Clinical Trial: A Pragmatic Randomised Controlled Study to Assess the Effectiveness of Two Patient Management Strategies in Mild to Moderate Ulcerative Colitis—The OPTIMISE Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Objectives and Endpoints
2.2. Study Population
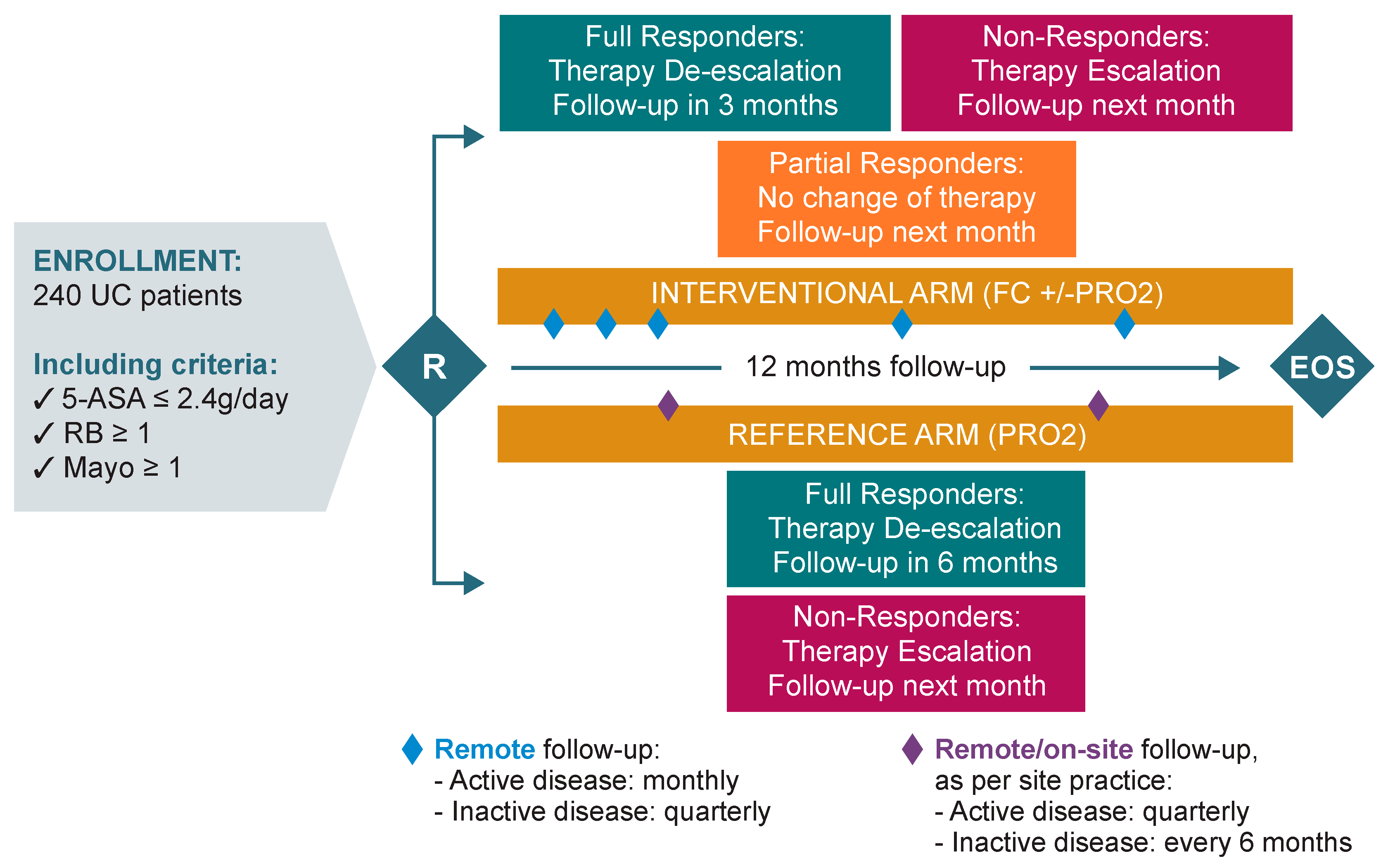
2.3. Interventions
- Interventional arm: monthly monitoring during the active phase of the disease, and quarterly during remission. UC treatment optimisation (escalation/de-escalation) was performed by the investigator and triggered based on patient self monitoring of FC values and/or Patient Reported Outcome-2 (PRO2) scoring.
