Multimodality Imaging for Right Ventricular Function Assessment in Severe Tricuspid Regurgitation
Abstract
:1. Introduction
2. Anatomy and Physiology of Right Ventricle
3. Multimodality Imaging in Right Ventricular Function Evaluation
4. Echocardiography
5. Cardiac Magnetic Resonance
6. Cardiac Computed Tomography
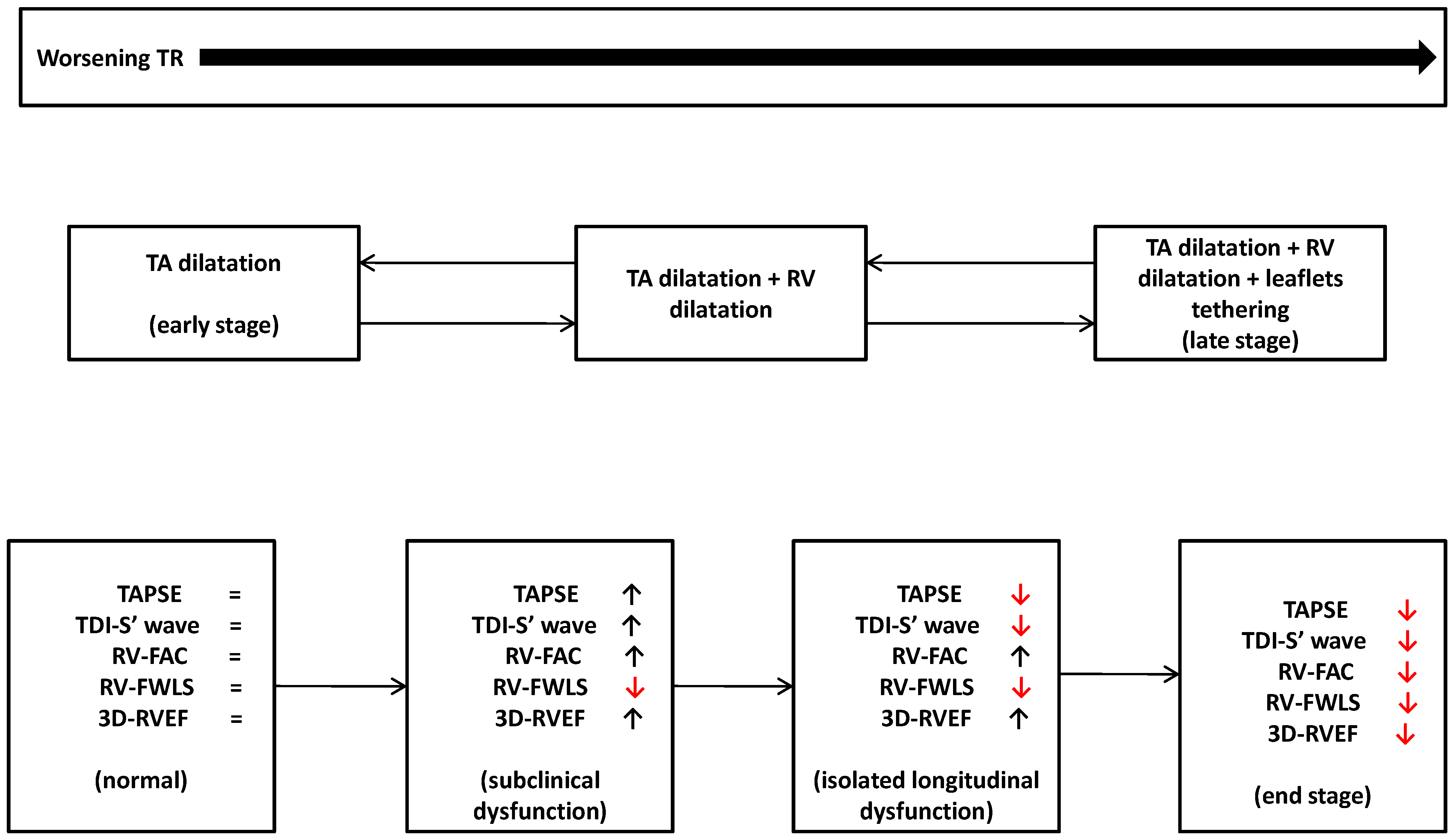
7. Right Ventricle and Severe Tricuspid Regurgitation: A Comprehensive Assessment
8. Conventional Echocardiographic Indices
9. Right Ventricular Free Wall Longitudinal Strain
10. Three-Dimensional Echocardiography Right Ventricular Ejection Fraction
11. Right Ventricular–Pulmonary Arterial Coupling
12. CMR
13. CCT
14. Use of Multimodality Imaging to Assess Rv Function
15. Future Perspective
16. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Topilsky, Y.; Maltais, S.; Medina-Inojosa, J.; Oguz, D.; Michelena, H.; Maalouf, J.; Mahoney, D.W.; Enriquez-Sarano, M. Burden of tricuspid regurgitation in patients diagnosed in the community setting. J. Am. Coll. Cardiol. Imaging 2019, 12, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Zack, C.J.; Fender, E.A.; Chandrashekar, P.; Reddy, Y.N.V.; Bennett, C.E.; Stulak, J.M.; Miller, V.M.; Nishimura, R.A. National Trends and Outcomes in Isolated Tricuspid Valve Surgery. J. Am. Coll. Cardiol. 2017, 70, 2953–2960. [Google Scholar] [CrossRef] [PubMed]
- Scotti, A.; Sturla, M.; Granada, J.F.; Kodali, S.K.; Coisne, A.; Mangieri, A.; Godino, C.; Ho, E.; Goldberg, Y.; Chau, M.; et al. Outcomes of isolated tricuspid valve replacement: A systematic review and meta-analysis of 5,316 patients from19 35 studies. EuroIntervention 2022, 18, 840–851. [Google Scholar] [CrossRef] [PubMed]
- Hahn, R.T.; Lawlor, M.K.; Davidson, C.J.; Badhwar, V.; Sannino, A.; Spitzer, E.; Lurz, P.; Lindman, B.R.; Topilsky, Y.; Baron, S.J.; et al. Tricuspid Valve Academic Research Consortium Definitions for Tricuspid Regurgitation and Trial Endpoints. J. Am. Coll. Cardiol. 2023, 82, 1711–1735. [Google Scholar] [CrossRef]
- Dreyfus, J.; Flagiello, M.; Bazire, B.; Eggenspieler, F.; Viau, F.; Riant, E.; Mbaki, Y.; Bohbot, Y.; Eyharts, D.; Senage, T.; et al. Isolated tricuspid valve surgery: Impact of aetiology and clinical presentation on outcomes. Eur. Heart J. 2020, 41, 4304–4317. [Google Scholar] [CrossRef] [PubMed]
- Topilsky, Y.; Khanna, A.D.; Oh, J.K.; Nishimura, R.A.; Enriquez-Sarano, M.; Jeon, Y.B.; Sundt, T.M.; Schaff, H.V.; Park, S.J. Preoperative factors associated with adverse outcome after tricuspid valve replacement. Circulation 2011, 123, 1929–1939. [Google Scholar] [CrossRef]
- Patlolla, S.H.; Schaff, H.V.; Greason, K.L.; Pochettino, A.; Daly, R.C.; Frye, R.L.; Nishimura, R.A.; Dearani, J.A. Early Right Ventricular Reverse Remodeling Predicts Survival after Isolated Tricuspid Valve Surgery. Ann. Thorac. Surg. 2021, 112, 1402–1409. [Google Scholar] [CrossRef]
- Miura, M.; Alessandrini, H.; Alkhodair, A.; Attinger-Toller, A.; Biasco, L.; Lurz, P.; Braun, D.; Brochet, E.; Connelly, K.A.; de Bruijn, S.; et al. Impact of Massive or Torrential Tricuspid Regurgitation in Patients Undergoing Transcatheter Tricuspid Valve Intervention. JACC Cardiovasc. Interv. 2020, 13, 1999–2009. [Google Scholar] [CrossRef] [PubMed]
- Sala, A.; Hahn, R.T.; Kodali, S.K.; Mack, M.J.; Maisano, F. Tricuspid Valve Regurgitation: Current Understanding and Novel Treatment Options. J. Soc. Cardiovasc. Angiogr. Interv. 2023, 2, 101041. [Google Scholar] [CrossRef]
- Preda, A.; Melillo, F.; Liberale, L.; Montecucco, F.; Agricola, E. Right ventricle dysfunction assessment for transcatheter tricuspid valve repair: A matter of debate. Eur. J. Clin. Investig. 2021, 51, e13653. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef]
- Lorenz, C.H.; Walker, E.S.; Morgan, V.L.; Klein, S.S.; Graham, T.P., Jr. Normal human right and left ventricular mass, systolic function, and gender differences by cine magnetic resonance imaging. J. Cardiovasc. Magn. Reson. 1999, 1, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Haddad, F.; Hunt, S.A.; Rosenthal, D.N.; Murphy, D.J. Right ventricular function in cardiovascular disease, part I: Anatomy, physiology, aging, and functional assessment of the right ventricle. Circulation 2008, 117, 1436–1448. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Hur, M.S. Morphological classification of the moderator band and its relationship with the anterior papillary muscle. Anat. Cell Biol. 2019, 52, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Hahn, R.T.; Lerakis, S.; Delgado, V.; Addetia, K.; Burkhoff, D.; Muraru, D.; Pinney, S.; Friedberg, M.K. Multimodality Imaging of Right Heart Function: JACC Scientific Statement. J. Am. Coll. Cardiol. 2023, 81, 1954–1973. [Google Scholar] [CrossRef]
