Population Analysis of Masseter Muscle Tension Using Shear Wave Ultrasonography across Different Disease States
Abstract
1. Introduction
2. Method
2.1. Inclusion and Exclusion Criteria
- Age range: Patients aged between 20 and 70 years, covering a broad demographic to evaluate age-related trends in masseter muscle stiffness.
- Presence of TMJ pain: Individuals experiencing temporomandibular joint (TMJ) pain or TMJ-related pain radiating to the head and neck, localized around and extending from the TMJ.
- Diagnosis of TMJ dysfunction: Patients with clinical signs of TMJ dysfunction, such as disc displacement, arthrosis, or exudate in the joint, as confirmed by ultrasonographic examination.
- Masseter muscle evaluation: Participants who are suitable for shear wave elastography (SWE) to assess masseter muscle stiffness, ensuring that their condition allows for accurate ultrasonographic measurement.
- Symptomatic individuals: Patients reporting persistent TMJ pain, even in the absence of visible ultrasonographic changes, to explore potential psychosomatic contributions to muscle stiffness.
- Patients with inflammatory diseases or chronic infections (e.g., Borrelia infections)—as factors which can alter muscle tension values.
- Patients with recent trauma—trauma can change muscle tension with mechanical influence on muscle structure.
- Patients presenting with diagnosed or visible neurological diseases—changes in efferent nervous signals influences muscle tension.
- Patients who had undergone surgery in the facial/neck region—iatrogenic muscle trauma can influence muscle structure.
- Patients using analgesics or muscle relaxants—drugs which directly influence muscle function changes muscle tension values.
- Patients who had recently undergone physiotherapy—physiotherapy is usually directed towards muscle tension decrease.
2.2. Examination Procedure
2.3. Muscle Tension Measurement
2.4. Data Collection and Analysis
2.5. Studied Patients Group
3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sarnat, B.G.; Laskin, D.M. The Temporomandibular Joint: A Biological Basis for Clinical Practice, 4th ed.; WB Saunders: Philadelphia, PA, USA, 1992; pp. 78–163. [Google Scholar]
