Behavioral and Electrophysiological Markers of Attention Fluctuations in Children with Hypersomnolence
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Diagnostic Procedure for Hypersomnolence
2.3. BLAST Paradigm
2.4. Resting-State EEG Recording and Analyses
2.5. Statistical Analysis
3. Results
3.1. Descriptive Analysis
3.2. Group Comparisons
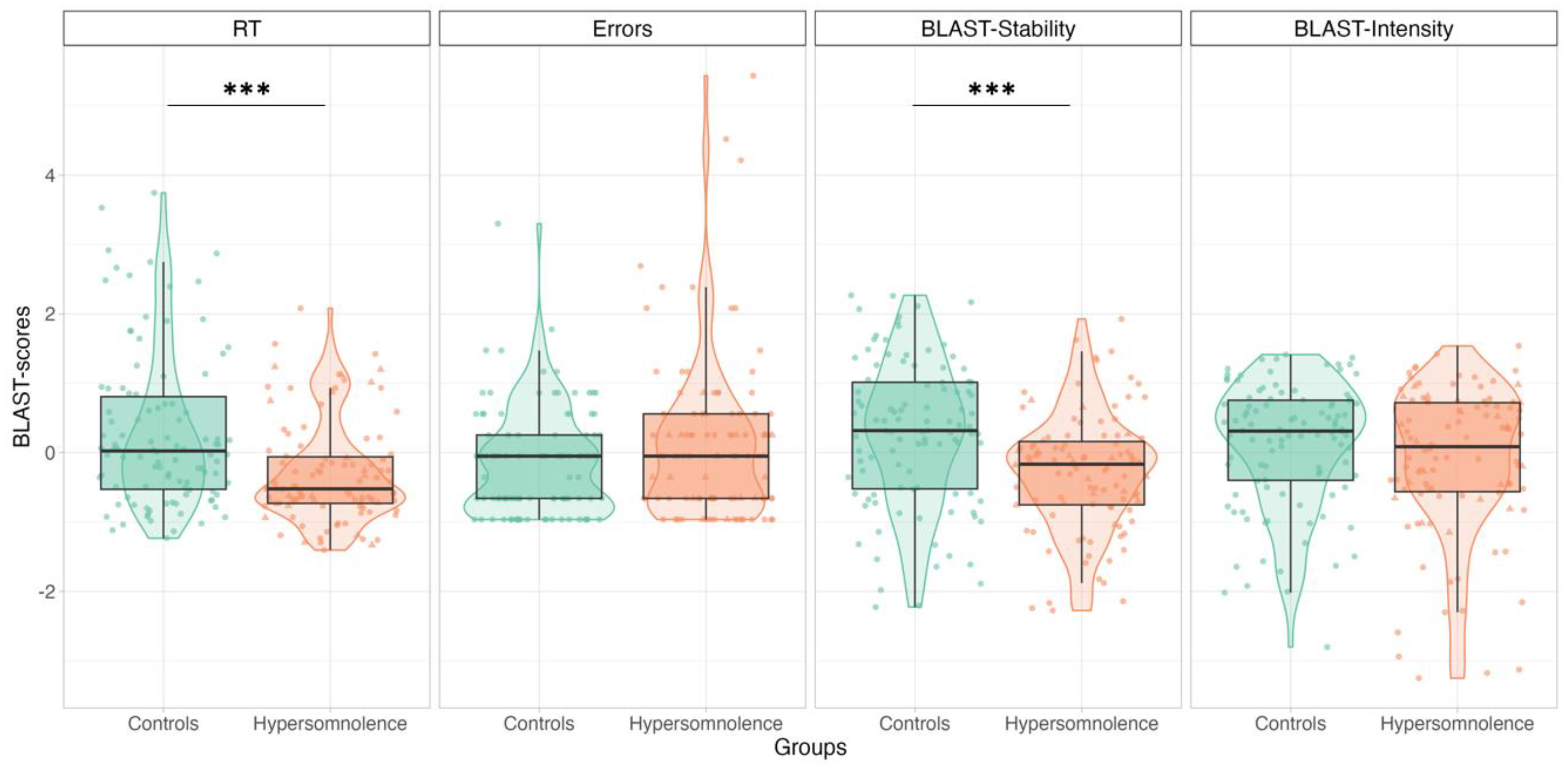
3.2.1. HYP vs. Controls: BLAST-Color
3.2.2. HYP vs. ADHD: BLAST-Classic and EEG
3.3. Correlations between BLAST, EEG Parameters, and Sleepiness in Children with HYP
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dinges, D.F. An Overview of Sleepiness and Accidents. J. Sleep Res. 1995, 4, 4–14. [Google Scholar] [CrossRef]
- Bioulac, S.; Micoulaud Franchi, J.A.; Arnaud, M.; Sagaspe, P.; Moore, N.; Salvo, F.; Philip, P. Risk of Motor Vehicle Accidents Related to Sleepiness at the Wheel: A Systematic Review and Meta-Analysis. Sleep 2017, 40, zsx134. [Google Scholar] [CrossRef]
- Leger, D. The Cost of Sleep-Related Accidents: A Report for the National Commission on Sleep Disorders Research. Sleep 1994, 17, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Doran, S.M.; Van Dongen, H.P.; Dinges, D.F. Sustained Attention Performance during Sleep Deprivation: Evidence of State Instability. Arch. Ital. Biol. 2001, 139, 253–267. [Google Scholar]
- D’Ambrosio, S.; Castelnovo, A.; Guglielmi, O.; Nobili, L.; Sarasso, S.; Garbarino, S. Sleepiness as a Local Phenomenon. Front. Neurosci. 2019, 13, 1086. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Dinges, D.F. Sleep Deprivation and Vigilant Attention. Ann. N. Y. Acad. Sci. 2008, 1129, 305–322. [Google Scholar] [CrossRef]
- Killgore, W.D.S. Effects of Sleep Deprivation on Cognition; Elsevier B.V.: Amsterdam, The Netherlands, 2010; Volume 185, ISBN 9780444537027. [Google Scholar]
- Hudson, A.N.; Van Dongen, H.P.A.; Honn, K.A. Sleep Deprivation, Vigilant Attention, and Brain Function: A Review. Neuropsychopharmacology 2020, 45, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, T.S.; Cade, B.E.; Wolfe, J.M.; Czeisler, C.A. Searching Night and Day: A Dissociation of Effects of Circadian Phase and Time Awake on Visual Selective Attention and Vigilance. Psychol. Sci. 2003, 14, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Madiouni, C.; Lopez, R.; Gély-Nargeot, M.C.; Lebrun, C.; Bayard, S. Mind-Wandering and Sleepiness in Adults with Attention-Deficit/Hyperactivity Disorder. Psychiatry Res. 2020, 287. [Google Scholar] [CrossRef]
