Atherosclerosis Prevalence among Different Physical Activity Patterns in Adult Men
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Physical Activity Assessment
2.3. Subclinical Atherosclerosis Imaging
2.4. Covariables and Definitions
2.5. Statistical Analysis
3. Results
4. Discussion
4.1. Physical Activity and Atherosclerosis
4.2. Methodology Used to Assess Physical Activity
4.3. Clinical Relevance
4.4. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fernández-Friera, L.; Peñalvo, J.L.; Fernandez-Ortiz, A.; Ibañez, B.; López-Melgar, B.; Laclaustra, M.; Oliva, B.; Mocoroa, A.; Mendiguren, J.; Martinez de Vega, V.; et al. Prevalence, Vascular Distribution and Multi-Territorial Extent of Subclinical Atherosclerosis in a Middle-Aged Cohort: The PESA (Progression of Early Subclinical Atherosclerosis) Study. Circulation 2015, 131, 2104–2113. [Google Scholar] [CrossRef]
- Laclaustra, M.; Casasnovas, J.A.; Fernandez-Ortiz, A.; Fuster, V.; León-Latre, M.; Jimenez Borreguero, J.; Pocovi, M.; Hurtado-roca, Y.; Ordovas, J.M.; Jarauta, E.; et al. Femoral and Carotid Subclinical Atherosclerosis: Risk Factors and Coronary Calcium. J. Am. Coll. Cardiol. 2016, 67, 1263–1274. [Google Scholar] [CrossRef] [PubMed]
- Lechner, K.; Schacky, C.V.; Mckenzie, A.L.; Worm, N.; Nixdorff, U.; Halle, M.; Lechner, B.; Kra, N. Lifestyle Factors and High-Risk Atherosclerosis: Pathways and Mechanisms beyond Traditional Risk Factors. Eur. J. Prev. Cardiol. 2020, 27, 394–406. [Google Scholar] [CrossRef] [PubMed]
- Uzhova, I.; Mateo-Gallego, R.; Moreno-Franco, B.; Molina-Montes, E.; Leon-Latre, M.; Casasnovas Lenguas, J.A.; Civeira, F.; Peñalvo, J.L. The Additive Effect of Adherence to Multiple Healthy Lifestyles on Subclinical Atherosclerosis: Insights from the AWHS. J. Clin. Lipidol. 2018, 12, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Kyu, H.H.; Bachman, V.F.; Alexander, L.T.; Mumford, J.E.; Afshin, A.; Estep, K.; Veerman, J.L.; Delwiche, K.; Iannarone, M.L.; Moyer, M.L.; et al. Physical Activity and Risk of Breast Cancer, Colon Cancer, Diabetes, Ischemic Heart Disease, and Ischemic Stroke Events: Systematic Review and Dose-Response Meta-Analysis for the Global Burden of Disease Study 2013. BMJ 2016, 9, i3857. [Google Scholar] [CrossRef]
- Bull, F.C.; Al-, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.-P.; Chastin, S.; Chou, R.; et al. World Health Organization 2020 Guidelines on Physical Activity and Sedentary Behaviour. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef]