- Reference arm: quarterly monitoring during the active phase of the disease, and every 6 months during remission. UC treatment optimisation (escalation/de-escalation) was performed by the investigator and triggered based on PRO-2 scoring only.
- If patients were non-responders (defined as FC ≥ 150 μg/g, irrespective of PRO-2 scoring), therapy was escalated to the next escalation step (Table 1). If current therapy administered had not reached the minimum duration of therapy as per the SmPC, the investigator could decide to continue the current therapy or to escalate, in the best interest of the patient. In all cases, the next follow-up call was scheduled for the following month.
- If patients were partial responders (defined as FC < 150 μg/g and PRO-2 ≥ 2), the investigator maintained the current therapy administered if the SmPC allowed (or de-escalated to oral 5-ASA ≥ 2.4 g/day if not). The next follow-up call was scheduled for the following month.
- If patients were full responders (defined as FC < 150 μg/g and PRO-2 ≤ 1, with RB = 0), the investigator first de-escalated the current therapy to oral 5-ASA ≥ 2.4 g/day. De-escalation started only once current therapy administered had reached the minimum duration of therapy as per the SmPC. The next follow-up call was scheduled in 3 months. At the next visit, therapy was managed as follows:
- For full responders, the investigator proceeded to the second de-escalation step/maintenance therapy of 5-ASA ≤ 2.4 g/day and the next follow-up call was scheduled in 3 months.
- For partial responders, the investigator maintained the therapy unchanged and scheduled the next follow-up call in 1 month.
- For non-responders, the investigator escalated the therapy to the next level and scheduled the next follow-up call in 1 month.
- For non-responders (defined as PRO-2 ≥ 2), the investigator escalated to the next step (Table 1) and scheduled the next follow-up visit in 3 months.