- Poh, J.H.; Chong, P.L.H.; Chee, M.W.L. Sleepless Night, Restless Mind: Effects of Sleep Deprivation on Mind Wandering. J. Exp. Psychol. Gen. 2016, 145, 1312–1318. [Google Scholar] [CrossRef]
- Andrillon, T.; Windt, J.; Silk, T.; Drummond, S.P.A.; Bellgrove, M.A.; Tsuchiya, N. Does the Mind Wander When the Brain Takes a Break? Local Sleep in Wakefulness, Attentional Lapses and Mind-Wandering. Front. Neurosci. 2019, 13, 949. [Google Scholar] [CrossRef] [PubMed]
- Andrillon, T.; Burns, A.; Mackay, T.; Windt, J.; Tsuchiya, N. Predicting Lapses of Attention with Sleep-like Slow Waves. Nat. Commun. 2021, 12, 3657. [Google Scholar] [CrossRef] [PubMed]
- Weissman, D.H.; Roberts, K.C.; Visscher, K.M.; Woldorff, M.G. The Neural Bases of Momentary Lapses in Attention. Nat. Neurosci. 2006, 9, 971–978. [Google Scholar] [CrossRef] [PubMed]
- Filardi, M.; D’Anselmo, A.; Agnoli, S.; Rubaltelli, E.; Mastria, S.; Mangiaruga, A.; Franceschini, C.; Pizza, F.; Corazza, G.E.; Plazzi, G. Cognitive Dysfunction in Central Disorders of Hypersomnolence: A Systematic Review. Sleep Med. Rev. 2021, 59, 101510. [Google Scholar] [CrossRef]
- Bayard, S.; Croisier Langenier, M.; Cochen De Cock, V.; Scholz, S.; Dauvilliers, Y. Executive Control of Attention in Narcolepsy. PLoS ONE 2012, 7, e33525. [Google Scholar] [CrossRef]
- Zamarian, L.; Högl, B.; Delazer, M.; Hingerl, K.; Gabelia, D.; Mitterling, T.; Brandauer, E.; Frauscher, B. Subjective Deficits of Attention, Cognition and Depression in Patients with Narcolepsy. Sleep Med. 2015, 16, 45–51. [Google Scholar] [CrossRef]
- Des Champs de Boishebert, L.; Pradat, P.; Bastuji, H.; Ricordeau, F.; Gormand, F.; Le Cam, P.; Stauffer, E.; Petitjean, T.; Peter-Derex, L. Microsleep versus Sleep Onset Latency during Maintenance Wakefulness Tests: Which One Is the Best Marker of Sleepiness? Clocks Sleep 2021, 3, 259–273. [Google Scholar] [CrossRef]
- Petton, M.; Perrone-Bertolotti, M.; Mac-Auliffe, D.; Bertrand, O.; Aguera, P.E.; Sipp, F.; Batthacharjee, M.; Isnard, J.; Minotti, L.; Rheims, S.; et al. BLAST: A Short Computerized Test to Measure the Ability to Stay on Task. Normative Behavioral Data and Detailed Cortical Dynamics. Neuropsychologia 2019, 134. [Google Scholar] [CrossRef]
- Thieux, M.; Jung, J.; Bouet, R.; Gerard, D.; Bauer, P.R.; Bertrand, O.; Perrone-Bertolotti, M.; Arzimanoglou, A.; Kahane, P.; Lachaux, J.P.; et al. BLAST Paradigm: A New Test to Assess Brief Attentional Fluctuations in Children with Epilepsy, ADHD, and Normally Developing Children. Epilepsy Behav. 2019, 99, 106470. [Google Scholar] [CrossRef]