- Thompson, P.D.; Buchner, D.; Piña, I.L.; Balady, G.J.; Williams, M.A.; Marcus, B.H.; Berra, K.; Blair, S.N.; Costa, F.; Franklin, B.; et al. Exercise and Physical Activity in the Prevention and Treatment of Atherosclerotic Cardiovascular Disease: A Statement from the Council on Clinical Cardiology (Subcommittee on Exercise, Rehabilitation, and Prevention) and the Council on Nutrition, Physical. Circulation 2003, 107, 3109–3116. [Google Scholar] [CrossRef]
- Kozákova, M.; Palombo, C.; Morizzo, C.; Nolan, J.J.; Konrad, T.; Balkau, B. Effect of Sedentary Behaviour and Vigorous Physical Activity on Segment-Specific Carotid Wall Thickness and Its Progression in a Healthy Population. Eur. Heart J. 2010, 31, 1511–1519. [Google Scholar] [CrossRef]
- Boss, H.M.; van der Graaf, Y.; Visseren, F.L.J.; Van den Berg-Vos, R.M.; Bots, M.L.; de Borst, G.J.; Cramer, M.J.; Kappelle, L.J.; Geerlings, M.I. Physical Activity and Characteristics of the Carotid Artery Wall in High-Risk Patients—The SMART (Second Manifestations of Arterial Disease) Study. J. Am. Heart Assoc. 2017, 6, e005143. [Google Scholar] [CrossRef]
- Stein, R.; Rockman, C.; Guo, Y.; Adelman, M.; Riles, T.; Hiatt, W.; Berger, J. Association between Physical Activity and Peripheral Artery Disease and Carotid Artery Stenosis in a Self-Referred Population of 3 Million Adults. Arter. Thromb. Vasc. Biol. 2015, 35, 206–212. [Google Scholar] [CrossRef]
- Chen, L.; Bi, Y.; Su, J.; Cui, L.; Han, R.; Tao, R.; Zhou, J.; Wu, M.; Qin, Y. Physical Activity and Carotid Atherosclerosis Risk Reduction in Population with High Risk for Cardiovascular Diseases: A Cross-Sectional Study. BMC Public Health 2022, 22, 250. [Google Scholar] [CrossRef] [PubMed]
- Aengevaeren, V.L.; Mosterd, A.; Braber, T.L.; Prakken, N.H.J.; Doevendans, P.A.; Grobbee, D.E.; Thompson, P.D.; Eijsvogels, T.M.H.; Velthuis, B.K. The Relationship between Lifelong Exercise Volume and Coronary Atherosclerosis in Athletes. Circulation 2017, 136, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Aengevaeren, V.L.; Mosterd, A.; Sharma, S.; Prakken, N.H.J.; Möhlenkamp, S.; Thompson, P.D.; Velthuis, B.K.; Eijsvogels, T.M.H. Exercise and Coronary Atherosclerosis: Observations, Explanations, Relevance, and Clinical Management. Circulation 2020, 141, 1338–1350. [Google Scholar] [CrossRef] [PubMed]
- Aengevaeren, V.L.; Mosterd, A.; Bakker, E.A.; Braber, T.L.; Nathoe, H.M.; Sharma, S.; Thompson, P.D.; Velthuis, B.K.; Eijsvogels, T.M.H. Exercise Volume Versus Intensity and the Progression of Coronary Atherosclerosis in Middle-Aged and Older Athletes: Findings From the MARC-2 Study. Circulation 2023, 147, 993–1003. [Google Scholar] [CrossRef]