- For full responders (defined as PRO-2 ≤ 1 with RB = 0), the investigator first de-escalated therapy to oral 5-ASA ≥ 2.4 g/day (waiting for the time indicated in the SmPC), and the next follow-up visit was scheduled in 6 months. If the patient was still a full responder during the following visit, the investigator proceeded to the second de-escalation step/maintenance therapy of 5-ASA ≤ 2.4 g/day, and the next follow-up visit was scheduled in 6 months, or at the end of the study visit, whichever came first. Whereas if the patient was a non-responder at the following study visit, therapy was escalated to the next level, and the subsequent follow-up visit was scheduled in 3 months.
2.4. Statistical Analysis
2.4.1. Sample Size
2.4.2. Effectiveness Analysis Set
2.4.3. Statistical Tests
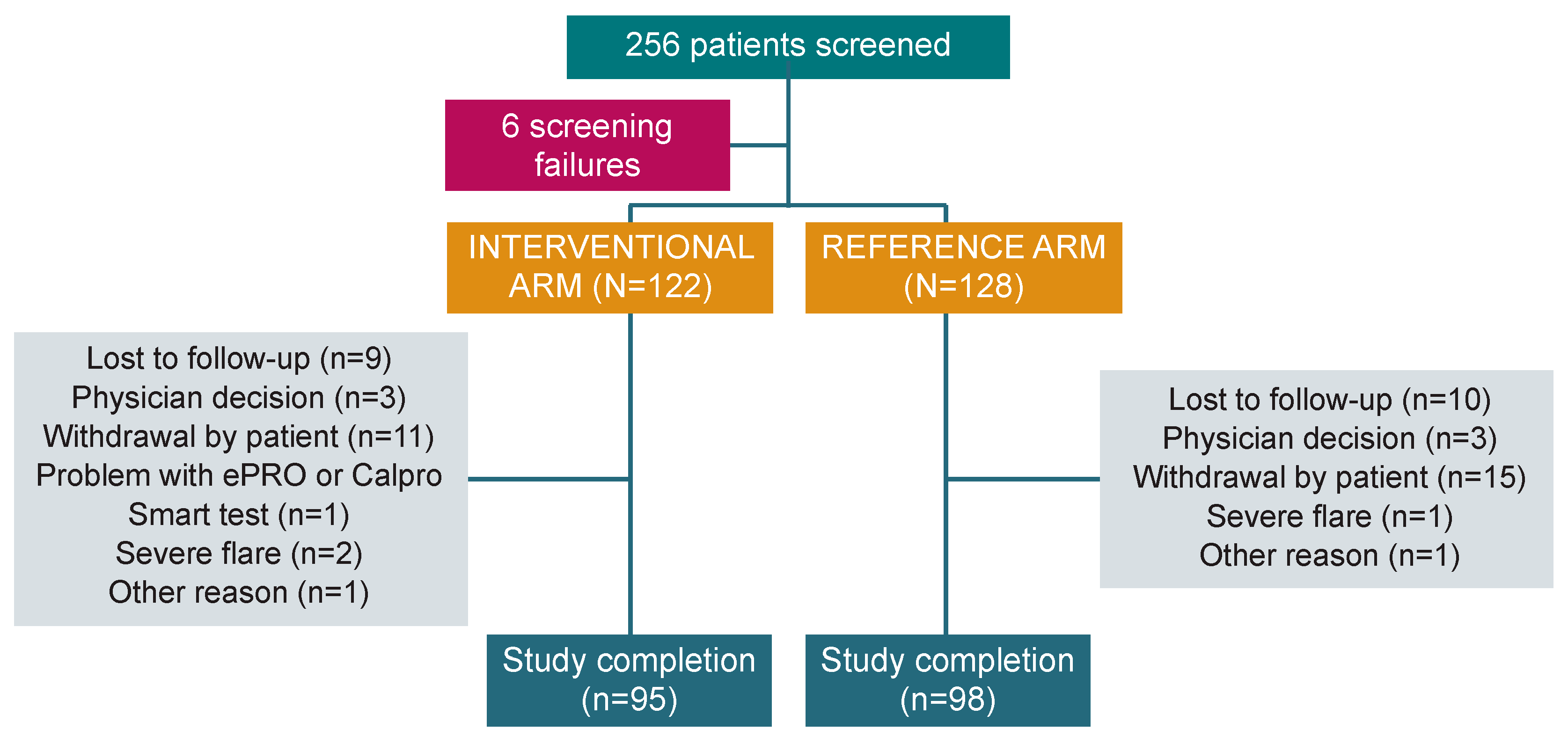
3. Results
3.1. mITT Analysis
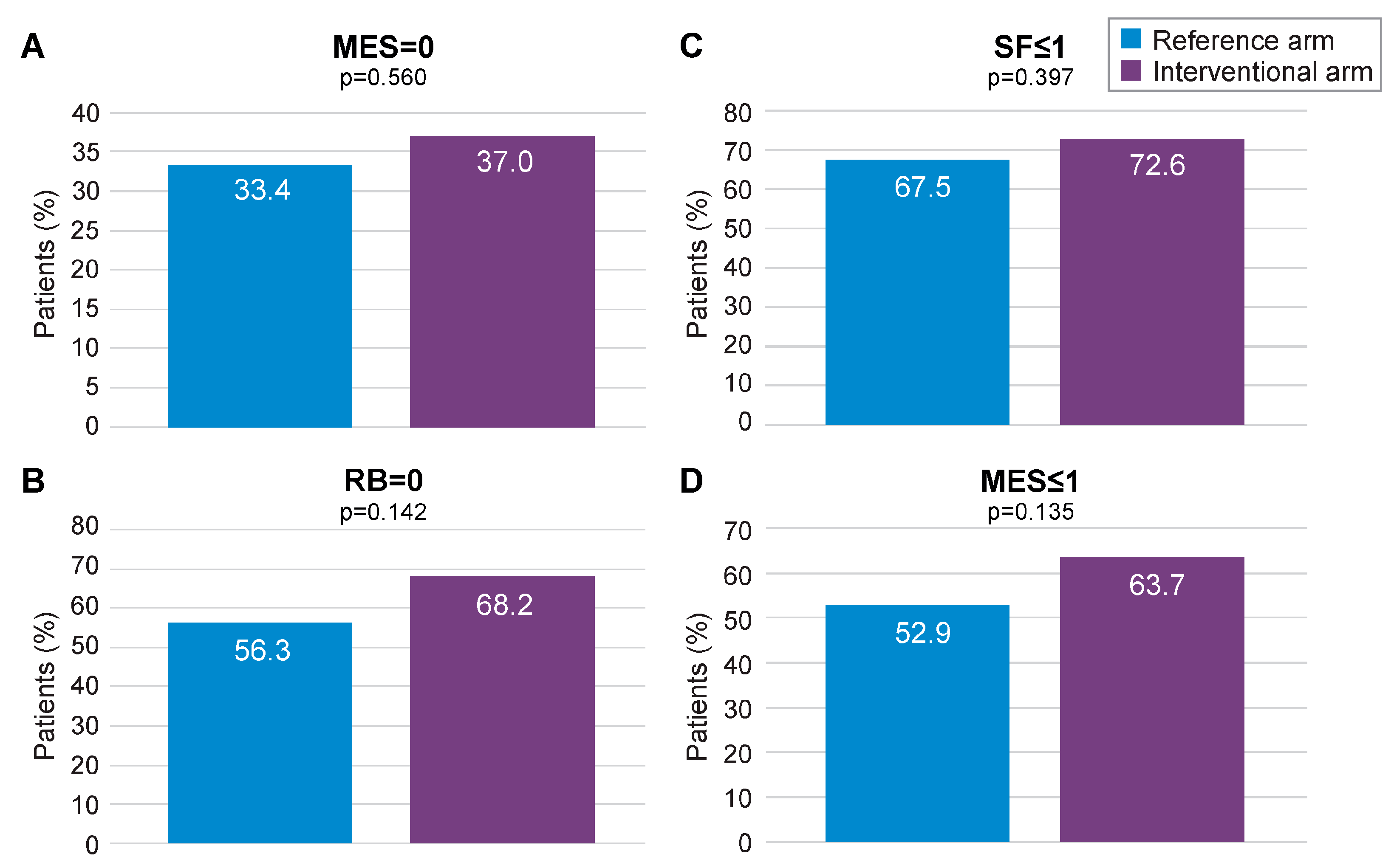
3.2. PP Analysis
Complementary Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Magro, F.; Gionchetti, P.; Eliakim, R.; Ardizzone, S.; Armuzzi, A.; Barreiro-de Acosta, M.; Burisch, J.; Gecse, K.B.; Hart, A.L.; Hindryckx, P.; et al. Third European Evidence-Based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 1: Definitions, Diagnosis, Extra-Intestinal Manifestations, Pregnancy, Cancer Surveillance, Surgery, and Ileo-Anal Pouch Disorders. J. Crohns Colitis 2017, 11, 649–670. [Google Scholar] [CrossRef] [PubMed]
- Qin, X. Etiology of Inflammatory Bowel Disease: A Unified Hypothesis. World J. Gastroenterol. 2012, 18, 1708–1722. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, F.; Fasulo, E.; Jairath, V.; Paridaens, K.; Peyrin-Biroulet, L.; Danese, S. Management and Treatment Optimization of Patients with Mild to Moderate Ulcerative Colitis. Expert. Rev. Clin. Immunol. 2024, 20, 277–290. [Google Scholar] [CrossRef]
