Grad-CAM-Based Investigation into Acute-Stage Fluorescein Angiography Images to Predict Long-Term Visual Prognosis of Branch Retinal Vein Occlusion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Deep Learning-Based Prediction of logMAR BCVA in Remission from FA Images
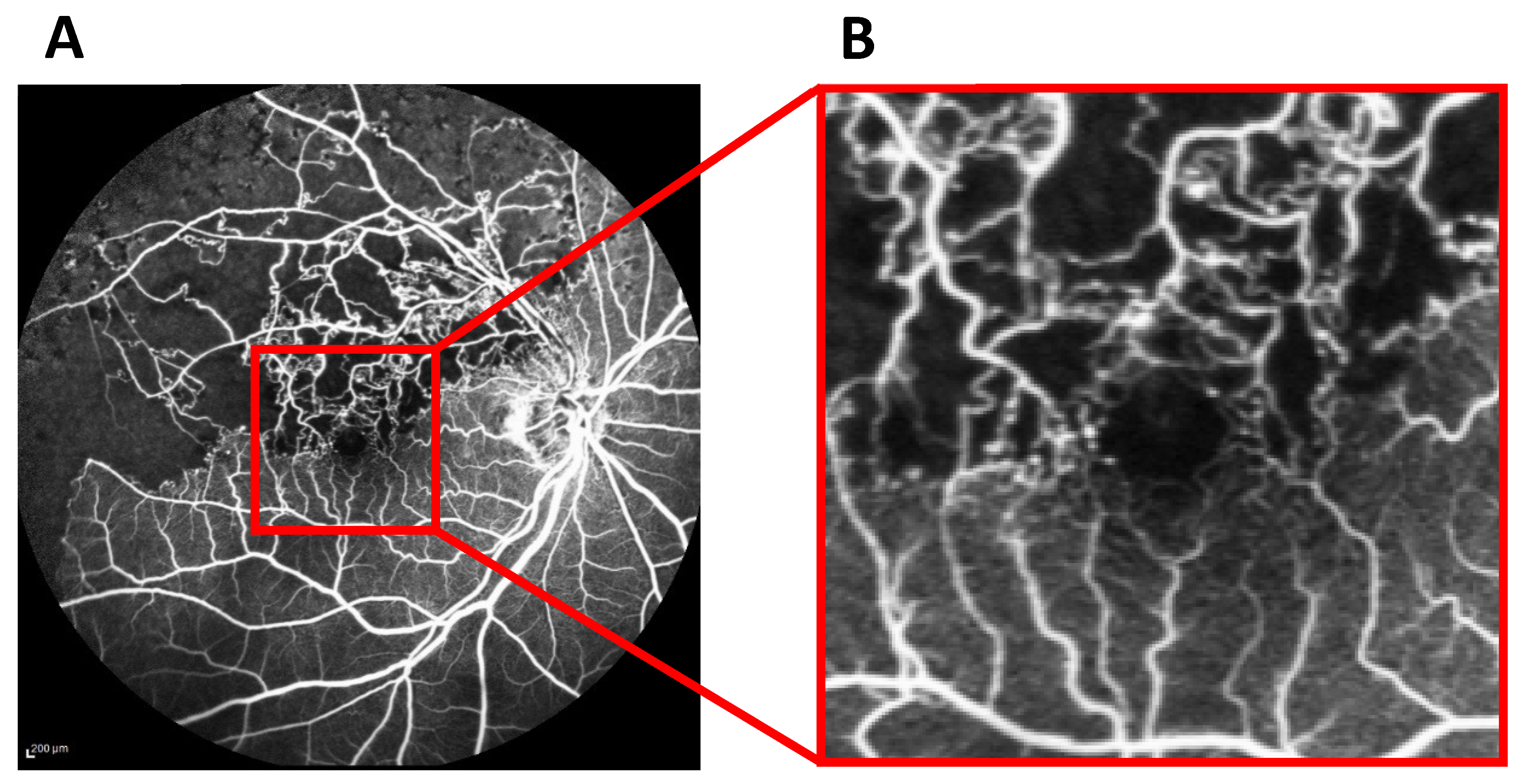
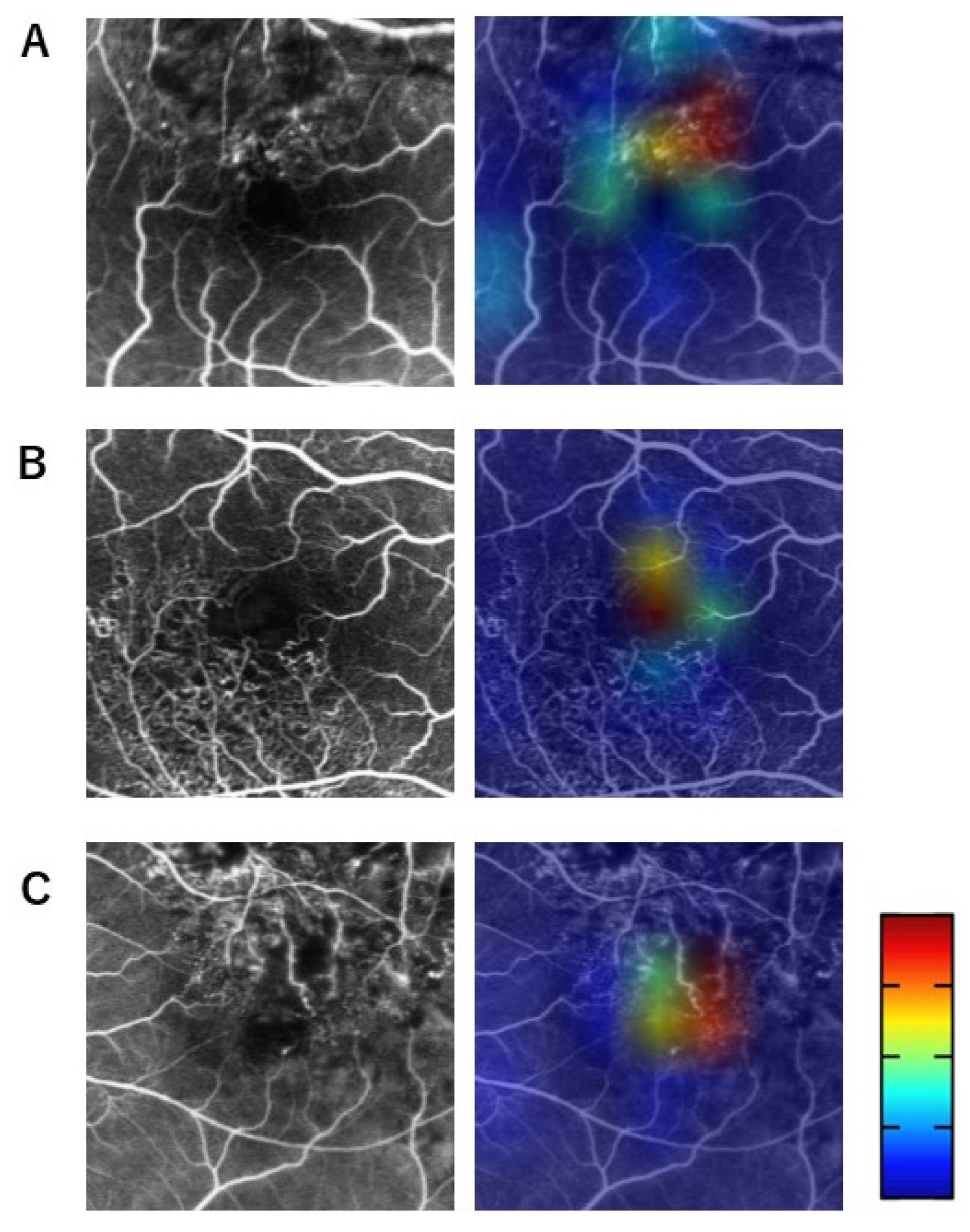