- Graber, R.; Rakosi, T. Petrovic. Functional analysis- examination of temporomandibular joint and condylar movement. In Dentofacial Orthopedics with Functional Appliances, 2nd ed.; Mosby: St. Louis, MO, USA, 2009; pp. 135–140. [Google Scholar]
- Athanasiou, A.E. Orthodontics and craniomandibular disorders. In Textbook of Orthodontics, 2nd ed.; Samire, B., Ed.; Saunders: Philadelphia, PA, USA, 2003; pp. 478–493. [Google Scholar]
- Irby, W.B.; Baldwin, K.H. Emergencies and Urgent Complications in Dentistry; The C. V. Mosby Company: St. Louis, MO, USA, 1965; pp. 167–173. [Google Scholar]
- Donovan, T.E.; Becker, W.; Brodine, A.H.; Burgess, J.O.; Cronin, R.J.; Summitt, J.B. Annual review of selected dental literature: Report of the Committee on Scientific Investigation of the American Academy of Restorative Dentistry. J. Prosthet. Dent. 2007, 98, 36–67. [Google Scholar] [CrossRef]
- Ahlberg, J.; Nikkilä, H.; Könönen, M.; Partinen, M.; Lindholm, H.; Sarna, S.; Savolainen, A. Associations of perceived pain and painless TMD-relatedsymptoms with alexithymia and depressive mood in media personnel with or without irregular shift work. Acta Odontol. Scand. 2004, 62, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Parker, M.W. A dynamic model of etiology in temporomandibular disorders. J. Am. Dent. Assoc. 1990, 120, 283–290. [Google Scholar] [CrossRef]
- Gianniri, A.I.; Melsen, B.; Nielsen, L.; Athanasiou, A.E. Occlusal contacts in maximum intercuspation and craniomandibular dysfunction in 16 to 17 year old adolescents. J. Oral. Rehabil. 1991, 18, 49–59. [Google Scholar] [CrossRef]
- Gauer, R.L.; Semidey, M.J. Diagnosis and Treatment of temporomandibular disorders. Am. Fam. Physician 2015, 91, 378–386. [Google Scholar] [PubMed]
- Tanaka, E.; Detamore, M.S.; Mercuri, L.G. Degenerative disorders of the temporomandibular joint: Etiology, diagnosis, and treatment. J. Dent. Res. 2008, 87, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Wieckiewicz, M.; Boening, K.; Wiland, P.; Shiau, Y.Y.; Paradowska-Stolarz, A. Reported concepts for the treatment modalities and pain management of temporomandibular disorders. J. Headache Pain. 2015, 16, 106. [Google Scholar] [CrossRef] [PubMed]