- Petitjean, C.; Rougon, N.; Cluzel, P. Assessment of myocardial function: A review of quantification methods and results using tagged MRI. J. Cardiovasc. Magn. Rieson. 2005, 7, 501–516. [Google Scholar] [CrossRef]
- Friedberg, M.K. Imaging Right-Left Ventricular Interactions. JACC Cardiovasc. Imaging 2018, 11, 755–771. [Google Scholar] [CrossRef] [PubMed]
- Harjola, V.P.; Mebazaa, A.; Čelutkienė, J.; Bettex, D.; Bueno, H.; Chioncel, O.; Crespo-Leiro, M.G.; Falk, V.; Filippatos, G.; Gibbs, S.; et al. Contemporary management of acute right ventricular failure: A statement from the Heart Failure Association and the Working Group on Pulmonary Circulation and Right Ventricular Function of the European Society of Cardiology. Eur. J. Heart Fail. 2016, 18, 226–241. [Google Scholar] [CrossRef]
- Dell’Italia, L.J. The right ventricule anatomy, physiology and clinical importance. Curr. Probl. Cardiol. 1991, 16, 658–720. [Google Scholar] [CrossRef]
- Ling, L.F.; Obuchowski, N.A.; Rodriguez, L.; Popovic, Z.; Kwon, D.; Marwick, T.H. Accuracy and interobserver concordance of echocardiographic assessment of right ventricular size and systolic function: A quality control exercise. J. Am. Soc. Echocardiogr. 2012, 25, 709–713. [Google Scholar] [CrossRef]
- Mor-Avi, V.; Lang, R.M.; Badano, L.P.; Belohlavek, M.; Cardim, N.M.; Derumeaux, G.; Galderisi, M.; Marwick, T.; Nagueh, S.F.; Sengupta, P.P.; et al. Current and evolving echocardiographic techniques for the quantitative evaluation of cardiac mechanics: ASE/EAE consensus statement on methodology and indications endorsed by the Japanese Society of Echocardiography. J. Am. Soc. Echocardiogr. 2011, 24, 277–313. [Google Scholar] [CrossRef] [PubMed]
- Giusca, S.; Dambrauskaite, V.; Scheurwegs, C.; D’Hooge, J.; Claus, P.; Herbots, L.; Magro, M.; Rademakers, F.; Meyns, B.; Delcroix, M.; et al. Deformation imaging describes right ventricular function better than longitudinal displacement of the tricuspid ring. Heart 2010, 96, 281–288. [Google Scholar] [CrossRef]
- Modin, D.; Møgelvang, R.; Andersen, D.M.; Biering-Sørensen, T. Right Ventricular Function Evaluated by Tricuspid Annular Plane Systolic Excursion Predicts Cardiovascular Death in the General Population. J. Am. Heart Assoc. 2019, 8, e012197. [Google Scholar] [CrossRef]
- Di Mauro, M.; Scrofani, R.; Antona, C.; Nicolò, F.; Cappabianca, G.; Beghi, C.; Santarpino, G.; Gregorini, R.; Di Marco, L.; Pacini, D.; et al. Right ventricular assessment can improve prognostic value of Euroscore II. J. Card. Surg. 2020, 35, 1548–1555. [Google Scholar] [CrossRef] [PubMed]
- Focardi, M.; Cameli, M.; Carbone, S.F.; Massoni, A.; De Vito, R.; Lisi, M.; Mondillo, S. Traditional and innovative echocardiographic parameter for the analysis of right ventricular performance in comparison with cardiac magnetic resonance. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 47–52. [Google Scholar] [CrossRef]
- Zornoff, L.A.; Skali, H.; Pfeffer, M.A.; St John Sutton, M.; Rouleau, J.L.; Lamas, G.A.; Plappert, T.; Rouleau, J.R.; Moyé, L.A.; Lewis, S.J.; et al. Right ventricular dysfunction and risk of heart failure and mortality after myocardial infarction. J. Am. Coll. Cardiol. 2002, 39, 1450–1455. [Google Scholar] [CrossRef]
- Anavekar, N.S.; Skali, H.; Bourgoun, M.; Ghali, J.K.; Kober, L.; Maggioni, A.P.; McMurray, J.J.; Velazquez, E.; Califf, R.; Pfeffer, M.A.; et al. Usefulness of right ventricular fractional area change to predict death, heart failure, and stroke following myocardial infarction (from the VALIANT ECHO Study). Am. J. Cardiol. 2008, 101, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Grapsa, J.; Pereira Nunes, M.C.; Tan, T.C.; Cabrita, I.Z.; Coulter, T.; Smith, B.C.; Dawson, D.; Gibbs, J.S.; Nihoyannopoulos, P. Echocardiographic and Hemodynamic Predictors of Survival in Precapillary Pulmonary Hypertension: Seven-Year Follow-Up. Circ. Cardiovasc. Imaging 2015, 8, e002107. [Google Scholar] [CrossRef]