- Merghani, A.; Maestrini, V.; Rosmini, S.; Cox, A.T.; Dhutia, H.; Bastiaenan, R.; David, S.; Yeo, T.J.; Narain, R.; Malhotra, A.; et al. Prevalence of Subclinical Coronary Artery Disease in Masters Endurance Athletes with a Low Atherosclerotic Risk Profile. Circulation 2017, 136, 126–137. [Google Scholar] [CrossRef]
- Zouhal, H.; Jacob, C.; Delamarche, P.; Gratas-Delamarche, A. Catecholamines and the Effects of Exercise, Training and Gender. Sports Med. 2008, 38, 401–423. [Google Scholar] [CrossRef]
- Gleeson, M.; Bishop, N.C.; Stensel, D.J.; Lindley, M.R.; Mastana, S.S.; Nimmo, M.A. The Anti-Inflammatory Effects of Exercise: Mechanisms and Implications for the Prevention and Treatment of Disease. Nat. Rev. Immunol. 2011, 11, 607–610. [Google Scholar] [CrossRef]
- Van Der Heijden, C.D.C.C.; Groh, L.; Keating, S.T.; Kaffa, C.; Noz, M.P.; Kersten, S.; Van Herwaarden, A.E.; Hoischen, A.; Joosten, L.A.B.; Timmers, H.J.L.M.; et al. Catecholamines Induce Trained Immunity in Monocytes in Vitro and in Vivo. Circ. Res. 2020, 127, 269–283. [Google Scholar] [CrossRef]
- Casasnovas, J.A.; Alcalde, V.; Civeira, F.; Guallar, E.; Ibañez, B.; Jimenez Borreguero, J.; Laclaustra, M.; León, M.; Peñalvo, J.L.; Ordovás, J.M.; et al. Aragon Workers’ Health Study—Design and Cohort Description. BMC Cardiovasc. Disord. 2012, 12, 45. [Google Scholar] [CrossRef]
- Sasaki, J.E.; John, D.; Freedson, P.S. Validation and Comparison of ActiGraph Activity Monitors. J. Sci. Med. Sport 2011, 14, 411–416. [Google Scholar] [CrossRef]
- Junyent, M.; Gilabert, R.; Zambon, D.; Pocovı, M.; Mallen, M.; Cofán, M.; Nuñez, I.; Civeira, F.; Tejedor, D.; Ros, E. Femoral Atherosclerosis in Heterozygous Familial Hypercholesterolemia: Influence of the Genetic Defect. Arter. Thromb. Vasc. Biol. 2008, 28, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Inaba, Y.; Chen, J.A.; Bergmann, S.R. Carotid Plaque, Compared with Carotid Intima-Media Thickness, More Accurately Predicts Coronary Artery Disease Events: A Meta-Analysis. Atherosclerosis 2012, 220, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Perez-Lasierra, J.L.; Laclaustra, M.; Guallar-Castillón, P.; Casasnovas, J.A.; Casajús, J.A.; Jarauta, E.; Gonzalez-Agüero, A.; Moreno-Franco, B. Daily Sitting for Long Periods Increases the Odds for Subclinical Atheroma Plaques. J. Clin. Med. 2021, 10, 1229. [Google Scholar] [CrossRef] [PubMed]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the Concentration of Low-Density Lipoprotein Cholesterol in Plasma, without Use of the Preparative Ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef]