- Torsvall, L.; åAkerstedt, T. Sleepiness on the Job: Continuously Measured EEG Changes in Train Drivers. Electroencephalogr. Clin. Neurophysiol. 1987, 66, 502–511. [Google Scholar] [CrossRef]
- Strijkstra, A.M.; Beersma, D.G.M.; Drayer, B.; Halbesma, N.; Daan, S. Subjective Sleepiness Correlates Negatively with Global Alpha (8–12 Hz) and Positively with Central Frontal Theta (4–8 Hz) Frequencies in the Human Resting Awake Electroencephalogram. Neurosci. Lett. 2003, 340, 17–20. [Google Scholar] [CrossRef]
- Gorgoni, M.; Ferlazzo, F.; Ferrara, M.; Moroni, F.; D’Atri, A.; Fanelli, S.; Gizzi Torriglia, I.; Lauri, G.; Marzano, C.; Rossini, P.M.; et al. Topographic Electroencephalogram Changes Associated with Psychomotor Vigilance Task Performance after Sleep Deprivation. Sleep Med. 2014, 15, 1132–1139. [Google Scholar] [CrossRef]
- Cajochen, C.; Brunner, D.P.; Krauchi, K.; Graw, P.; Wirz-Justice, A. Power Density in Theta/Alpha Frequencies of the Waking EEG Progressively Increases during Sustained Wakefulness. Sleep 1995, 18, 890–894. [Google Scholar] [CrossRef]
- Åkerstedt, T.; Gillberg, M. Subjective and Objective Sleepiness in the Active Individual. Int. J. Neurosci. 1990, 52, 29–37. [Google Scholar] [CrossRef]
- Cajochen, C.; Khalsa, S.B.S.; Wyatt, J.K.; Czeisler, C.A.; Dijk, D.J. EEG and Ocular Correlates of Circadian Melatonin Phase and Human Performance Decrements during Sleep Loss. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1999, 277. [Google Scholar] [CrossRef]
- Makeig, S.; Jung, T.P.; Sejnowski, T.J. Awareness during Drowsiness: Dynamics and Electrophysiological Correlates. Can. J. Exp. Psychol. 2000, 54, 266–273. [Google Scholar] [CrossRef]
- Quercia, A.; Zappasodi, F.; Committeri, G.; Ferrara, M. Local Use-Dependent Sleep in Wakefulness Links Performance Errors to Learning. Front. Hum. Neurosci. 2018, 12, 122. [Google Scholar] [CrossRef]
- Hung, C.S.; Sarasso, S.; Ferrarelli, F.; Riedner, B.; Ghilardi, M.F.; Cirelli, C.; Tononi, G. Local Experience-Dependent Changes in the Wake EEG after Prolonged Wakefulness. Sleep 2013, 36, 59–72. [Google Scholar] [CrossRef]
- Bernardi, G.; Siclari, F.; Yu, I.; Zennig, C.; Bellesi, M.; Ricciardi, E.; Cirelli, C.; Ghilardi, M.F.; Pietrini, P.; Tononi, G. Neural and Behavioral Correlates of Extended Training during Sleep Deprivation in Humans: Evidence for Local, Task-Specific Effects. J. Neurosci. 2015, 35, 4487–4500. [Google Scholar] [CrossRef]
- Fattinger, S.; Kurth, S.; Ringli, M.; Jenni, O.G.; Huber, R. Theta Waves in Children’s Waking Electroencephalogram Resemble Local Aspects of Sleep during Wakefulness. Sci. Rep. 2017, 7, 11187. [Google Scholar] [CrossRef]