- Rudski, L.G.; Lai, W.W.; Afilalo, J.; Hua, L.; Handschumacher, M.D.; Chandrasekaran, K.; Solomon, S.D.; Louie, E.K.; Schiller, N.B. Guidelines for the echocardiographic assessment of the right heart in adults: A report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J. Am. Soc. Echocardiogr. 2010, 23, 685–713. [Google Scholar]
- Badano, L.P.; Muraru, D.; Parati, G.; Haugaa, K.; Voigt, J.U. How to do right ventricular strain. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 825–827. [Google Scholar] [CrossRef]
- Verhaert, D.; Mullens, W.; Borowski, A.; Popović, Z.B.; Curtin, R.J.; Thomas, J.D.; Tang, W.H. Right ventricular response to intensive medical therapy in advanced decompensated heart failure. Circ. Heart Fail. 2010, 3, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Carluccio, E.; Biagioli, P.; Alunni, G.; Murrone, A.; Zuchi, C.; Coiro, S.; Riccini, C.; Mengoni, A.; D’Antonio, A.; Ambrosio, G. Prognostic Value of Right Ventricular Dysfunction in Heart Failure with Reduced Ejection Fraction: Superiority of Longitudinal Strain over Tricuspid Annular Plane Systolic Excursion. Circ. Cardiovasc. Imaging 2018, 11, e006894. [Google Scholar] [CrossRef]
- Antoni, M.L.; Scherptong, R.W.; Atary, J.Z.; Boersma, E.; Holman, E.R.; van der Wall, E.E.; Schalij, M.J.; Bax, J.J. Prognostic value of right ventricular function in patients after acute myocardial infarction treated with primary percutaneous coronary intervention. Circ. Cardiovasc. Imaging 2010, 3, 264–271. [Google Scholar] [CrossRef]
- Tadic, M.; Nita, N.; Schneider, L.; Kersten, J.; Buckert, D.; Gonska, B.; Scharnbeck, D.; Reichart, C.; Belyavskiy, E.; Cuspidi, C.; et al. The Predictive Value of Right Ventricular Longitudinal Strain in Pulmonary Hypertension, Heart Failure, and Valvular Diseases. Front. Cardiovasc. Med. 2021, 8, 698158. [Google Scholar] [CrossRef]
- Fukuda, Y.; Tanaka, H.; Sugiyama, D.; Ryo, K.; Onishi, T.; Fukuya, H.; Nogami, M.; Ohno, Y.; Emoto, N.; Kawai, H.; et al. Utility of right ventricular free wall speckle-tracking strain for evaluation of right ventricular performance in patients with pulmonary hypertension. J. Am. Soc. Echocardiogr. 2011, 24, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Addetia, K.; Miyoshi, T.; Amuthan, V.; Citro, R.; Daimon, M.; Gutierrez Fajardo, P.; Kasliwal, R.R.; Kirkpatrick, J.N.; Monaghan, M.J.; Muraru, D.; et al. Normal Values of Three-Dimensional Right Ventricular Size and Function Measurements: Results of the World Alliance Societies of Echocardiography Study. J. Am. Soc. Echocardiogr. 2023, 36, 858–866.e1. [Google Scholar] [CrossRef] [PubMed]
- Namisaki, H.; Nabeshima, Y.; Kitano, T.; Otani, K.; Takeuchi, M. Prognostic Value of the Right Ventricular Ejection Fraction, Assessed by Fully Automated Three-Dimensional Echocardiography: A Direct Comparison of Analyses Using Right Ventricular-Focused Views versus Apical Four-Chamber Views. J. Am. Soc. Echocardiogr. 2021, 34, 117–126. [Google Scholar] [CrossRef]
- Muraru, D.; Badano, L.P.; Nagata, Y.; Surkova, E.; Nabeshima, Y.; Genovese, D.; Otsuji, Y.; Guida, V.; Azzolina, D.; Palermo, C.; et al. Development and prognostic validation of partition values to grade right ventricular dysfunction severity using 3D echocardiography. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 10–21. [Google Scholar] [CrossRef]
- Shimada, Y.J.; Shiota, M.; Siegel, R.J.; Shiota, T. Accuracy of right ventricular volumes and function determined by three-dimensional echocardiography in comparison with magnetic resonance imaging: A meta-analysis study. J. Am. Soc. Echocardiogr. 2010, 23, 943–953. [Google Scholar] [CrossRef]
- Dissabandara, T.; Lin, K.; Forwood, M.; Sun, J. Validating real-time three-dimensional echocardiography against cardiac magnetic resonance, for the determination of ventricular mass, volume and ejection fraction: A meta-analysis. Clin. Res. Cardiol. 2024, 113, 367–392. [Google Scholar] [CrossRef]
- Surkova, E.; Muraru, D.; Iliceto, S.; Badano, L.P. The use of multimodality cardiovascular imaging to assess right ventricular size and function. Int. J. Cardiol. 2016, 214, 54–69. [Google Scholar] [CrossRef]
- Mak, S.M.; Gopalan, D. Right ventricle in adulthood: CT and MR assessment. Postgrad. Med. J. 2020, 96, 487–494. [Google Scholar] [CrossRef]
- Kramer, C.M.; Barkhausen, J.; Bucciarelli-Ducci, C.; Flamm, S.D.; Kim, R.J.; Nagel, E. Standardized cardiovascular magnetic resonance imaging (CMR) protocols: 2020 update. J. Cardiovasc. Magn. Reson. 2020, 22, 17. [Google Scholar] [CrossRef] [PubMed]