- Harbord, M.; Eliakim, R.; Bettenworth, D.; Karmiris, K.; Katsanos, K.; Kopylov, U.; Kucharzik, T.; Molnár, T.; Raine, T.; Sebastian, S.; et al. Third European Evidence-Based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 2: Current Management. J. Crohns Colitis 2017, 11, 769–784. [Google Scholar] [CrossRef]
- Lewis, J.D.; Parlett, L.E.; Jonsson Funk, M.L.; Brensinger, C.; Pate, V.; Wu, Q.; Dawwas, G.K.; Weiss, A.; Constant, B.D.; McCauley, M.; et al. Incidence, Prevalence, and Racial and Ethnic Distribution of Inflammatory Bowel Disease in the United States. Gastroenterology 2023, 165, 1197–1205.e2. [Google Scholar] [CrossRef] [PubMed]
- Zamani, M.; Alizadeh-Tabari, S.; Murad, M.H.; Ananthakrishnan, A.N.; Malekzadeh, R.; Talley, N.J. Meta-Analysis: Risk of Pancreatic Cancer in Patients with Inflammatory Bowel Disease. Aliment. Pharmacol. Ther. 2024, 59, 918–927. [Google Scholar] [CrossRef]
- Le Berre, C.; Honap, S.; Peyrin-Biroulet, L. Ulcerative Colitis. Lancet 2023, 402, 571–584. [Google Scholar] [CrossRef]
- Awadhi, S.A.; Alboraie, M.; Albaba, E.A.; Almutairdi, A.; Alsaad, M.; Azzam, N.; Barakat, H.; D’Amico, F.; Danese, S.; El Kady, M.; et al. Treatment of Patients with Mild to Moderate Ulcerative Colitis: A Middle East Expert Consensus. J. Clin. Med. 2023, 12, 6929. [Google Scholar] [CrossRef]
- Raine, T.; Bonovas, S.; Burisch, J.; Kucharzik, T.; Adamina, M.; Annese, V.; Bachmann, O.; Bettenworth, D.; Chaparro, M.; Czuber-Dochan, W.; et al. ECCO Guidelines on Therapeutics in Ulcerative Colitis: Medical Treatment. J. Crohns Colitis 2022, 16, 2–17. [Google Scholar] [CrossRef]
- Peyrin-Biroulet, L.; Sandborn, W.; Sands, B.E.; Reinisch, W.; Bemelman, W.; Bryant, R.V.; D’Haens, G.; Dotan, I.; Dubinsky, M.; Feagan, B.; et al. Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE): Determining Therapeutic Goals for Treat-to-Target. Am. J. Gastroenterol. 2015, 110, 1324–1338. [Google Scholar] [CrossRef]