- Pearson, T.A.; Palaniappan, L.P.; Artinian, N.T.; Carnethon, M.R.; Criqui, M.H.; Daniels, S.R.; Fonarow, G.C.; Fortmann, S.P.; Franklin, B.A.; Galloway, J.M.; et al. American Heart Association Guide for Improving Cardiovascular Health at the Community Level, 2013 Update: A Scientific Statement for Public Health Practitioners, Healthcare Providers, and Health Policy Makers. Circulation 2013, 127, 1730–1753. [Google Scholar] [CrossRef]
- National Cholesterol Education Program (US). Expert Panel on Detection, & Treatment of High Blood Cholesterol in Adults. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Final Report. Circulation 2002, 106, 3143–3421. [Google Scholar]
- Laddu, D.R.; Rana, J.S.; Murillo, R.; Sorel, M.E.; Quesenberry, C.P.; Allen, N.B.; Gabriel, K.P.; Carnethon, M.R.; Liu, K.; Reis, J.P.; et al. 25-Year Physical Activity Trajectories and Development of Subclinical Coronary Artery Disease as Measured by Coronary Artery Calcium: The Coronary Artery Risk Development in Young Adults (CARDIA) Study. Mayo Clin. Proc. 2017, 92, 1660–1670. [Google Scholar] [CrossRef] [PubMed]
- Warren, J.M.; Ekelund, U.; Besson, H.; Mezzani, A.; Geladas, N.; Vanhees, L. Assessment of Physical Activity—A Review of Methodologies with Reference to Epidemiological Research: A Report of the Exercise Physiology Section of the European Association of Cardiovascular Prevention and Rehabilitation. Eur. J. Cardiovasc. Prev. Rehabil. 2010, 17, 127–139. [Google Scholar] [CrossRef]
- Ainsworth, B.; Cahalin, L.; Buman, M.; Ross, R. The Current State of Physical Activity Assessment Tools. Prog. Cardiovasc. Dis. 2015, 57, 387–395. [Google Scholar] [CrossRef]
- Gabriel, K.P.; Matthews, K.A.; Pérez, A.; Edmundowicz, D.; Kohl, H.W.; Hawkins, M.S.; Janak, J.C.; Kriska, A.M.; Kuller, L.H. Self-Reported and Accelerometer Physical Activity Levels and Coronary Artery Calcification Progression in Older Women: Results from the Healthy Women Study. Menopause 2013, 20, 152–161. [Google Scholar] [CrossRef]
- Welk, G.J. Harmonizing Monitor- and Report-Based Estimates of Physical Activity through Calibration. Kinesiol. Rev. 2019, 8, 16–24. [Google Scholar] [CrossRef]
| Vigorous Physical Activity Level (m/w) | |||||
|---|---|---|---|---|---|