- Kawel-Boehm, N.; Hetzel, S.J.; Ambale-Venkatesh, B.; Captur, G.; Francois, C.J.; Jerosch-Herold, M.; Salerno, M.; Teague, S.D.; Valsangiacomo-Buechel, E.; van der Geest, R.J.; et al. Reference ranges (“normal values”) for cardiovascular magnetic resonance (CMR) in adults and children: 2020 update. J. Cardiovasc. Magn. Reson. 2020, 22, 87. [Google Scholar] [CrossRef]
- Bourfiss, M.; Steensma, B.R.; TeRiele, A.; Leiner, T.; Velthuis, B.K.; Raaijmakers, A.J.E. Feature-tracking cardiac magnetic resonance of the right ventricle: Effect of field strength, resolution and imaging sequence. Eur. J. Radiol. 2021, 138, 109671. [Google Scholar] [CrossRef] [PubMed]
- Corrado, D.; Perazzolo Marra, M.; Zorzi, A.; Beffagna, G.; Cipriani, A.; Lazzari, M.; Migliore, F.; Pilichou, K.; Rampazzo, A.; Rigato, I.; et al. Diagnosis of arrhythmogeniccardiomyopathy: The Paduacriteria. Int. J. Cardiol. 2020, 319, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Corrado, D.; Anastasakis, A.; Basso, C.; Bauce, B.; Blomström-Lundqvist, C.; Bucciarelli-Ducci, C.; Cipriani, A.; De Asmundis, C.; Gandjbakhch, E.; Jiménez-Jáimez, J.; et al. Proposed diagnostic criteria for arrhythmogenic cardiomyopathy: European Task Force consensus report. Int. J. Cardiol. 2024, 395, 131447. [Google Scholar] [CrossRef] [PubMed]
- Kochav, J.; Simprini, L.; Weinsaft, J.W. Imaging of the right heart—CT and CMR. Echocardiography 2015, 32 (Suppl. S1), S53–S68. [Google Scholar] [CrossRef]
- Revels, J.W.; Wang, S.S.; Gharai, L.R.; Febbo, J.; Fadl, S.; Bastawrous, S. The role of CT in planning percutaneous structural heart interventions: Where to measure and why. Clin. Imaging 2021, 76, 247–264. [Google Scholar] [CrossRef]
- Suh, Y.J.; Kim, D.; Shim, C.Y.; Han, K.; Chang, B.-C.; Lee, S.; Hong, G.-R.; Choi, B.W.; Kim, Y.J. Tricuspid annular diameter and right ventricular volume on preoperative cardiac CT can predict postoperative right ventricular dysfunction in patients who undergo tricuspid valve surgery. Int. J. Cardiol. 2019, 288, 44–50. [Google Scholar] [CrossRef]
- La Fazia, V.M.; Lepone, A.; Pierucci, N.; Gianni, C.; Barletta, V.; Mohanty, S.; Della Rocca, D.G.; La Valle, C.; Torlapati, P.G.; Al-Ahmad, M.; et al. Low prevalence of new-onset severe tricuspid regurgitation following leadless pacemaker implantation in a large series of consecutive patients. Heart Rhythm, 2024; in press. [Google Scholar]
- Muraru, D.; Addetia, K.; Guta, A.C.; Ochoa-Jimenez, R.C.; Genovese, D.; Veronesi, F.; Basso, C.; Iliceto, S.; Badano, L.P.; Lang, R.M. Right atrial volume is a major determinant of tricuspid annulus area in functional tricuspid regurgitation: A three-dimensional echocardiographic study. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Topilsky, Y.; Nkomo, V.T.; Vatury, O.; Michelena, H.I.; Letourneau, T.; Suri, R.M.; Pislaru, S.; Park, S.; Mahoney, D.W.; Biner, S.; et al. Clinical outcome of isolated tricuspid regurgitation. JACC Cardiovasc. Imaging 2014, 7, 1185–1194. [Google Scholar] [CrossRef] [PubMed]
- Kresoja, K.-P.; Rommel, K.-P.; Lücke, C.; Unterhuber, M.; Besler, C.; von Roeder, M.; Schöber, A.R.; Noack, T.; Gutberlet, M.; Thiele, H.; et al. Right ventricular contraction patterns in patients undergoing transcatheter tricuspid valve repair for severe tricuspid regurgitation. J. Am. Coll. Cardiol. Interv. 2021, 14, 1551–1561. [Google Scholar] [CrossRef] [PubMed]
- Antunes, M.J.; Barlow, J.B. Management of tricuspid valve regurgitation. Heart 2007, 93, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Sanz, J.; Sánchez-Quintana, D.; Bossone, E.; Bogaard, H.J.; Naeije, R. Anatomy, Function, and Dysfunction of the Right Ventricle: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 73, 1463–1482. [Google Scholar] [CrossRef]
- de Agustin, J.A.; Martinez-Losas, P.; de Diego, J.J.G.; Mahia, P.; Marcos-Alberca, P.; Nuñez-Gil, I.J.; Rodrigo, J.L.; Luaces, M.; Islas, F.; Garcia-Fernandez, M.A.; et al. Tricuspid annular plane systolic excursion inaccuracy to assess right ventricular function in patients with previous tricuspid annulopasty. Int. J. Cardiol. 2016, 223, 713–716. [Google Scholar] [CrossRef]
- Dietz, M.F.; Prihadi, E.A.; van der Bijl, P.; Goedemans, L.; Mertens, B.J.A.; Gursoy, E.; van Genderen, O.S.; Ajmone Marsan, N.; Delgado, V.; Bax, J.J. Prognostic Implications of Right Ventricular Remodeling and Function in Patients with Significant Secondary Tricuspid Regurgitation. Circulation 2019, 140, 836–845. [Google Scholar] [CrossRef]