- Bioulac, S.; Taillard, J.; Philip, P.; Sagaspe, P. Excessive Daytime Sleepiness Measurements in Children with Attention Deficit Hyperactivity Disorder. Front. Psychiatry 2020, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Loo, S.K.; Makeig, S. Clinical Utility of EEG in Attention-Deficit/Hyperactivity Disorder: A Research Update. Neurotherapeutics 2012, 9, 569–587. [Google Scholar] [CrossRef] [PubMed]
- Braboszcz, C.; Delorme, A. Lost in Thoughts: Neural Markers of Low Alertness during Mind Wandering. Neuroimage 2011, 54, 3040–3047. [Google Scholar] [CrossRef] [PubMed]
- van Son, D.; De Blasio, F.M.; Fogarty, J.S.; Angelidis, A.; Barry, R.J.; Putman, P. Frontal EEG Theta/Beta Ratio during Mind Wandering Episodes. Biol. Psychol. 2019, 140, 19–27. [Google Scholar] [CrossRef]
- Barry, R.J.; Clarke, A.R.; Johnstone, S.J.; McCarthy, R.; Selikowitz, M. Electroencephalogram θ/β Ratio and Arousal in Attention-Deficit/Hyperactivity Disorder: Evidence of Independent Processes. Biol. Psychiatry 2009, 66, 398–401. [Google Scholar] [CrossRef] [PubMed]
- Saad, J.F.; Kohn, M.R.; Clarke, S.; Lagopoulos, J.; Hermens, D.F. Is the Theta/Beta EEG Marker for ADHD Inherently Flawed? J. Atten. Disord. 2018, 22, 815–826. [Google Scholar] [CrossRef]
- Lubar, J.F. Discourse on the Development of EEG Diagnostics and Biofeedback for Attention-Deficit/Hyperactivity Disorders. Biofeedback Self Regul. 1991, 16, 201–225. [Google Scholar] [CrossRef]
- Clarke, A.R.; Barry, R.J.; McCarthy, R.; Selikowitz, M. EEG-Defined Subtypes of Children with Attention-Deficit/Hyperactivity Disorder. Clin. Neurophysiol. 2001, 112, 2098–2105. [Google Scholar] [CrossRef]
- Newson, J.J.; Thiagarajan, T.C. EEG Frequency Bands in Psychiatric Disorders: A Review of Resting State Studies. Front. Hum. Neurosci. 2019, 12, 521. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®); American Psychiatric Pub.: Washington, DC, USA, 2013; ISBN 0890425574. [Google Scholar]
- DuPaul, G.J.; Power, T.J.; Anastopoulos, A.D.; Reid, R. ADHD Rating Scale—IV: Checklists, Norms, and Clinical Interpretation; Guilford Press: New York, NY, USA, 1998; ISBN 1-57230-423-5. [Google Scholar]
- Snow, A.; Gozal, E.; Malhotra, A.; Tiosano, D.; Perlman, R.; Vega, C.; Shahar, E.; Gozal, D.; Hochberg, Z.; Pillar, G. Severe Hypersomnolence after Pituitary/Hypothalamic Surgery in Adolescents: Clinical Characteristics and Potential Mechanisms. Pediatrics 2002, 110, e74. [Google Scholar] [CrossRef]