- Turner, D.; Ricciuto, A.; Lewis, A.; D’Amico, F.; Dhaliwal, J.; Griffiths, A.M.; Bettenworth, D.; Sandborn, W.J.; Sands, B.E.; Reinisch, W.; et al. STRIDE-II: An Update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): Determining Therapeutic Goals for Treat-to-Target Strategies in IBD. Gastroenterology 2021, 160, 1570–1583. [Google Scholar] [CrossRef] [PubMed]
- Røseth, A.G.; Schmidt, P.N.; Fagerhol, M.K. Correlation between Faecal Excretion of Indium-111-Labelled Granulocytes and Calprotectin, a Granulocyte Marker Protein, in Patients with Inflammatory Bowel Disease. Scand. J. Gastroenterol. 1999, 34, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Røseth, A.G.; Fagerhol, M.K.; Aadland, E.; Schjønsby, H. Assessment of the Neutrophil Dominating Protein Calprotectin in Feces. A Methodologic Study. Scand. J. Gastroenterol. 1992, 27, 793–798. [Google Scholar] [CrossRef]
- D’Amico, F.; Bonovas, S.; Danese, S.; Peyrin-Biroulet, L. Review Article: Faecal Calprotectin and Histologic Remission in Ulcerative Colitis. Aliment. Pharmacol. Ther. 2020, 51, 689–698. [Google Scholar] [CrossRef] [PubMed]
- af Björkesten, C.-G.; Nieminen, U.; Turunen, U.; Arkkila, P.E.; Sipponen, T.; Färkkilä, M.A. Endoscopic Monitoring of Infliximab Therapy in Crohn’s Disease. Inflamm. Bowel Dis. 2011, 17, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Fiorino, G.; Danese, S.; Peyrin-Biroulet, L.; Sans, M.; Bonelli, F.; Calleri, M.; Zierold, C.; Pollastro, R.; Moretti, F.; Malesci, A. LIAISON® Calprotectin for the Prediction of Relapse in Quiescent Ulcerative Colitis: The EuReCa Study. United European Gastroenterol. J. 2022, 10, 836–843. [Google Scholar] [CrossRef]