- Dreyfus, J.; Audureau, E.; Bohbot, Y.; Coisne, A.; Lavie-Badie, Y.; Bouchery, M.; Flagiello, M.; Bazire, B.; Eggenspieler, F.; Viau, F.; et al. TRI-SCORE: A new risk score for in-hospital mortality prediction after isolated tricuspid valve surgery. Eur. Heart J. 2022, 43, 654–662. [Google Scholar] [CrossRef]
- Subbotina, I.; Girdauskas, E.; Bernhardt, A.M.; Sinning, C.; Reichenspurner, H.; Sill, B. Comparison of Outcomes of Tricuspid Valve Surgery in Patients with Reduced and Normal Right Ventricular Function. Thorac. Cardiovasc. Surg. 2017, 65, 617–625. [Google Scholar] [PubMed]
- Algarni, K.D.; Arafat, A.; Algarni, A.D.; Alfonso, J.J.; Alhossan, A.; Elsayed, A.; Kheirallah, H.M.; Albacker, T.B. Degree of right ventricular dysfunction dictates outcomes after tricuspid valve repair concomitant with left-side valve surgery. Gen. Thorac. Cardiovasc. Surg. 2021, 69, 911–918. [Google Scholar] [CrossRef]
- Karam, N.; Mehr, M.; Taramasso, M.; Besler, C.; Ruf, T.; Connelly, K.A.; Weber, M.; Yzeiraj, E.; Schiavi, D.; Mangieri, A.; et al. Value of Echocardiographic Right Ventricular and Pulmonary Pressure Assessment in Predicting Transcatheter Tricuspid Repair Outcome. JACC Cardiovasc. Interv. 2020, 13, 1251–1261. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Sugiura, A.; Kavsur, R.; Öztürk, C.; Wilde, N.; Zimmer, S.; Nickenig, G.; Weber, M.; Vogelhuber, J. Changes in right ventricular function and clinical outcomes following tricuspid transcatheter edge-to-edge repair. Eur. J. Heart Fail. 2024, 26, 1015–1024. [Google Scholar] [CrossRef]
- Agricola, E.; Fiore, G. Right ventricular function profits from tricuspid regurgitation reduction following tricuspid transcatheter edge-to-edge repair: When is it never too late? Eur. J. Heart Fail. 2024, 26, 1628–1630. [Google Scholar] [CrossRef] [PubMed]
- Prihadi, E.A.; van der Bijl, P.; Dietz, M.; Abou, R.; Vollema, E.M.; Marsan, N.A.; Delgado, V.; Bax, J.J. Prognostic Implications of Right Ventricular Free Wall Longitudinal Strain in Patients with Significant Functional Tricuspid Regurgitation. Circ. Cardiovasc. Imaging 2019, 12, e008666. [Google Scholar] [CrossRef] [PubMed]
- Ancona, F.; Melillo, F.; Calvo, F.; El Halabieh, N.A.; Stella, S.; Capogrosso, C.; Ingallina, G.; Tafciu, E.; Pascaretta, A.; Ancona, M.B.; et al. Right ventricular systolic function in severe tricuspid regurgitation: Prognostic relevance of longitudinal strain. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 868–875. [Google Scholar] [CrossRef]
- Hinojar, R.; Zamorano, J.L.; González Gómez, A.; García-Martin, A.; Monteagudo, J.M.; García Lunar, I.; Sanchez Recalde, A.; Fernández-Golfín, C. Prognostic Impact of Right Ventricular Strain in Isolated Severe Tricuspid Regurgitation. J. Am. Soc. Echocardiogr. 2023, 36, 615–623. [Google Scholar] [CrossRef]
- Bannehr, M.; Kahn, U.; Liebchen, J.; Okamoto, M.; Hähnel, V.; Georgi, C.; Dworok, V.; Edlinger, C.; Lichtenauer, M.; Kücken, T.; et al. Right Ventricular Longitudinal Strain Predicts Survival in Patients with Functional Tricuspid Regurgitation. Can. J. Cardiol. 2021, 37, 1086–1093. [Google Scholar] [CrossRef]
- Wang, T.K.M.; Akyuz, K.; Reyaldeen, R.; Griffin, B.P.; Popovic, Z.B.; Pettersson, G.B.; Gillinov, A.M.; Flamm, S.D.; Xu, B.; Desai, M.Y. Prognostic Value of Complementary Echocardiography and Magnetic Resonance Imaging Quantitative Evaluation for Isolated Tricuspid Regurgitation. Circ. Cardiovasc. Imaging 2021, 14, e012211. [Google Scholar] [CrossRef]
- Akintoye, E.; Wang, T.K.M.; Nakhla, M.; Ali, A.H.; Fava, A.M.; Akyuz, K.; Popovic, Z.B.; Pettersson, G.B.; Gillinov, A.M.; Xu, B.; et al. Quantitative Echocardiographic Assessment and Optimal Criteria for Early Intervention in Asymptomatic Tricuspid Regurgitation. JACC Cardiovasc. Imaging 2023, 16, 13–24. [Google Scholar] [CrossRef]
- Kim, M.; Lee, H.; Park, J.; Kim, J.; Lee, S.; Kim, Y.; Chang, S.; Kim, H. Preoperative Right Ventricular Free-Wall Longitudinal Strain as a Prognosticator in Isolated Surgery for Severe Functional Tricuspid Regurgitation. J. Am. Heart Assoc. 2021, 10, e019856. [Google Scholar] [CrossRef]
- Hirasawa, K.; van Rosendael, P.J.; Dietz, M.F.; Ajmone Marsan, N.; Delgado, V.; Bax, J.J. Comparison of the Usefulness of Strain Imaging by Echocardiography versus Computed Tomography to Detect Right Ventricular Systolic Dysfunction in Patients with Significant Secondary Tricuspid Regurgitation. Am. J. Cardiol. 2020, 134, 116–122. [Google Scholar] [CrossRef]