- Iber, C.; Iber, C. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications; American Academy of Sleep Medicine: Westchester, IL, USA, 2007; Volume 1. [Google Scholar]
- Thieux, M.; Zhang, M.; Marcastel, A.; Herbillon, V.; Guignard-Perret, A.; Seugnet, L.; Lin, J.-S.; Guyon, A.; Plancoulaine, S.; Franco, P. Intellectual Abilities of Children with Narcolepsy. J. Clin. Med. 2020, 9, 4075. [Google Scholar] [CrossRef] [PubMed]
- American Academy of Sleep Medicine. International Classification of Sleep Disorders, 3rd ed.; ICSD-3; American Academy of Sleep Medicine: Darien, IL, USA, 2014. [Google Scholar] [CrossRef]
- Bouet, R. Tool BLAST EEG. Available online: https://github.com/rbouet/Tool_BLAST_EEG (accessed on 25 July 2024).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 25 July 2024).
- Lopez, R.; Micoulaud-Franchi, J.A.; Camodeca, L.; Gachet, M.; Jaussent, I.; Dauvilliers, Y. Association of Inattention, Hyperactivity, and Hypersomnolence in Two Clinic-Based Adult Cohorts. J. Atten. Disord. 2018, 24, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Lecendreux, M.; Konofal, E.; Bouvard, M.; Falissard, B.; Mouren-Siméoni, M.C. Sleep and Alertness in Children with ADHD. J. Child. Psychol. Psychiatry 2000, 41, 803–812. [Google Scholar] [CrossRef] [PubMed]
- Lecendreux, M.; Lavault, S.; Lopez, R.; Inocente, C.O.; Konofal, E.; Cortese, S.; Franco, P.; Arnulf, I.; Dauvilliers, Y. Attention-Deficit/Hyperactivity Disorder (ADHD) Symptoms in Pediatric Narcolepsy: A Cross-Sectional Study. Sleep 2015, 38, 1285–1295. [Google Scholar] [CrossRef]
- Miano, S.; Amato, N.; Foderaro, G.; Pezzoli, V.; Ramelli, G.P.; Toffolet, L.; Manconi, M. Sleep Phenotypes in Attention Deficit Hyperactivity Disorder. Sleep Med. 2019, 60, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Arns, M.; Kenemans, J.L. Neurofeedback in ADHD and Insomnia: Vigilance Stabilization through Sleep Spindles and Circadian Networks. Neurosci. Biobehav. Rev. 2014, 44, 183–194. [Google Scholar] [CrossRef]
- Sander, C.; Arns, M.; Olbrich, S.; Hegerl, U. EEG-Vigilance and Response to Stimulants in Paediatric Patients with Attention Deficit/Hyperactivity Disorder. Clin. Neurophysiol. 2010, 121, 1511–1518. [Google Scholar] [CrossRef]
- Weinberg, W.A.; Brumback, R.A. Primary Disorder of Vigilance: A Novel Explanation of Inattentiveness, Daydreaming, Boredom, Restlessness, and Sleepiness. J. Pediatr. 1990, 116, 720–725. [Google Scholar] [CrossRef]
- Beebe, D.W.; Fallone, G.; Godiwala, N.; Flanigan, M.; Martin, D.; Schaffner, L.; Amin, R. Feasibility and Behavioral Effects of an At-Home Multi-Night Sleep Restriction Protocol for Adolescents. J. Child. Psychol. Psychiatry 2008, 49, 915–923. [Google Scholar] [CrossRef]