- Widmer, C.G.; English, A.W.; Morris-Wiman, J. Developmental and functional considerations of masseter muscle partitioning. Arch. Oral Biol. 2007, 52, 305–308. [Google Scholar] [CrossRef]
- Ingervall, B.; Helkimo, E. Masticatory muscle force and facial morphology in man. Arch. Oral. Biol. 1978, 23, 203–206. [Google Scholar] [CrossRef]
- Olchowy, A.; Wieckiewicz, M.; Winocur, E.; Dominiak, M.; Dekkers, I.; Łasecki, M.; Olchowy, C. Great potential of ultrasound elastography for the assessment of the masseter muscle in patients with temporomandibular disorders. Syst. Review. Dento Maxillo Facial Radiol. 2020, 49, 20200024. [Google Scholar] [CrossRef]
- Almukhtar, R.M.; Fabi, S.G. A masseter muscle and its role in facial contouring, aging, and quality of life. Plast. Reconstr. Surg. 2019, 143, 39e–48e. [Google Scholar] [CrossRef] [PubMed]
- Taljanovic, M.S.; Gimber, L.H.; Becker, G.W.; Latt, L.D.; Klauser, A.S.; Melville, D.M.; Gao, L.; Witte, R.S. Shear-Wave elastography: Basic physics and musculoskeletal applications. Radiographics 2017, 37, 855–870. [Google Scholar] [CrossRef]
- Arda, K.; Ciledag, N.; Aktas, E.; Aribas, B.K.; Köse, K. Quantitative assessment of normal soft-tissue elasticity using shear-wave ultrasound elastography. AJR Am. J. Roentgenol. 2011, 197, 532–536. [Google Scholar] [CrossRef] [PubMed]
- Obuchowicz, R.; Ambrozinski, L.; Kohut, P. Influence of Load and Transducer Bandwidth on the Repeatability of In Vivo Tendon Stiffness Evaluation Using Shear Wave Elastography. J. Diagn. Med. Sonogr. 2020, 36, 409–420. [Google Scholar] [CrossRef]
- Romano, A.; Staber, D.; Grimm, A.; Kronlage, C.; Marquetand, J. Limitations of muscle ultrasound shear wave elastography for clinical routine—Positioning and muscle selection. Sensors 2021, 21, 8490. [Google Scholar] [CrossRef] [PubMed]
- Olchowy, C.; Grzech-Leśniak, K.; Hadzik, J.; Olchowy, A.; Łasecki, M. Monitoring of changes in masticatory muscle stiffness after gum chewing using shear wave elastography. J. Clin. Med. 2021, 10, 2480. [Google Scholar] [CrossRef] [PubMed]