- Sayour, A.A.; Tokodi, M.; Celeng, C.; Takx, R.A.P.; Fábián, A.; Lakatos, B.K.; Friebel, R.; Surkova, E.; Merkely, B.; Kovács, A. Association of Right Ventricular Functional Parameters with Adverse Cardiopulmonary Outcomes: A Meta-analysis. J. Am. Soc. Echocardiogr. 2023, 36, 624–633.e8. [Google Scholar] [CrossRef] [PubMed]
- Orban, M.; Wolff, S.; Braun, D.; Stolz, L.; Higuchi, S.; Stark, K.; Mehr, M.; Stocker, T.J.; Dischl, D.; Scherer, C.; et al. Right Ventricular Function in Transcatheter Edge-to-Edge Tricuspid Valve Repair. JACC Cardiovasc. Imaging 2021, 14, 2477–2479. [Google Scholar] [CrossRef] [PubMed]
- Guazzi, M.; Naeije, R.; Arena, R.; Corrà, U.; Ghio, S.; Forfia, P.; Rossi, A.; Cahalin, L.P.; Bandera, F.; Temporelli, P. Echocardiography of Right Ventriculoarterial Coupling Combined with Cardiopulmonary Exercise Testing to Predict Outcome in Heart Failure. Chest 2015, 148, 226–234. [Google Scholar] [CrossRef]
- Colalillo, A.; Hoffmann-Vold, A.M.; Pellicano, C.; Romaniello, A.; Gabrielli, A.; Hachulla, E.; Smith, V.; Simeón-Aznar, C.P.; Castellví, I.; Airò, P.; et al. The role of TAPSE/sPAP ratio in predicting pulmonary hypertension and mortality in the systemic sclerosis EUSTAR cohort. Autoimmun. Rev. 2023, 22, 103290. [Google Scholar] [CrossRef]
- Fortuni, F.; Butcher, S.C.; Dietz, M.F.; van der Bijl, P.; Prihadi, E.A.; De Ferrari, G.M.; Marsan, N.A.; Bax, J.J.; Delgado, V. Right Ventricular–Pulmonary Arterial Coupling in Secondary Tricuspid Regurgitation. Am. J. Cardiol. 2021, 148, 138–145. [Google Scholar] [CrossRef]
- Brener, M.I.; Grayburn, P.; Lindenfeld, J.; Burkhoff, D.; Liu, M.; Zhou, Z.; Alu, M.C.; Medvedofsky, D.A.; Asch, F.M.; Weissman, N.J.; et al. Right Ventricular-Pulmonary Arterial Coupling in Patients with HF Secondary MR: Analysis from the COAPT Trial. JACC Cardiovasc. Interv. 2021, 14, 2231–2242. [Google Scholar] [CrossRef]
- Ancona, F.; Margonato, D.; Menzà, G.; Bellettini, M.; Melillo, F.; Stella, S.; Capogrosso, C.; Ingallina, G.; Biondi, F.; Boccellino, A.; et al. Ratio between right ventricular longitudinal strain and pulmonary arterial systolic pressure: A novel prognostic parameter in patients with severe tricuspid regurgitation. Int. J. Cardiol. 2023, 384, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Gerçek, M.; Körber, M.I.; Narang, A.; Friedrichs, K.P.; Puthumana, J.J.; Rudolph, T.K.; Thomas, J.D.; Pfister, R.; Davidson, C.J.; Rudolph, V. Echocardiographic Pulmonary Artery Systolic Pressure Is Not Reliable for RV-PA Coupling in Transcatheter Tricuspid Valve Annuloplasty. JACC Cardiovasc. Interv. 2022, 15, 2578–2580. [Google Scholar] [CrossRef]
- Lurz, P.; Orban, M.; Besler, C.; Braun, D.; Schlotter, F.; Noack, T.; Desch, S.; Karam, N.; Kresoja, K.P.; Hagl, C.; et al. Clinical characteristics, diagnosis, and risk stratification of pulmonary hypertension in severe tricuspid regurgitation and implications for transcatheter tricuspid valve repair. Eur. Heart J. 2020, 41, 2785–2795. [Google Scholar] [CrossRef]
- Brener, M.I.; Lurz, P.; Hausleiter, J.; Rodés-Cabau, J.; Fam, N.; Kodali, S.K.; Rommel, K.P.; Muntané-Carol, G.; Gavazzoni, M.; Nazif, T.M.; et al. Right Ventricular-Pulmonary Arterial Coupling and Afterload Reserve in Patients Undergoing Transcatheter Tricuspid Valve Repair. J. Am. Coll. Cardiol. 2022, 79, 448–461. [Google Scholar] [CrossRef] [PubMed]
- Gavazzoni, M.; Badano, L.P.; Cascella, A.; Heilbron, F.; Tomaselli, M.; Caravita, S.; Baratto, C.; Perelli, F.; Radu, N.; Perger, E.; et al. Clinical Value of a Novel Three-Dimensional Echocardiography-Derived Index of Right Ventricle-Pulmonary Artery Coupling in Tricuspid Regurgitation. J. Am. Soc. Echocardiogr. 2023, 36, 1154–1166.e3. [Google Scholar] [CrossRef] [PubMed]
- Petersen, S.E.; Aung, N.; Sanghvi, M.M.; Zemrak, F.; Fung, K.; Paiva, J.M.; Francis, J.M.; Khanji, M.Y.; Lukaschuk, E.; Lee, A.M.; et al. Reference ranges for cardiac structure and function using cardiovascular magnetic resonance (CMR) in Caucasians from the UK Biobank population cohort. J. Cardiovasc. Magn. Reson. 2017, 19, 18. [Google Scholar] [CrossRef] [PubMed]
- Park, J.B.; Kim, H.K.; Jung, J.H.; Klem, I.; Yoon, Y.E.; Lee, S.P.; Park, E.A.; Hwang, H.Y.; Lee, W.; Kim, K.H.; et al. Prognostic Value of Cardiac MR Imaging for Preoperative Assessment of Patients with Severe Functional Tricuspid Regurgitation. Radiology 2016, 280, 723–734. [Google Scholar] [CrossRef] [PubMed]