- Beebe, D.W.; Rose, D.; Amin, R. Attention, Learning, and Arousal of Experimentally Sleep-Restricted Adolescents in a Simulated Classroom. J. Adolesc. Health 2010, 47, 523–525. [Google Scholar] [CrossRef]
- Sadeh, A.; Gruber, R.; Raviv, A. The Effects of Sleep Restriction and Extension on School-Age Children: What a Difference an Hour Makes. Child. Dev. 2003, 74, 444–455. [Google Scholar] [CrossRef]
- Fallone, G.; Acebo, C.; Seifer, R.; Carskadon, M.A. Experimental Restriction of Sleep Opportunity in Children: Effects on Teacher Ratings. Sleep 2005, 28, 1561–1567. [Google Scholar] [CrossRef] [PubMed]
- Petersen, S.E.; Posner, M.I. The Attention System of the Human Brain: 20 Years After. Annu. Rev. Neurosci. 2012, 35, 73–89. [Google Scholar] [CrossRef] [PubMed]
- Fox, M.D.; Snyder, A.Z.; Vincent, J.L.; Corbetta, M.; Van Essen, D.C.; Raichle, M.E. The Human Brain Is Intrinsically Organized into Dynamic, Anticorrelated Functional Networks. Proc. Natl. Acad. Sci. USA 2005, 102, 9673–9678. [Google Scholar] [CrossRef]
- Krause, A.J.; Ben Simon, E.; Mander, B.A.; Greer, S.M.; Saletin, J.M.; Goldstein-Piekarski, A.N.; Walker, M.P. The Sleep-Deprived Human Brain. Nat. Rev. Neurosci. 2017, 18, 404–418. [Google Scholar] [CrossRef]
- Drummond, S.P.A.; Bischoff-Grethe, A.; Dinges, D.F.; Ayalon, L.; Mednick, S.C.; Meloy, M.J. The Neural Basis of the Psychomotor Vigilance Task. Sleep 2005, 28, 1059–1068. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Shuai, D.; Bu, X.; Hu, X.; Tang, S.; Zhang, L.; Li, H.; Hu, X.; Lu, L.; Gong, Q.; et al. Impairments of Large-Scale Functional Networks in Attention-Deficit/Hyperactivity Disorder: A Meta-Analysis of Resting-State Functional Connectivity. Psychol. Med. 2019, 49, 2475–2485. [Google Scholar] [CrossRef]
- Cortese, S.; Kelly, C.; Chabernaud, C.; Proal, E.; Di Martino, A.; Milham, M.P.; Castellanos, F.X. Toward Systems Neuroscience of ADHD: A Meta-Analysis of 55 FMRI Sudies. Am. J. Psychiatry 2012, 169, 1038–1055. [Google Scholar] [CrossRef]
- Witt, S.T.; Drissi, N.M.; Tapper, S.; Wretman, A.; Szakács, A.; Hallböök, T.; Landtblom, A.M.; Karlsson, T.; Lundberg, P.; Engström, M. Evidence for Cognitive Resource Imbalance in Adolescents with Narcolepsy. Brain Imaging Behav. 2018, 12, 411–424. [Google Scholar] [CrossRef]
- Xiao, F.; Lu, C.; Zhao, D.; Zou, Q.; Xu, L.; Li, J.; Zhang, J.; Han, F. Independent Component Analysis and Graph Theoretical Analysis in Patients with Narcolepsy. Neurosci. Bull. 2019, 35, 743–755. [Google Scholar] [CrossRef]
