly improve freedom from AA compared with PVI alone [HR, 0.99 [95% CI, 0.73–1.36]; p = 0.98). |
| Kim 2015 (prospective RCT) | 120 PeAF patients followed for 12 months after RFCA | Linear ablation + CPVI + PWI (n = 60) vs. linear ablation + CPVI (n = 60) | PWI (+) group had higher AF termination during procedure (p = 0.035), and lower AF recurrence on follow-up (17% vs. 37%, p = 0.02) | |
| Tamborero 2008 (prospective RCT) | 120 patients with AF | Prospective, randomized to CPVI + PWI (n = 60) vs. CPVI alone (n = 60) | PWI did not improve freedom from AA recurrence in patients undergoing CPVI. (log-rank test p = 0.943) | |
| Wong 2023 (PEF-HOT trial) | 67 patients with PeAF using high-power short- duration ablation (HPSD) | Multicenter, open-label, single-blind, randomized to PVI + PWI (n = 39) vs. PVI alone (n = 28) | AA recurrence rates did not significantly differ between PVI + PWI and PVI-only groups (25.6% vs. 28.6%; p = 0.7895) | |
| Left atrial appendage (LAA) | Di Biase 2016 (BELIEF Trial) | 173 patients with long-standing PeAF | Open-label, randomized to empirical LAAEI + extensive ablation (n = 85) vs. extensive ablation alone (n = 88) | LAAEI improved long-term freedom from AAs without increasing complications. (unadjusted HR: 2.24; 95% CI: 1.3 to 3.8; log-rank p = 0.003) |
| Lakkireddy 2024 (aMAZE RCT) | 404 patients with non-paroxysmal AF | Multicenter, prospective, open-label, randomized 2:1 to PVI + LAA ligation (n = 404) vs. PVI alone (n = 206) | Freedom from AAs was 64.3% with LAAI + PVI and was not superior to 59.9% with PVI only (difference, 4.3% [Bayesian 95% credible interval, −4.2% to 13.2%]; posterior superiority probability, 0.835) | |
| Superior vena cava (SVC) | Dong 2024 (RCT) | 130 patients undergoing paroxysmal AF ablation | Prospective, multi-center, randomized 1:1 to CPVI + SVCI (n = 50) vs. CPVI alone (n = 50) | In patients without provoked SVC triggers, SVCI, in addition to CPVI, did not increase freedom from atrial tachyarrhythmias (87.9 vs. 79.6%, log-rank p = 0.28) |
| Crista terminalis (CT) | Dixit 2012 (RASTA study) | 156 patients with PeAF. Standard approach: PVI + ablation of non-PV triggers identified using a stimulation protocol | Single-center, randomized to either arm 1 (PVI + standard approach), arm 2, (standard approach + empirical ablation at common NPVTs (including CT)), or arm 3 (standard approach + ablation of LA CFAEs) | Freedom from AAs after single ablation was lowest with arm 3 (29%) vs. arm 1 (49%; p = 0.04) and arm 2 (58%; p = 0.004) |
| Vein of Marshall (VOM) | Valderrábano 2020 (VENUS Randomized Clinical Trial) | 343 patients with PeAF | Single-blinded trial, randomized 1:1.15 to CA alone (n = 158) or CA + VOM ethanol infusion (n = 185) | Higher freedom from AF/atrial tachycardia with catheter ablation + VOM ethanol ablation vs. catheter ablation alone at 6 and 12 months (difference, 11.2% [95% CI, 0.8%–21.7%]; p = 0.04) |
| Low voltage areas (LVAs) | Kaiser 2024 (RCT) | 100 patients with PeAF | Randomized 1:1 to A or B. A: PVI + substrate modification if LVAs present, B: PVI + additional ablation if AF persisted | Freedom from AAs in 34 (68%) patients of group A vs.28 (56%) patients in group B (p = ns). Low complication rate in both groups. |
| Huo 2022 (RCT) | 324 patients with PeAF | Multicenter trial, randomized 1:1 to PVI alone (n = 163) vs. PVI + substrate modification (n = 161) | Lower first AA recurrence with PVI + substrate modification (35%) vs. PVI-only (50%) (Kaplan–Meier event rate estimates: HR = 0.62, 95% CI = 0.43 to 0.88, log-rank p = 0.006) |
| Ablation Target | Study | Study Population | Outcome |
|---|---|---|---|
| Left atrial posterior wall (LAPW) | Kanitsoraphan et al., 2022 | 8 RCTs with 1024 patients with AF | Adjunctive PWI decreased AF recurrence compared to controlled approaches (RR 0.88, 95% CI: 0.81–0.96, I2 = 48.2%, p-value 0.004) without decrease in overall AA recurrence (RR 0.96, 95% CI: 0.88–1.05, I2 = 31.6%, p-value 0.393) |
| Left atrial appendage (LAA) | Romero et al., 2018 | 7 studies that enrolled a total of 930 patients [mean age 63 ± 5 years; male: 69%]. | LAA ablation + standard ablation appears to improve freedom from AAs in patients with PeAF and long-standing PeAF without raising complication risks. [56% relative reduction and 31.6% absolute reduction; RR 0.44, 95% CI 0.31–0.64; p < 0.0001] |
| Superior vena cava (SVC) | Sharma et al., 2017 | 3 RCTs with 526 participants with AF | No difference in AF recurrence rate between PVI + SVCI versus PVI alone (39% vs. 60%; OR 0.68; 95% CI 0.43–1.07; p = 0.73; I2 = 0%) |
| Low voltage areas (LVAs) | Rivera et al., 2024 | 10 RCTs with 1780 patients with AF | Adjunctive LVA ablation significantly reduced recurrence of AA, as compared with conventional ablation (RR 0.76; 95% CI 0.67−0.88; p < 0.01) |
| Toumpourleka et al., 2023 | 5 RCTs with 989 participants with PeAF | Higher probability for freedom from arrhythmia with additional LVA substrate modification when compared to conventional AF ablation (OR 1.73; 95% CI 1.03–2.90; I2 = 0%; p = 0.04). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baqal, O.; Shafqat, A.; Kulthamrongsri, N.; Sanghavi, N.; Iyengar, S.K.; Vemulapalli, H.S.; El Masry, H.Z. Ablation Strategies for Persistent Atrial Fibrillation: Beyond the Pulmonary Veins. J. Clin. Med. 2024, 13, 5031. https://doi.org/10.3390/jcm13175031
Baqal O, Shafqat A, Kulthamrongsri N, Sanghavi N, Iyengar SK, Vemulapalli HS, El Masry HZ. Ablation Strategies for Persistent Atrial Fibrillation: Beyond the Pulmonary Veins. Journal of Clinical Medicine. 2024; 13(17):5031. https://doi.org/10.3390/jcm13175031
Chicago/Turabian StyleBaqal, Omar, Areez Shafqat, Narathorn Kulthamrongsri, Neysa Sanghavi, Shruti K. Iyengar, Hema S. Vemulapalli, and Hicham Z. El Masry. 2024. "Ablation Strategies for Persistent Atrial Fibrillation: Beyond the Pulmonary Veins" Journal of Clinical Medicine 13, no. 17: 5031. https://doi.org/10.3390/jcm13175031
APA StyleBaqal, O., Shafqat, A., Kulthamrongsri, N., Sanghavi, N., Iyengar, S. K., Vemulapalli, H. S., & El Masry, H. Z. (2024). Ablation Strategies for Persistent Atrial Fibrillation: Beyond the Pulmonary Veins. Journal of Clinical Medicine, 13(17), 5031. https://doi.org/10.3390/jcm13175031