- Hinojar, R.; Gómez, A.G.; García-Martin, A.; Monteagudo, J.M.; Fernández-Méndez, M.A.; de Vicente, A.G.; Salinas, G.L.A.; Zamorano, J.L.; Fernández-Golfín, C. Impact of right ventricular systolic function in patients with significant tricuspid regurgitation. A cardiac magnetic resonance study. Int. J. Cardiol. 2021, 339, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Cipriani, A.; Mattesi, G.; Bariani, R.; Cecere, A.; Martini, N.; De Michieli, L.; Da Pozzo, S.; Corradin, S.; De Conti, G.; Zorzi, A.; et al. Cardiac magnetic resonance imaging of arrhythmogenic cardiomyopathy: Evolving diagnostic perspectives. Eur. Radiol. 2023, 33, 270–282. [Google Scholar] [CrossRef]
- Romano, S.; Dell’atti, D.; Judd, R.M.; Kim, R.J.; Weinsaft, J.W.; Kim, J.; Heitner, J.F.; Hahn, R.T.; Farzaneh-Far, A. Prognostic Value of Feature-Tracking Right Ventricular Longitudinal Strain in Severe Functional Tricuspid Regurgitation: A Multicenter Study. JACC Cardiovasc. Imaging 2021, 14, 1561–1568. [Google Scholar] [CrossRef]
- Dupont, M.V.; Drăgean, C.A.; Coche, E.E. Right ventricle function assessment by MDCT. AJR Am. J. Roentgenol. 2011, 196, 77–86. [Google Scholar] [CrossRef]
- Praz, F.; Khalique, O.K.; Macedo, L.G.D.R.; Pulerwitz, T.C.; Jantz, J.; Wu, I.Y.; Kantor, A.; Patel, A.; Vahl, T.; Bapat, V.; et al. Comparison between three-dimensional echocardiography and computed tomography for comprehensive tricuspid annulus and valve assessment in severe tricuspid regurgitation: Implications for tricuspid regurgitation grading and transcatheter therapies. J. Am. Soc. Echocardiogr. 2018, 31, 1190–1202.e3. [Google Scholar] [CrossRef]
- Plumhans, C.; Mühlenbruch, G.; Rapaee, A.; Sim, K.H.; Seyfarth, T.; Günther, R.W.; Mahnken, A.H. Assessment of global right ventricular function on 64-MDCT compared with MRI. AJR Am. J. Roentgenol. 2008, 190, 1358–1361. [Google Scholar] [CrossRef]
- Tanaka, T.; Sugiura, A.; Kavsur, R.; Öztürk, C.; Vogelhuber, J.; Wilde, N.; Kütting, D.; Meyer, C.; Zimmer, S.; Grube, E.; et al. Right ventricular ejection fraction assessed by computed tomography in patients undergoing transcatheter tricuspid valve repair. Eur. Heart J. Cardiovasc. Imaging 2023, 24, 1501–1508. [Google Scholar] [CrossRef] [PubMed]
- Hell, M.M.; Emrich, T.; Kreidel, F.; Kreitner, K.F.; Schoepf, U.J.; Münzel, T.; von Bardeleben, R.S. Computed tomography imaging needs for novel transcatheter tricuspid valve repair and replacement therapies. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Winkel, M.G.; Brugger, N.; Khalique, O.K.; Gräni, C.; Huber, A.; Pilgrim, T.; Billinger, M.; Windecker, S.; Hahn, R.T.; Praz, F. Imaging and Patient Selection for Transcatheter Tricuspid Valve Interventions. Front. Cardiovasc. Med. 2020, 7, 60. [Google Scholar] [CrossRef] [PubMed]
- Surkova, E.; Cosyns, B.; Gerber, B.; Gimelli, A.; La Gerche, A.; Ajmone Marsan, N. The dysfunctional right ventricle: The importance of multi-modality imaging. Eur. Heart J. Cardiovasc. Imaging 2022, 23, 885–897. [Google Scholar] [CrossRef]
- Lahm, T.; Douglas, I.S.; Archer, S.L.; Bogaard, H.J.; Chesler, N.C.; Haddad, F.; Hemnes, A.R.; Kawut, S.M.; Kline, J.A.; Kolb, T.M.; et al. Assessment of Right Ventricular Function in the Research Setting: Knowledge Gaps and Pathways Forward. An Official American Thoracic Society Research Statement. Am. J. Respir. Crit. Care Med. 2018, 198, e15–e43. [Google Scholar] [CrossRef]
- Moceri, P.; Duchateau, N.; Gillon, S.; Jaunay, L.; Baudouy, D.; Squara, F.; Ferrari, E.; Sermesant, M. Three-dimensional right ventricular shape and strain in congenital heart disease patients with right ventricular chronic volume loading. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 1174–1181. [Google Scholar] [CrossRef]
- Butcher, S.C.; Fortuni, F.; Montero-Cabezas, J.M.; Abou, R.; El Mahdiui, M.; van der Bijl, P.; van der Velde, E.T.; Ajmone Marsan, N.; Bax, J.J.; Delgado, V. Right ventricular myocardial work: Proof-of-concept for non-invasive assessment of right ventricular function. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 142–152. [Google Scholar] [CrossRef]

| RV Function Indices | Advantages | Disadvantages |
|---|---|---|
| TAPSE | Easy and fast Wide availability Reproducibility Prognostic role | Angle and load dependency Reflects only RV systolic longitudinal function Excludes the contribution of interventricular septum and RVOT to systolic phase |