- Aydin Aksu, S.; Kursoglu, P.; Turker, I.; Baskak, F.; Ozen Sutuven, E.; Meric, K.; Cabbar, F. Dynamic quantitative imaging of the masseter muscles in bruxism patients with myofascial pain: Could it be an objective biomarker? J. Pers. Med. 2023, 13, 1467. [Google Scholar] [CrossRef] [PubMed]
- Pihut, M.; Gala, A.; Obuchowicz, R.; Chmura, K. Influence of ultrasound examination on diagnosis and treatment of temporomandibular disorders. J. Clin. Med. 2022, 11, 1202. [Google Scholar] [CrossRef] [PubMed]
- Alfuraih, A.M.; O’Connor, P.; Hensor, E.; Tan, A.L.; Emery, P.; Wakefield, R.J. The effect of unit, depth, and probe load on the reliability of muscle shear wave elastography: Variables affecting reliability of SWE. J. Clin. Ultrasound 2018, 46, 108–115. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, M.; Lee, H. The measurement of stiffness for major muscles with shear wave elastography and myoton: A quantitative analysis study. Diagnostics 2021, 11, 524. [Google Scholar] [CrossRef]
- Inami, T.; Kawakami, Y. Assessment of individual muscle hardness and stiffness using ultrasound elastography. J. Phys. Fit. Sports Med. 2016, 5, 313–317. [Google Scholar] [CrossRef][Green Version]
- Haueise, A.; Le Sant, G.; Eisele-Metzger, A.; Dieterich, A.V. Is musculoskeletal pain associated with increased muscle stiffness? Evidence map and critical appraisal of muscle measurements using shear wave elastography. Clin. Physiol. Funct. Imaging 2024, 44, 187–204. [Google Scholar] [CrossRef]
- Kolding, L.T.; Do, T.P.; Ewertsen, C.; Schytz, H.W. Muscle stiffness in tension-type headache patients with pericranial tenderness: A shear wave elastography study. Cephalalgia Rep. 2018, 1, 2515816318760293. [Google Scholar] [CrossRef]
- Špalj, S.; Šlaj, M.; Athanasiou, A.E.; Žak, I.; Šimunović, M.; Šlaj, M. Temporomandibular disorders and orthodontic treatment need in orthodontically untreated children and adolescents. Coll. Antropol. 2015, 39, 151–158. [Google Scholar] [PubMed]
- Karkazi, F.; Özdemir, F. Temporomandibular Disorders: Fundamental Questions and Answers. Turk. J. Orthod. 2020, 33, 246–252. [Google Scholar] [CrossRef]