| Total | 0 | >0–60 | >60 | p-Value | |
| N | 428 | 188 | 198 | 42 | |
| Age, years | 52.8 (4.3) | 53.9 (3.4) | 52.1 (4.6) | 51.5 (5.2) | <0.001 ab |
| BMI, kg/m2 | 24.2 (3.3) | 24.3 (3.4) | 24.2 (3.3) | 24.0 (2.9) | 0.766 |
| Systolic BP, mmHg | 125.0 (13.9) | 126.1 (14.7) | 124.6 (13.7) | 122.4 (10.7) | 0.249 |
| Diastolic BP, mmHg | 82.0 (9.7) | 83.0 (9.9) | 81.6 (9.6) | 79.9 (8.4) | 0.132 |
| Total cholesterol, mg/dL | 217.2 (35.6) | 218. 3 (34.7) | 215.0 (36.6) | 222.6 (34.3) | 0.385 |
| HDL-c, mg/dL | 53.3 (11.4) | 51.7 (10.1) | 54.3 (12.3) | 56.0 (11.2) | 0.021 |
| LDL-c, mg/dL | 135.0 (29.7) | 136.5 (30.5) | 132.4 (28.8) | 140.3 (29.6) | 0.188 |
| Triglycerides, mg/dL | 148.6 (100.4) | 152.4 (82.4) | 148.6 (119.9) | 131.5 (67.8) | 0.475 |
| Glucose, mg/dL | 96.3 (18.7) | 97.7 (18.9) | 96.0 (19.4) | 91.1 (13.4) | 0.115 |
| Hypertension, % | 155 [36.2] | 71 [37.8] | 71 [35.9] | 13 [31.0] | 0.701 |
| MVPA, min/week | 443.2 (201.0) | 371.1 (164.1) | 470.2 (194.1) | 639.3 (224.1) | <0.001 abc |
| Dyslipidemia, % | 190 [44.4] | 95 [50.5] | 79 [39.9] | 16 [38.1] | 0.076 |
| Diabetes, % | 26 [6.1] | 11 [5.9] | 13 [6.6] | 2 [4.8] | 0.893 |
| Ever smokers, % | 341 [79.7] | 158 [84.0] | 153 [77.3] | 30 [71.4] | 0.096 |
| Obesity, % | 22 [5.1] | 13 [6.9] | 8 [4.0] | 1 [2.4] | 0.307 |
| Femoral plaque, % | 232 [54.2] | 113 [60.1] | 105 [53.0] | 14 [33.3] | 0.006 |
| Carotid plaque, % | 163 [38.1] | 78 [41.5] | 74 [37.4] | 11 [26.2] | 0.175 |
| Any plaque, % | 296 [69.2] | 144 [76.6] | 133 [67.2] | 19 [45.2] | <0.001 |
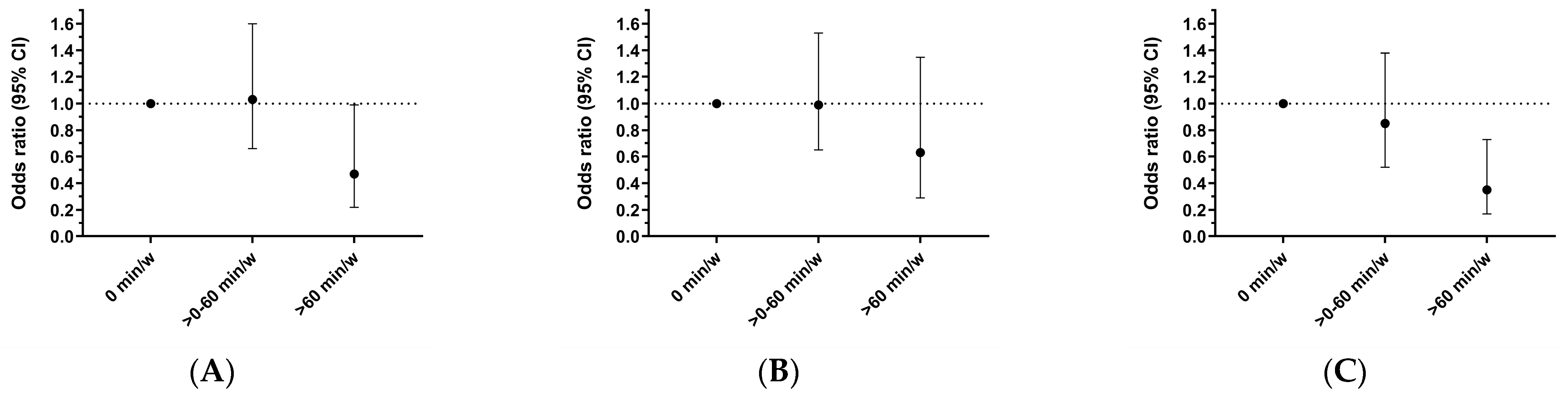
| Vigorous Physical Activity Level | |||||
| 0 min/Week | >0–60 min/Week | p-Value | >60 min/Week | p-Value | |
| Number with femoral plaque/Total | 113/188 | 105/198 | 14/42 | ||
| Unadjusted | 1.00 (ref) | 0.75 (0.50, 1.12) | 0.161 | 0.33 (0.16, 0.67) | 0.002 |
| Age-adjusted | 1.00 (ref) | 0.95 (0.62, 1.46) | 0.822 | 0.42 (0.20, 0.87) | 0.020 |
| Model 1 | 1.00 (ref) | 1.03 (0.66, 1.60) | 0.907 | 0.47 (0.22, 0.99) | 0.048 |
| Number with carotid plaque/Total | 78/188 | 74/198 | 11/42 | ||
| Unadjusted | 1.00 (ref) | 0.84 (0.56, 1.27) | 0.408 | 0.50 (0.24, 1.06) | 0.069 |
| Age-adjusted | 1.00 (ref) | 0.94 (0.62, 1.43) | 0.759 | 0.57 (0.27, 1.21) | 0.144 |
| Model 1 | 1.00 (ref) | 0.99 (0.65, 1.53) | 0.989 | 0.63 (0.29, 1.35) | 0.231 |
| Number with any plaque/Total | 144/188 | 133/198 | 19/42 | ||
| Unadjusted | 1.00 (ref) | 0.63 (0.40, 0.98) | 0.041 | 0.25 (0.13, 0.51) | <0.001 |
| Age-adjusted | 1.00 (ref) | 0.77 (0.48, 1.22) | 0.263 | 0.31 (0.15, 0.64) | 0.001 |