| TDI-S’ velocity | Easy and fast Wide availability Reproducibility Prognostic role | Angle and load dependency Reflects only RV systolic longitudinal function Excludes the contribution of interventricular septum and RVOT to systolic phase |
| RV-FAC | Easy Wide availability Reflects RV systolic radial function Correlation with RVEF-CMR derived Prognostic role | Load dependency Dependency on quality images Low reproducibility Time-consuming Excludes the contribution of RVOT to systolic phase |
| RV-FWLS | Less angle and load dependency Reproducibility Correlation with RVEF-CMR derived Strong prognostic role | Dependency on quality images Low availability Time-consuming Dependency of post-processing phase Dependency of vendor for reference values |
| 3D-RVEF | Not based on geometric assumptions Strong correlation with RVEF-CMR derived Strong prognostic role | Load dependency Dependency on quality images Low availability Time-consuming Necessity of regular cardiac rhythm and patient compliance (use of multibeat acquisition modality) Dependency of post-processing phase Dependency of specific analysis software |
| Authors | Protocol | Patients | Target | Parameters | Results |
|---|---|---|---|---|---|
| Dietz et al. [58] | Observational registry | 1298 | Severe functional TR treated medically | TAPSE |
|
| Dreyfus et al. [59] | Observational registry | 466 | Severe primary or functional TR treated surgically | TAPSE TDI-S’ RV-FAC |
|
| Subbotina et al. [60] | Observational retrospective study | 191 | Significant TR treated surgically | TAPSE |
|
| Algarni et al. [61] | Observational retrospective study | 548 | Functional TR treated surgically with concomitant left-side valve surgery | TAPSE |
|
| Karam et al. [62] | Observational study | 249 | Moderate/Severe TR treated with transcatheter edge-to-edge valve repair | TAPSE RV-FAC |
|
| Kresoja et al. [54] | Observational study | 79 | Significant symptomatic TR treated with transcatheter edge-to-edge valve repair | TAPSE |
|
| Tanaka et al. [63] | Observational retrospective study | 204 | Significant symptomatic TR treated with transcatheter edge-to-edge valve repair | RV-FAC |
|
| Authors | Protocol | Patients | Target | RV-FWLS Cut-Off (Absolute Values) | Results |
|---|---|---|---|---|---|
| Prihadi et al. [65] | Observational study | 896 | Moderate and severe functional TR | 23% |
|
| Ancona et al. [66] | Observational study | 250 | Severe TR (mostly functional) | 17% (hospitalization for RV heart failure) 14% (all-cause mortality) |
|
| Hinojar et al. [67] | Observational study | 151 | Severe, massive or torrential functional TR | - |
|
| Bannehr et al. [68] | Observational study | 1089 | Mild, moderate and severe TR | 18% |
|
| Wang et al. [69] | Observational study | 262 | Isolated severe TR | 11% |
|
| Kim et al. [71] | Observational study | 115 | Isolated severe functional TR treated surgically | 24% |
|
| Akintoye et al. [70] | Observational retrospective study | 325 | Moderate to severe or severe asymptomatic TR | 19% |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melillo, F.; Fabiani, D.; Santoro, A.; Oro, P.; Frecentese, F.; Salemme, L.; Tesorio, T.; Agricola, E.; De Bonis, M.; Lorusso, R. Multimodality Imaging for Right Ventricular Function Assessment in Severe Tricuspid Regurgitation. J. Clin. Med. 2024, 13, 5076. https://doi.org/10.3390/jcm13175076
Melillo F, Fabiani D, Santoro A, Oro P, Frecentese F, Salemme L, Tesorio T, Agricola E, De Bonis M, Lorusso R. Multimodality Imaging for Right Ventricular Function Assessment in Severe Tricuspid Regurgitation. Journal of Clinical Medicine. 2024; 13(17):5076. https://doi.org/10.3390/jcm13175076
Chicago/Turabian StyleMelillo, Francesco, Dario Fabiani, Alessandro Santoro, Pietro Oro, Francesca Frecentese, Luigi Salemme, Tullio Tesorio, Eustachio Agricola, Michele De Bonis, and Roberto Lorusso. 2024. "Multimodality Imaging for Right Ventricular Function Assessment in Severe Tricuspid Regurgitation" Journal of Clinical Medicine 13, no. 17: 5076. https://doi.org/10.3390/jcm13175076
APA StyleMelillo, F., Fabiani, D., Santoro, A., Oro, P., Frecentese, F., Salemme, L., Tesorio, T., Agricola, E., De Bonis, M., & Lorusso, R. (2024). Multimodality Imaging for Right Ventricular Function Assessment in Severe Tricuspid Regurgitation. Journal of Clinical Medicine, 13(17), 5076. https://doi.org/10.3390/jcm13175076