- Rugh, J.D.; Solberg, W.K. Oral health status in the United states: Temporomandibular disorders. J. Dent. Educ. 1985, 49, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Gupta, D.S.; Pal, U.S.; Jurel, S.K. Etiological factors of temporomandibular joint disorders. Natl. J. Maxillofac. Surg. 2011, 2, 116–119. [Google Scholar] [CrossRef]
- Olchowy, A.; Wieckiewicz, M.; Malysa, A.; Olchowy, C. Determination of Reference Values of the Masseter Muscle Stiffness in Healthy Adults Using Shear Wave Elastography. Int. J. Environ. Res. Public Health 2021, 18, 9371. [Google Scholar] [CrossRef]
- Dietsch, M.; Clark, H.M.; Steiner, J.N.; Solomon, N.P. Effects of age, sex, and body position on orofacial muscle tone in healthy adults. J. Speech Lang. Hear. Res. 2015, 58, 1145–1150. [Google Scholar] [CrossRef]
- Voscopoulos, C.; Lema, M. When does acute pain become chronic? Br. J. Anaest 2010, 105, 69–85. [Google Scholar] [CrossRef]
- Miettinen, O.; Lahti, S.; Sipilä, K. Psychosocial aspects of temporomandibular disorders and oral health-related quality-of-life. Acta Odontol. Scand. 2012, 70, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, D.M.A.O.; Costa, Y.M.; de Quevedo, H.M.; Bonjardim, L.R.; Conti, P.C.R. Experimental psychological stress on quantitative sensory testing response in patients with temporomandibular disorders. J. Oral. Facial Pain. Headache 2018, 32, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Wieckiewicz, M.; Grychowska, N.; Wojciechowski, K.; Pelc, A.; Augustyniak, M.; Sleboda, A.; Zietek, M. Prevalence and correlation between TMD based on RDC/TMD diagnoses, oral parafunctions and psychoemotional stress in Polish university students. BioMed Res. Int. 2014, 2014, 472346. [Google Scholar] [CrossRef] [PubMed]
- Shamim, T. The psychosomatic disorders pertaining to dental practice with revised working type classification. Korean J. Pain 2014, 27, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Sruthi, V.K.; Jimsha, S.V.; Srinivasan, J.M. Prevalence of Depression, Anxiety and Stress in Chronic Temporomandibular Joint Disorders Patients. J. Depress. Anxiety 2018, 7, 4. [Google Scholar]