- Gustavsson, A.; Järnerot, G.; Hertervig, E.; Friis-Liby, I.; Blomquist, L.; Karlén, P.; Grännö, C.; Vilien, M.; Ström, M.; Verbaan, H.; et al. Clinical Trial: Colectomy after Rescue Therapy in Ulcerative Colitis—3-Year Follow-up of the Swedish-Danish Controlled Infliximab Study. Aliment. Pharmacol. Ther. 2010, 32, 984–989. [Google Scholar] [CrossRef]
- Schnitzler, F.; Fidder, H.; Ferrante, M.; Noman, M.; Arijs, I.; Van Assche, G.; Hoffman, I.; Van Steen, K.; Vermeire, S.; Rutgeerts, P. Mucosal Healing Predicts Long-Term Outcome of Maintenance Therapy with Infliximab in Crohn’s Disease. Inflamm. Bowel Dis. 2009, 15, 1295–1301. [Google Scholar] [CrossRef]
- Abej, E.; El-Matary, W.; Singh, H.; Bernstein, C.N. The Utility of Fecal Calprotectin in the Real-World Clinical Care of Patients with Inflammatory Bowel Disease. Can. J. Gastroenterol. Hepatol. 2016, 2016, 2483261. [Google Scholar] [CrossRef]
- Rogler, G.; Aldeguer, X.; Kruis, W.; Lasson, A.; Mittmann, U.; Nally, K.; Peyrin-Biroulet, L.; Schoepfer, A.; Vatn, M.; Vavricka, S.; et al. Concept for a Rapid Point-of-Care Calprotectin Diagnostic Test for Diagnosis and Disease Activity Monitoring in Patients with Inflammatory Bowel Disease: Expert Clinical Opinion. J. Crohns Colitis 2013, 7, 670–677. [Google Scholar] [CrossRef]
- Røseth, A.G.; Aadland, E.; Jahnsen, J.; Raknerud, N. Assessment of Disease Activity in Ulcerative Colitis by Faecal Calprotectin, a Novel Granulocyte Marker Protein. Digestion 1997, 58, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Colombel, J.-F.; Panaccione, R.; Bossuyt, P.; Lukas, M.; Baert, F.; Vaňásek, T.; Danalioglu, A.; Novacek, G.; Armuzzi, A.; Hébuterne, X.; et al. Effect of Tight Control Management on Crohn’s Disease (CALM): A Multicentre, Randomised, Controlled Phase 3 Trial. Lancet 2017, 390, 2779–2789. [Google Scholar] [CrossRef]
- Testa, A.; Castiglione, F.; Nardone, O.M.; Colombo, G.L. Adherence in Ulcerative Colitis: An Overview. Patient Prefer. Adherence 2017, 11, 297–303. [Google Scholar] [CrossRef]