- Xiao, F.; Spruyt, K.; Lu, C.; Zhao, D.; Zhang, J.; Han, F. Resting-State Brain Network Topological Properties and the Correlation with Neuropsychological Assessment in Adolescent Narcolepsy. Sleep 2020, 43, zsaa018. [Google Scholar] [CrossRef]
- Kaufmann, T.; Elvsåshagen, T.; Alnæs, D.; Zak, N.; Pedersen, P.Ø.; Norbom, L.B.; Quraishi, S.H.; Tagliazucchi, E.; Laufs, H.; Bjørnerud, A.; et al. The Brain Functional Connectome Is Robustly Altered by Lack of Sleep. Neuroimage 2016, 127, 324–332. [Google Scholar] [CrossRef]
- Yeo, B.T.T.; Tandi, J.; Chee, M.W.L. Functional Connectivity during Rested Wakefulness Predicts Vulnerability to Sleep Deprivation. Neuroimage 2015, 111, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Miano, S.; Esposito, M.; Foderaro, G.; Ramelli, G.P.; Pezzoli, V.; Manconi, M. Sleep-Related Disorders in Children with Attention-Deficit Hyperactivity Disorder: Preliminary Results of a Full Sleep Assessment Study. CNS Neurosci. Ther. 2016, 22, 906–914. [Google Scholar] [CrossRef]
- Massimini, M.; Ferrarelli, F.; Huber, R.; Esser, S.K.; Singh, H.; Tononi, G. Neuroscience: Breakdown of Cortical Effective Connectivity during Sleep. Science 2005, 309, 2228–2232. [Google Scholar] [CrossRef] [PubMed]
- Naumann, A.; Bellebaum, C.; Daum, I. Cognitive Deficits in Narcolepsy. J. Sleep Res. 2006, 15, 329–338. [Google Scholar] [CrossRef]
- Yoon, S.M.; Joo, E.Y.; Kim, J.Y.; Hwang, K.J.; Hong, S.B. Is High IQ Protective against Cognitive Dysfunction in Narcoleptic Patients? J. Clin. Neurol. 2013, 9, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Fronczek, R.; Middelkoop, H.A.M.; Gert Van Dijk, J.; Lammers, G.J. Focusing on Vigilance Instead of Sleepiness in the Assessment of Narcolepsy: High Sensitivity of the Sustained Attention to Response Task (SART). Sleep 2006, 29, 187–191. [Google Scholar] [CrossRef]
- Delazer, M.; Högl, B.; Zamarian, L.; Wenter, J.; Gschliesser, V.; Ehrmann, L.; Brandauer, E.; Cevikkol, Z.; Frauscher, B. Executive Functions, Information Sampling, and Decision Making in Narcolepsy with Cataplexy. Neuropsychology 2011, 25, 477–487. [Google Scholar] [CrossRef]
- Ramm, M.; Boentert, M.; Lojewsky, N.; Jafarpour, A.; Young, P.; Heidbreder, A. Disease-Specific Attention Impairment in Disorders of Chronic Excessive Daytime Sleepiness. Sleep Med. 2019, 53, 133–140. [Google Scholar] [CrossRef]
- Posar, A.; Pizza, F.; Parmeggiani, A.; Plazzi, G. Neuropsychological Findings in Childhood Narcolepsy. J. Child. Neurol. 2014, 29, 1370–1376. [Google Scholar] [CrossRef] [PubMed]