- Reissmann, D.R.; John, M.T.; Seedorf, H.; Doering, S.; Schierz, O. Temporomandibular disorder pain is related to the general disposition to be anxious. J. Oral. Facial Pain. Headache 2014, 28, 322–330. [Google Scholar] [CrossRef]
- Giannakopoulos, N.N.; Keller, L.; Rammelsberg, P.; Kronmüller, K.T.; Schmitter, M. Anxiety and depression in patients with chronic temporomandibular pain and in controls. J. Dent. 2010, 38, 369–376. [Google Scholar] [CrossRef]
- Tu, H.; Li, Y.L. Inflammation balance in skeletal muscle damage and repair. Front. Immunol. 2023, 14, 1133355. [Google Scholar] [CrossRef]
- Alfuraih, A.M.; Tan, A.L.; O’Connor, P.; Emery, P.; Wakefield, R.J. Muscle stiffness in rheumatoid arthritis is not altered or associated with muscle weakness: A shear wave elastography study. Mod. Rheumatol. 2020, 30, 617–625. [Google Scholar] [CrossRef]
- Romero-Reyes, M.; Uyanik, J.M. Orofacial pain management: Current perspectives. J. Pain. Res. 2014, 7, 99–115. [Google Scholar] [CrossRef]
- Michelotti, A.; Iodice, G.; Vollaro, S.; Steenks, M.H.; Farella, M. Evaluation of the short-term effectiveness of education versus an occlusal splint for the treatment of myofascial pain of the jaw muscles. J. Am. Dent. Assoc. 2012, 143, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Costamagna, D.; Costelli, P.; Sampaolesi, M.; Penna, F. Role of Inflammation in Muscle Homeostasis and Myogenesis. Mediators Inflamm. 2015, 2015, 805172. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Li, M.; Chang, M.; Liu, R.; Qiu, J.; Wang, K.; Deng, C.; Shen, Y.; Zhu, J.; Wang, W.; et al. Inflammation: Roles in Skeletal Muscle Atrophy. Antioxidants 2022, 11, 1686. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wang, Z.-Y.; Zhang, Z.-J.; Shen, S.-H.; Guo, J.-Y.; Guo, Y.-X.; Feng, Y.R.; Zhang, L.; Wen, Y.B.; Zhang, Y.F.; et al. In hamstring muscles of patients with knee osteoarthritis an increased ultrasound shear modulus indicates a permanently elevated muscle tonus. Front. Physiol. 2021, 12, 752455. [Google Scholar] [CrossRef]
- Narici, M.V.; Maffulli, N. Sarcopenia: Characteristics, mechanisms and functional significance. Br. Med. Bull. 2010, 95, 139–159. [Google Scholar] [CrossRef]