- Cortesi, P.A.; Fiorino, G.; Peyrin-Biroulet, L.; Mantovani, L.G.; Jairath, V.; Paridaens, K.; Andersson, F.L.; Danese, S. Non-Invasive Monitoring and Treat-to-Target Approach Are Cost-Effective in Patients with Mild-Moderate Ulcerative Colitis. Aliment. Pharmacol. Ther. 2023, 57, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Danese, S.; Vermeire, S.; D’Haens, G.; Panés, J.; Dignass, A.; Magro, F.; Nazar, M.; Le Bars, M.; Lahaye, M.; Ni, L.; et al. Treat to Target versus Standard of Care for Patients with Crohn’s Disease Treated with Ustekinumab (STARDUST): An Open-Label, Multicentre, Randomised Phase 3b Trial. Lancet Gastroenterol. Hepatol. 2022, 7, 294–306. [Google Scholar] [CrossRef]
- D’Amico, F.; Fiorino, G.; Solitano, V.; Massarini, E.; Guillo, L.; Allocca, M.; Furfaro, F.; Zilli, A.; Bonovas, S.; Magro, F.; et al. Ulcerative Colitis: Impact of Early Disease Clearance on Long-Term Outcomes—A Multicenter Cohort Study. United Eur. Gastroenterol. J. 2022, 10, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Parigi, T.L.; Allocca, M.; Furfaro, F.; D’Amico, F.; Zilli, A.; Dal Buono, A.; Gabbiadini, R.; Bonovas, S.; Armuzzi, A.; Danese, S.; et al. Treat-to-Target and Regular Surveillance of Inflammatory Bowel Disease Are Associated with Low Incidence and Early-Stage Detection of Malignancies: A Retrospective Cohort Study. Cancers 2023, 15, 5754. [Google Scholar] [CrossRef]
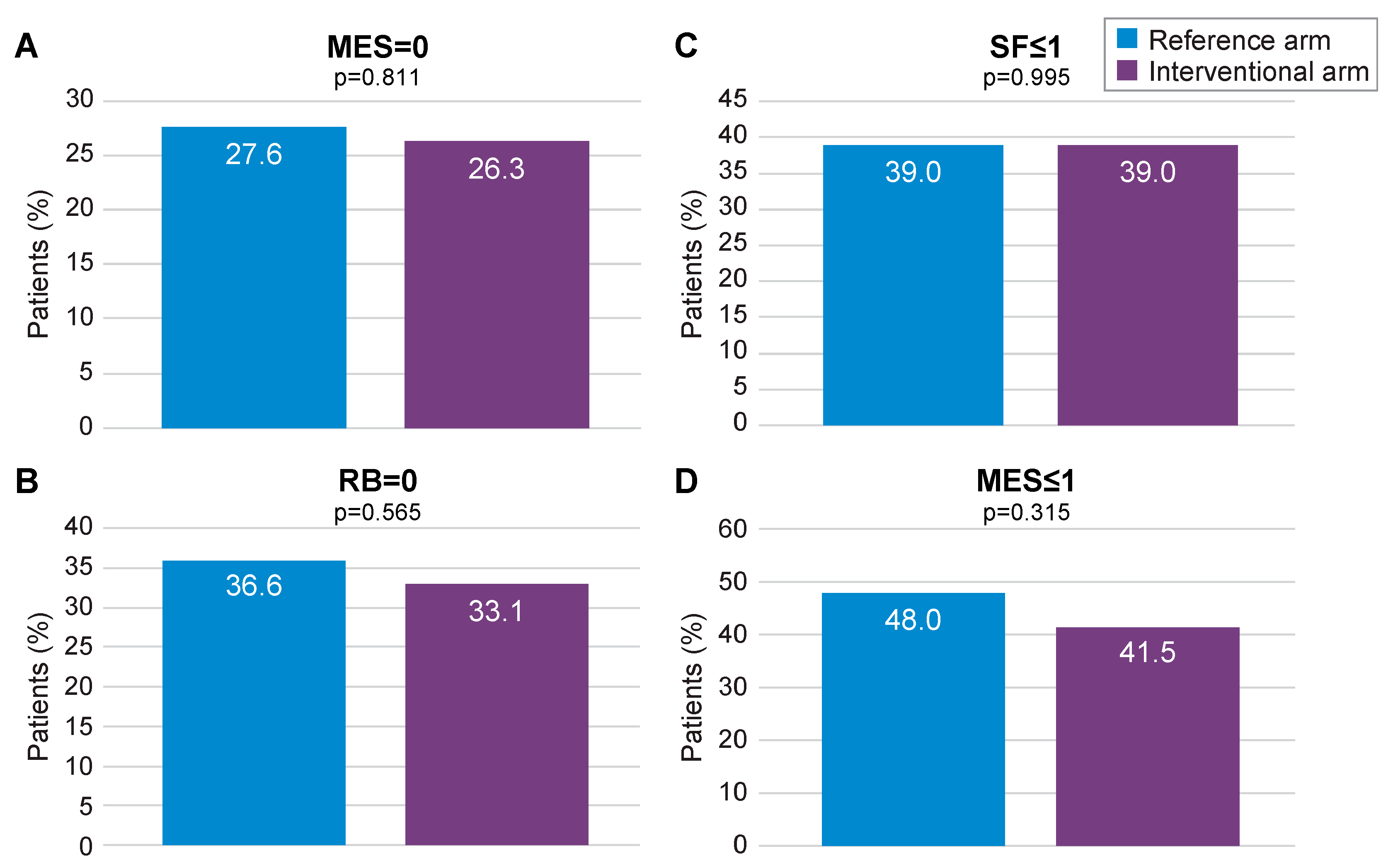

| Site of Disease | Prior Relapse (Screening) | Escalation Steps (Active Disease/Relapse) | De-Escalation Steps (Remission) | |||
|---|---|---|---|---|---|---|
| 1st Step (Baseline) | 2nd Step | 3rd Step | 1st Step | 2nd Step/ Maintenance | ||
| Proctitis UC | Oral 5-ASA 2.4 g/day or no treatment | 5-ASA suppository ≥ 1 g/day + oral 5-ASA ≥ 2.4 g/day | Rescue Therapy ** | Rescue Therapy ** | Oral 5-ASA ≥ 2.4 g/day | Oral 5-ASA < 2.4 g/day |