- Brunel, L.; Brossaud, E.; Lioret, J.; Jaffiol, A.; Vanderghote, L.; Cuisinier, L.; Peter-Derex, L.; Ricordeau, F.; Thieux, M.; Comajuan, M.; et al. Effectiveness of an Intervention Program on Physical Activity in Children with Narcolepsy Type 1. Sleep Med. 2024, 116, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Comsa, I.M.; Bekinschtein, T.A.; Chennu, S. Transient Topographical Dynamics of the Electroencephalogram Predict Brain Connectivity and Behavioural Responsiveness During Drowsiness. Brain Topogr. 2019, 32, 315–331. [Google Scholar] [CrossRef] [PubMed]

| Total HYP (n = 37) | Primary HYP (n = 7) | Secondary HYP (n = 30) | |
|---|---|---|---|
| Sleep characteristics—PSG | |||
| TST, min | 539 (393–628) | 562 (533–627) | 534 (393–628) |
| Sleep latency, min | 13.1 (0–146) | 0.2 (0–2.3) | 19.5 (0–146) |
| REM latency, min | 127 (0–272) | 2 (0–68) | 142 (1.5–271.5) |
| REM latency < 15 min, n | 19 (7) | 71 (5) | 7 (2) |
| Sleep efficiency, % | 91 (73–99) | 91 (88–98) | 91 (73–99) |
| N1, % | 6.8 (1.4–15.7) | 10.3 (3.5–15.6) | 6.7 (1.4–15.7) |
| N2, % | 53.6 (29.2–68) | 43.7 (29.2–64.7) | 53.7 (38.3–68) |
| N3, % | 17.4 (6.8–31.8) | 17.4 (7.2–31.8) | 17.3 (6.8–31) |
| REM, % | 22.4 (10.6–40.8) | 24.6 (21.1–40.8) | 21.8 (10.6–32.8) |
| Arousals and micro-arousals index, n/h | 11.8 (6.2–45) | 12.4 (9.2–30.9) | 10.8 (6.2–45) |
| Sleepiness characteristics | |||
| AESS | 13 (0–24) | 20 (16–24) | 12 (0–22) |
| Pathological AESS, n | 73 (27) | 100 (7) | 67 (20) |
| MSLT sleep latency, min | 15.8 (2.3–20) | 4.5 (2.3–13.9) | 17 (3.6–20) |
| MSLT sleep latency < 8 min, n | 19 (7) | 71 (5) | 7 (2) |
| MSLT SOREMP, % | 0 (0–100) | 75 (25–100) | 0 (0–50) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thieux, M.; Lioret, J.; Bouet, R.; Guyon, A.; Lachaux, J.-P.; Herbillon, V.; Franco, P. Behavioral and Electrophysiological Markers of Attention Fluctuations in Children with Hypersomnolence. J. Clin. Med. 2024, 13, 5077. https://doi.org/10.3390/jcm13175077
Thieux M, Lioret J, Bouet R, Guyon A, Lachaux J-P, Herbillon V, Franco P. Behavioral and Electrophysiological Markers of Attention Fluctuations in Children with Hypersomnolence. Journal of Clinical Medicine. 2024; 13(17):5077. https://doi.org/10.3390/jcm13175077
Chicago/Turabian StyleThieux, Marine, Julien Lioret, Romain Bouet, Aurore Guyon, Jean-Philippe Lachaux, Vania Herbillon, and Patricia Franco. 2024. "Behavioral and Electrophysiological Markers of Attention Fluctuations in Children with Hypersomnolence" Journal of Clinical Medicine 13, no. 17: 5077. https://doi.org/10.3390/jcm13175077