- Silva, J.M.; Alabarse, P.V.; Teixeira, V.D.; Freitas, E.C.; de Oliveira, F.H.; Chakr, R.M.; Xavier, R.M. Muscle wasting in osteoarthritis model induced by anterior cruciate ligament transection. PLoS ONE 2018, 13, e0196682. [Google Scholar] [CrossRef]
- Larsson, L.; Degens, H.; Li, M.; Salviati, L.; Lee, Y.I.; Thompson, W.; Kirkland, J.L.; Sandri, M. Sarcopenia: Aging-related loss of muscle mass and function. Physiol. Rev. 2019, 99, 427–511. [Google Scholar] [CrossRef]
- Brown, D.M.; Goljanek-Whysall, K. microRNAs: Modulators of the underlying pathophysiology of sarcopenia? Ageing Res. Rev. 2015, 24 Pt B, 263–273. [Google Scholar] [CrossRef]
- McComas, A.J. Oro-facial muscles: Internal structure, function and ageing. Gerodontology 1998, 15, 3–14. [Google Scholar] [CrossRef]
- Chang, K.V.; Wu, W.T.; Huang, K.C.; Jan, W.H.; Han, D.S. Limb muscle quality and quantity in elderly adults with dynapenia but not sarcopenia: An ultrasound imaging study. Exp. Gerontol. 2018, 108, 54–61. [Google Scholar] [CrossRef]
- Lee, K.; Chon, S. Assessments of Muscle Thickness and Tonicity of the Masseter and Sternocleidomastoid Muscles and Maximum Mouth Opening in Patients with Temporomandibular Disorder. Healthcare 2021, 9, 1640. [Google Scholar] [CrossRef] [PubMed]
- Strini, P.J.; Strini, P.J.; de Souza Barbosa, T.; Gavião, M.B. Assessment of thickness and function of masticatory and cervical muscles in adults with and without temporomandibular disorders. Arch. Oral Biol. 2013, 58, 1100–1108. [Google Scholar] [CrossRef] [PubMed]
- Busse, J.W.; Casassus, R.; Carrasco-Labra, A.; Durham, J.; Mock, D.; Zakrzewska, J.M.; Palmer, C.; Samer, C.F.; Coen, M.; Guevremont, B.; et al. Management of chronic pain associated with temporomandibular disorders: A clinical practice guideline. BMJ 2023, 383, e076227. [Google Scholar] [CrossRef] [PubMed]
- Wright, E.F.; North, S.L. Management and treatment of temporomandibular disorders: A clinical perspective. J. Man. Manip. Ther. 2009, 17, 247–254. [Google Scholar] [CrossRef]
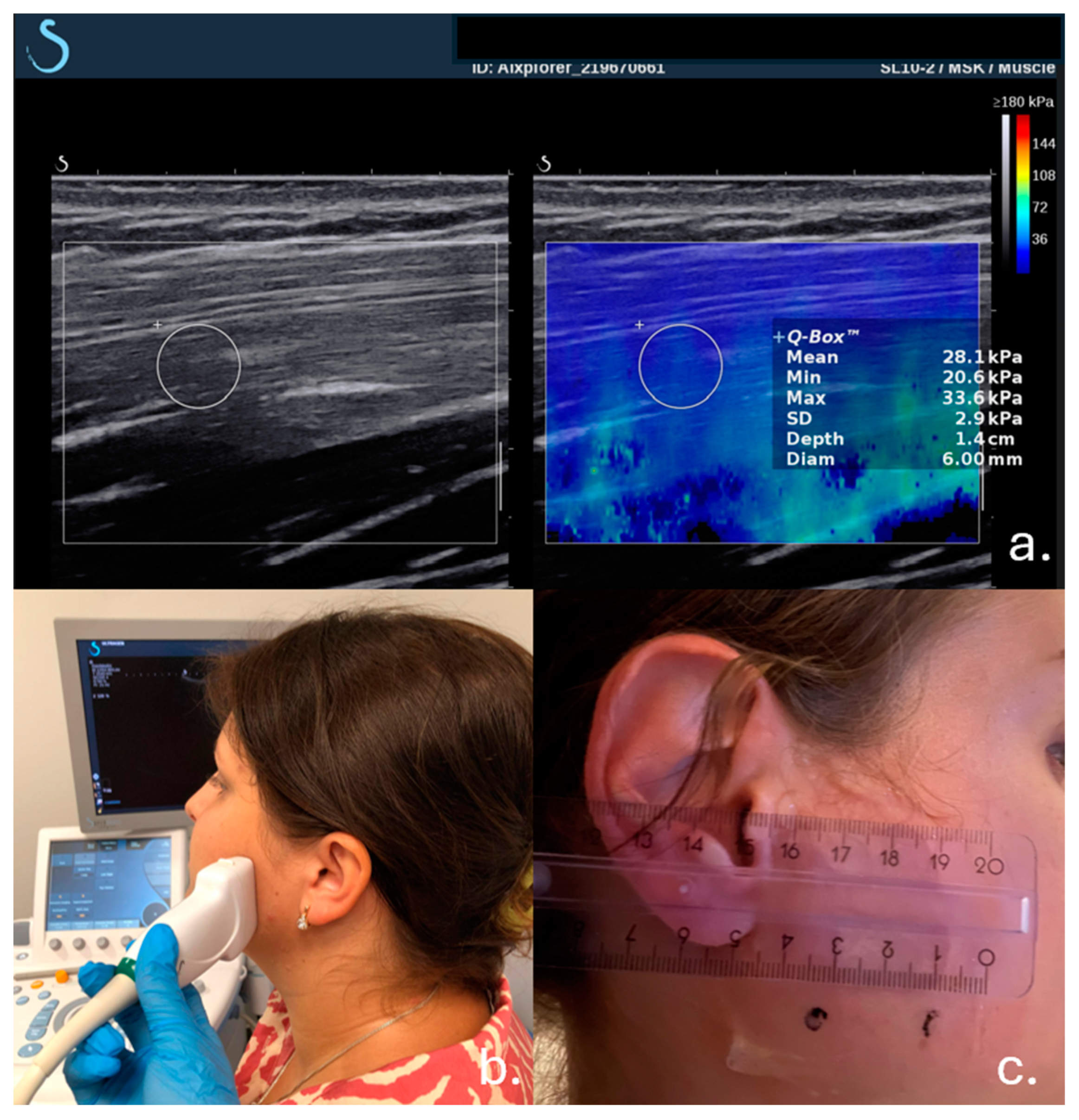
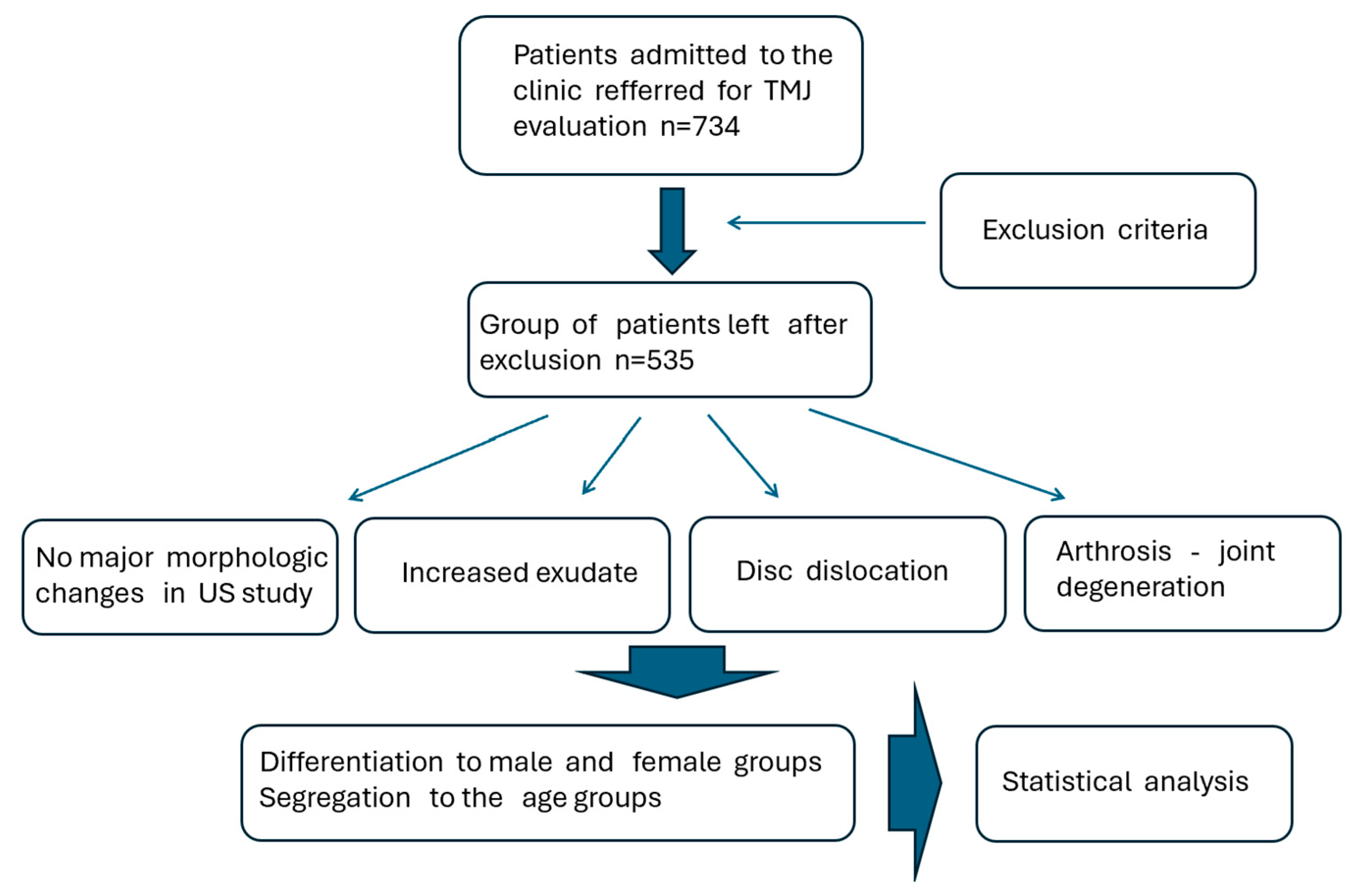


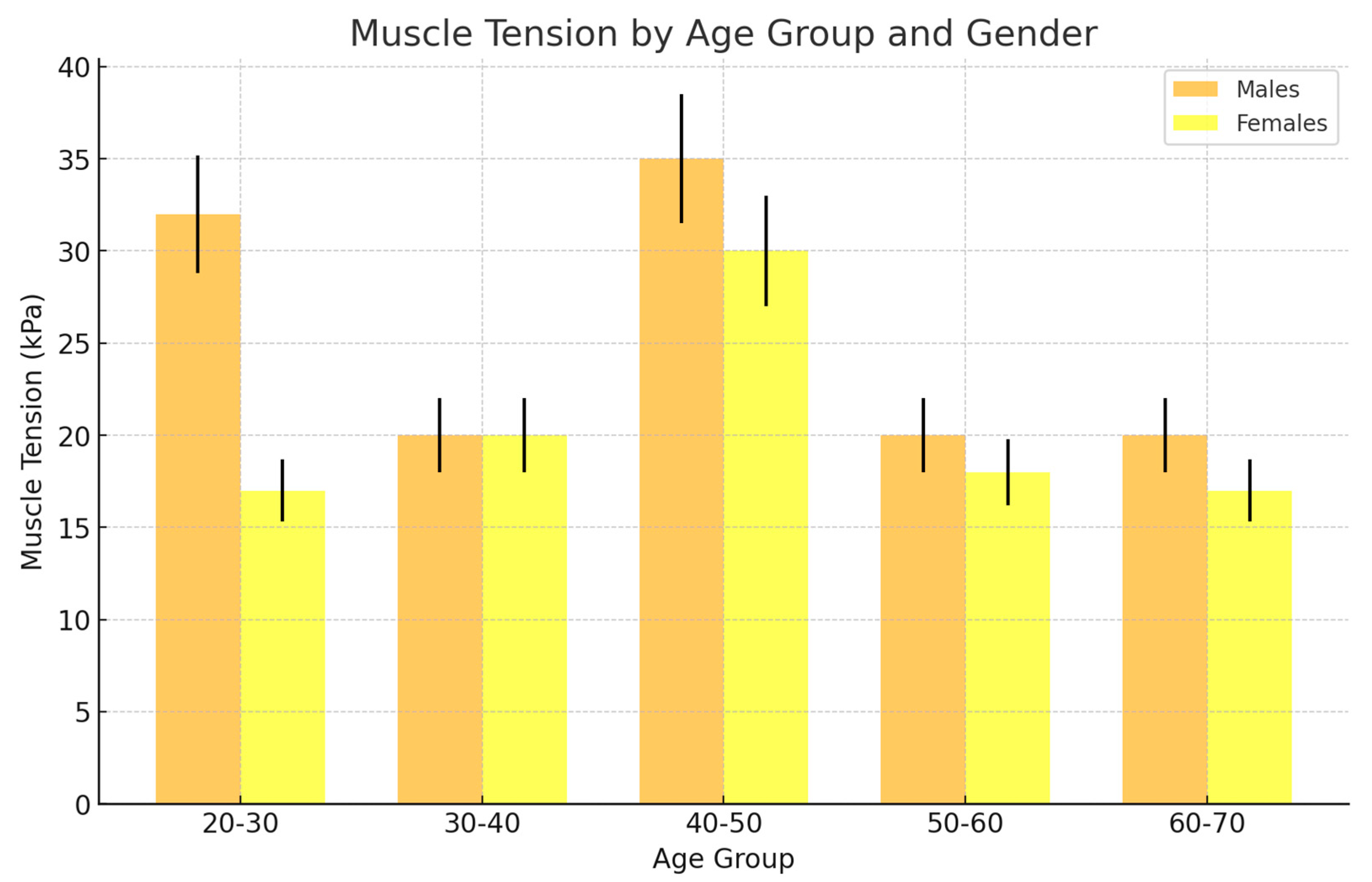