| Left-sided UC | 5-ASA enema ≥ 1 g/day + oral 5-ASA ≥ 2.4 g/day | 2nd generation corticosteroids * | ||||
| Extensive UC | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Danese, S.; Fiorino, G.; Vicaut, E.; Paridaens, K.; Ugur, A.; Clark, B.; Vanasek, T.; Stepek, D.; D’Amico, F.; West, R.; et al. Clinical Trial: A Pragmatic Randomised Controlled Study to Assess the Effectiveness of Two Patient Management Strategies in Mild to Moderate Ulcerative Colitis—The OPTIMISE Study. J. Clin. Med. 2024, 13, 5147. https://doi.org/10.3390/jcm13175147
Danese S, Fiorino G, Vicaut E, Paridaens K, Ugur A, Clark B, Vanasek T, Stepek D, D’Amico F, West R, et al. Clinical Trial: A Pragmatic Randomised Controlled Study to Assess the Effectiveness of Two Patient Management Strategies in Mild to Moderate Ulcerative Colitis—The OPTIMISE Study. Journal of Clinical Medicine. 2024; 13(17):5147. https://doi.org/10.3390/jcm13175147
Chicago/Turabian StyleDanese, Silvio, Gionata Fiorino, Eric Vicaut, Kristine Paridaens, Asiya Ugur, Brian Clark, Tomas Vanasek, David Stepek, Ferdinando D’Amico, Rachel West, and et al. 2024. "Clinical Trial: A Pragmatic Randomised Controlled Study to Assess the Effectiveness of Two Patient Management Strategies in Mild to Moderate Ulcerative Colitis—The OPTIMISE Study" Journal of Clinical Medicine 13, no. 17: 5147. https://doi.org/10.3390/jcm13175147
APA StyleDanese, S., Fiorino, G., Vicaut, E., Paridaens, K., Ugur, A., Clark, B., Vanasek, T., Stepek, D., D’Amico, F., West, R., Gilissen, L. P. L., Wisniewska Jarosinka, M., Drobinski, P., Fronik, G., Fic, M., Walczak, M., Kowalski, M., Korczowski, B., Wiatr, M., & Peyrin-Biroulet, L. (2024). Clinical Trial: A Pragmatic Randomised Controlled Study to Assess the Effectiveness of Two Patient Management Strategies in Mild to Moderate Ulcerative Colitis—The OPTIMISE Study. Journal of Clinical Medicine, 13(17), 5147. https://doi.org/10.3390/jcm13175147