| Model 1 | 1.00 (ref) | 0.85 (0.52, 1.38) | 0.512 | 0.35 (0.17, 0.73) | 0.005 |
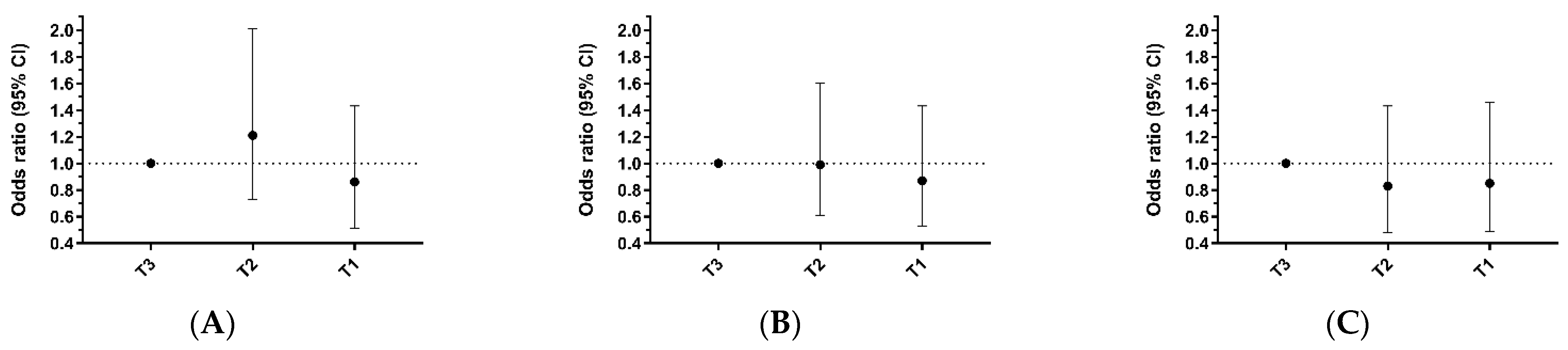
| Moderate to Vigorous Physical Activity Level | |||||
| T3 | T2 | p-Value | T1 | p-Value | |
| Number with femoral plaque/Total | 78/143 | 83/143 | 71/142 | ||
| Unadjusted | 1.00 (ref) | 1.15 (0.72, 1.84) | 0.551 | 0.83 (0.52, 1.33) | 0.443 |
| Age-adjusted | 1.00 (ref) | 1.09 (0.67, 1.77) | 0.732 | 0.79 (0.49, 1.29) | 0.345 |
| Model 1 | 1.00 (ref) | 1.21 (0.73, 2.01) | 0.453 | 0.86 (0.51, 1.43) | 0.551 |
| Number with carotid plaque/Total | 57/143 | 56/143 | 50/142 | ||
| Unadjusted | 1.00 (ref) | 0.97 (0.61, 1.56) | 0.904 | 0.82 (0.51, 1.33) | 0.418 |
| Age-adjusted | 1.00 (ref) | 0.94 (0.59, 1.52) | 0.811 | 0.81 (0.50, 1.31) | 0.380 |
| Model 1 | 1.00 (ref) | 0.99 (0.61, 1.60) | 0.962 | 0.87 (0.53, 1.43) | 0.590 |
| Number with any plaque/Total | 103/143 | 98/143 | 95/142 | ||
| Unadjusted | 1.00 (ref) | 0.85 (0.51, 1.41) | 0.518 | 0.79 (0.47, 1.30) | 0.348 |
| Age-adjusted | 1.00 (ref) | 0.79 (0.47, 1.33) | 0.372 | 0.76 (0.45, 1.28) | 0.305 |
| Model 1 | 1.00 (ref) | 0.83 (0.48, 1.43) | 0.501 | 0.85 (0.49, 1.46) | 0.554 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perez-Lasierra, J.L.; Casajús, J.A.; Gonzalez-Agüero, A.; Casasnovas, J.A.; Torrijo-Blanche, C.; Gimeno-Ruiz, S.; Moreno-Franco, B. Atherosclerosis Prevalence among Different Physical Activity Patterns in Adult Men. J. Clin. Med. 2024, 13, 5062. https://doi.org/10.3390/jcm13175062
Perez-Lasierra JL, Casajús JA, Gonzalez-Agüero A, Casasnovas JA, Torrijo-Blanche C, Gimeno-Ruiz S, Moreno-Franco B. Atherosclerosis Prevalence among Different Physical Activity Patterns in Adult Men. Journal of Clinical Medicine. 2024; 13(17):5062. https://doi.org/10.3390/jcm13175062
Chicago/Turabian StylePerez-Lasierra, Jose Luis, Jose Antonio Casajús, Alejandro Gonzalez-Agüero, Jose Antonio Casasnovas, Carolina Torrijo-Blanche, Sofia Gimeno-Ruiz, and Belén Moreno-Franco. 2024. "Atherosclerosis Prevalence among Different Physical Activity Patterns in Adult Men" Journal of Clinical Medicine 13, no. 17: 5062. https://doi.org/10.3390/jcm13175062
APA StylePerez-Lasierra, J. L., Casajús, J. A., Gonzalez-Agüero, A., Casasnovas, J. A., Torrijo-Blanche, C., Gimeno-Ruiz, S., & Moreno-Franco, B. (2024). Atherosclerosis Prevalence among Different Physical Activity Patterns in Adult Men. Journal of Clinical Medicine, 13(17), 5062. https://doi.org/10.3390/jcm13175062