| Age Group | M = 192 Participants (N) | M = 192 Participants (N) | |
|---|---|---|---|
| 1 | 20–30 | 98 | 51 |
| 2 | 30–40 | 65 | 77 |
| 3 | 40–50 | 55 | 34 |
| 4 | 50–60 | 93 | 21 |
| 5 | 60–70 | 32 | 9 |
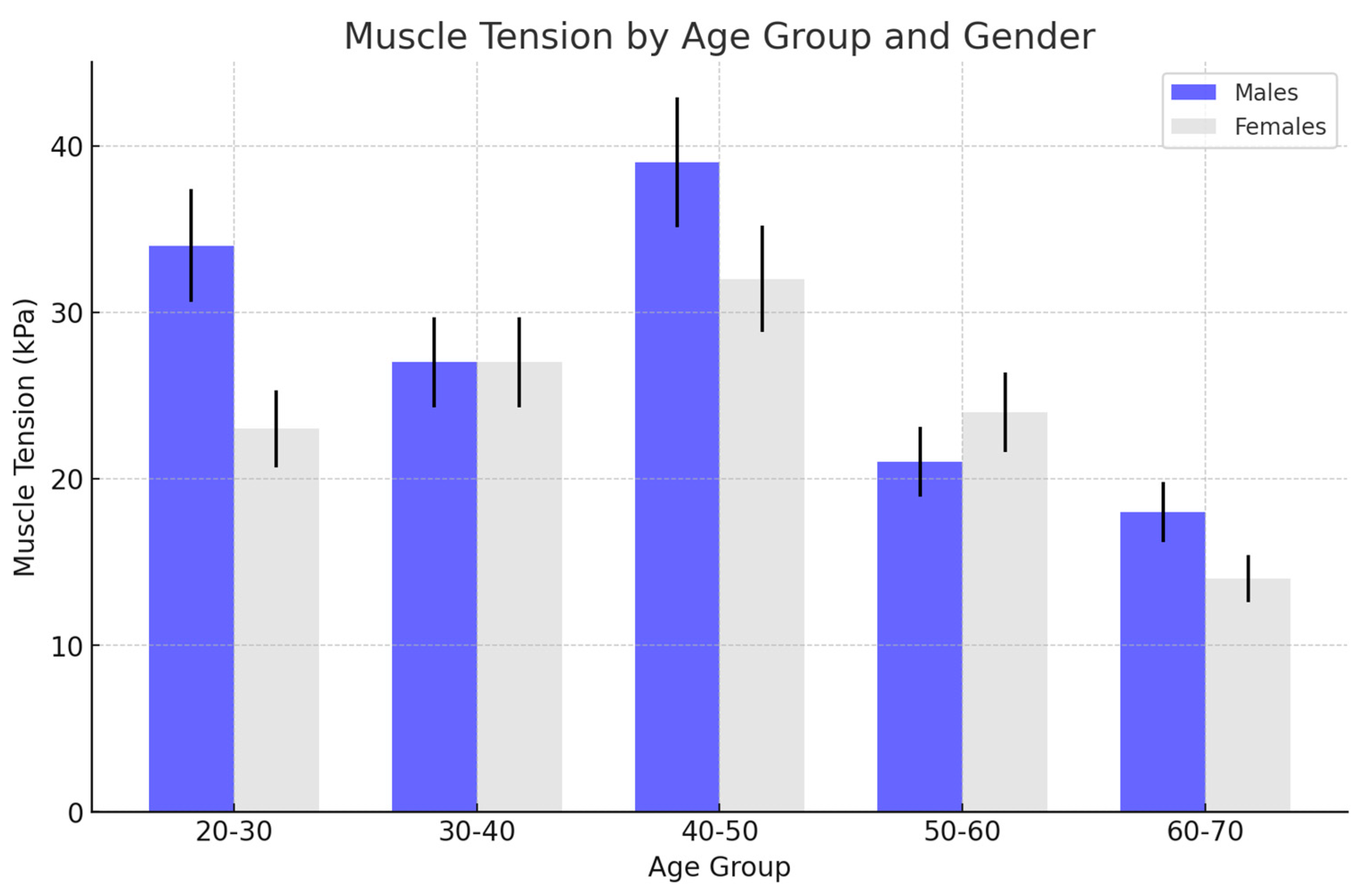
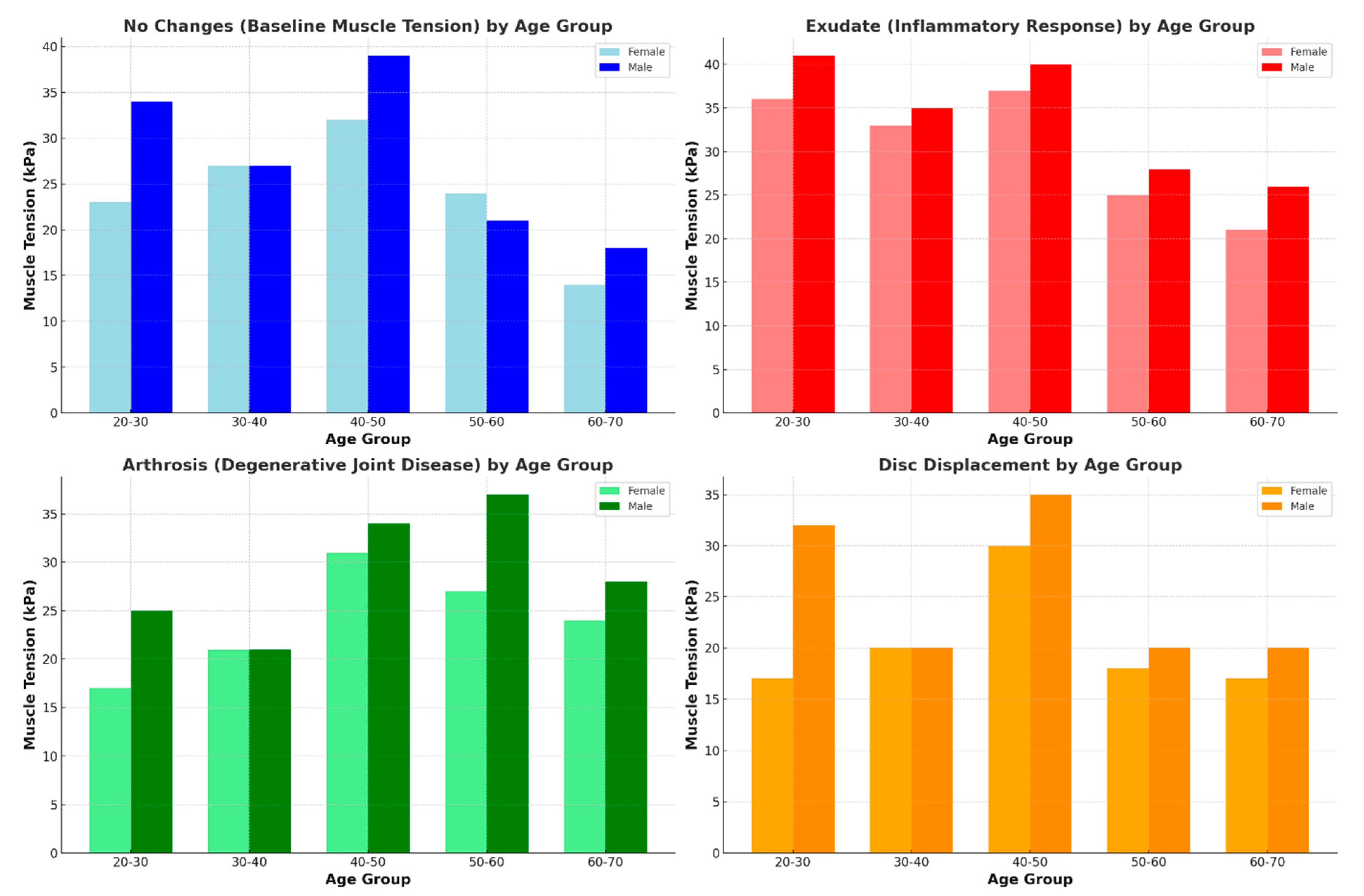
| Age Group | No Changes (kPa) | Exudate (kPa) | Arthrosis (kPa) | Disc Displacement (kPa) |
|---|---|---|---|---|
| 20–30 | 34 ± 5 | 41 ± 3 | 25 ± 6 | 32 ± 3 |
| 30–40 | 27 ± 3 | 35 ± 4 | 21 ± 3 | 20 ± 4 |
| 40–50 | 39 ± 4 | 40 ± 5 | 34 ± 4 | 35 ± 3 |
| 50–60 | 21 ± 2 | 28 ± 5 | 37 ± 4 | 20 ± 4 |
| 60–70 | 18 ± 3 | 26 ± 4 | 28 ± 5 | 20 ± 3 |
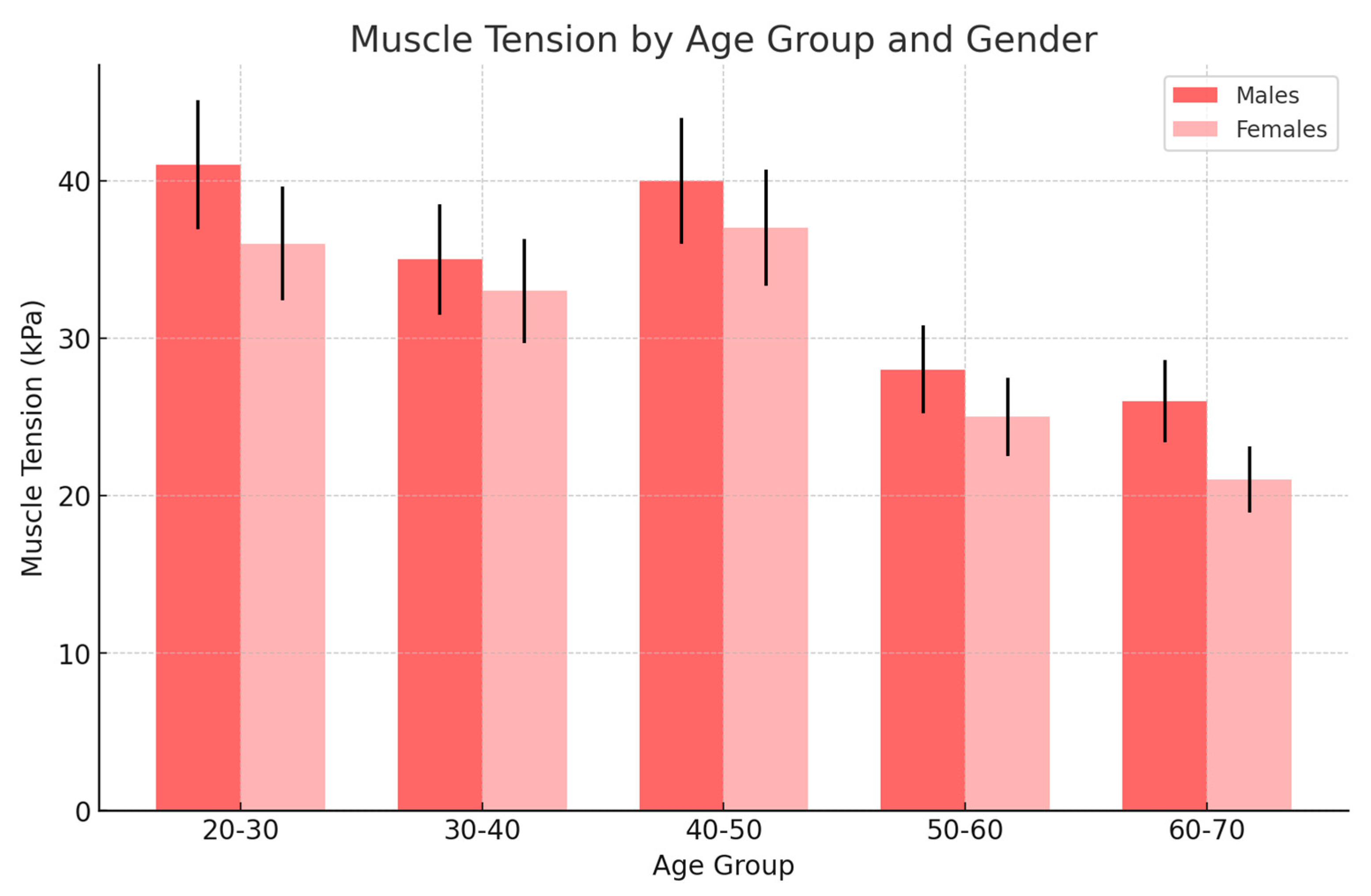
| Age Group | No Changes (kPa) | Exudate (kPa) | Arthrosis (kPa) | Disc Displacement (kPa) |
|---|---|---|---|---|
| 20–30 | 23 ± 4 | 36 ± 4 | 17 ± 5 | 17 ± 3 |
| 30–40 | 27 ± 4 | 33 ± 4 | 21 ± 4 | 20 ± 4 |
| 40–50 | 32 ± 3 | 37 ± 3 | 31 ± 3 | 30 ± 2 |
| 50–60 | 24 ± 4 | 25 ± 5 | 27 ± 4 | 18 ± 4 |
| 60–70 | 14 ± 2 | 21 ± 3 | 24 ± 2 | 17 ± 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obuchowicz, R.; Obuchowicz, B.; Nurzynska, K.; Urbanik, A.; Pihut, M. Population Analysis of Masseter Muscle Tension Using Shear Wave Ultrasonography across Different Disease States. J. Clin. Med. 2024, 13, 5259. https://doi.org/10.3390/jcm13175259
Obuchowicz R, Obuchowicz B, Nurzynska K, Urbanik A, Pihut M. Population Analysis of Masseter Muscle Tension Using Shear Wave Ultrasonography across Different Disease States. Journal of Clinical Medicine. 2024; 13(17):5259. https://doi.org/10.3390/jcm13175259
Chicago/Turabian StyleObuchowicz, Rafal, Barbara Obuchowicz, Karolina Nurzynska, Andrzej Urbanik, and Malgorzata Pihut. 2024. "Population Analysis of Masseter Muscle Tension Using Shear Wave Ultrasonography across Different Disease States" Journal of Clinical Medicine 13, no. 17: 5259. https://doi.org/10.3390/jcm13175259
APA StyleObuchowicz, R., Obuchowicz, B., Nurzynska, K., Urbanik, A., & Pihut, M. (2024). Population Analysis of Masseter Muscle Tension Using Shear Wave Ultrasonography across Different Disease States. Journal of Clinical Medicine, 13(17), 5259. https://doi.org/10.3390/jcm13175259