2.3. Visualization of Relevant Areas for BCVA Prediction Using Grad-CAM Heat Maps Merged with Acute-Stage FA Images
2.4. Endpoints and Statistical Analyses
3. Results
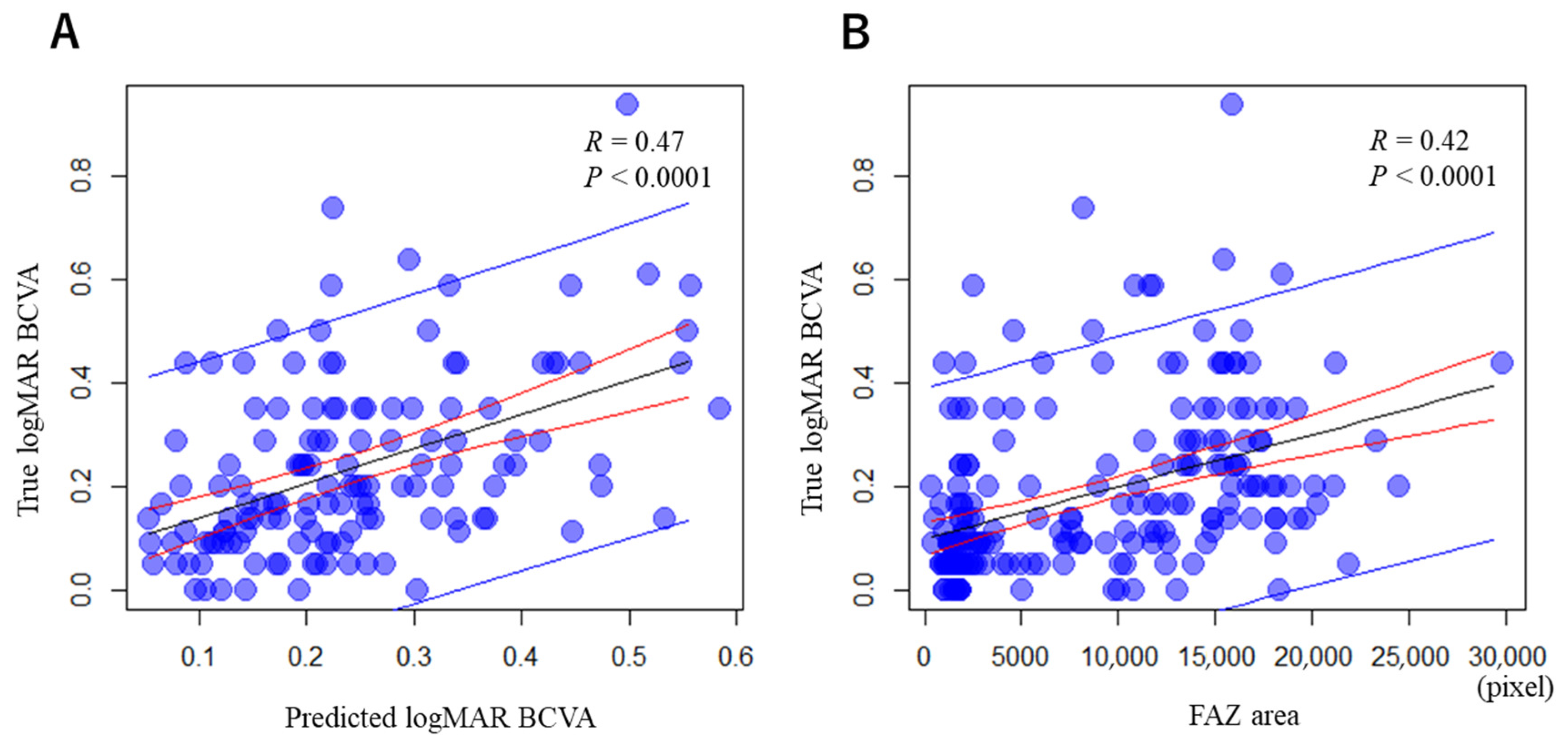
3.1. Correlations between the Predicted and True logMAR BCVAs in Remission, and between the Acute-Stage FAZ Area and True logMAR BCVA in Remission
3.2. Questionnaire on Grad-CAM Heat Maps
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yin, S.; Cui, Y.; Jiao, W.; Zhao, B. Potential prognostic indicators for patients with retinal vein occlusion. Front. Med. 2022, 9, 839082. [Google Scholar] [CrossRef] [PubMed]
- Samara, W.A.; Shahlaee, A.; Sridhar, J.; Khan, M.A.; Ho, A.C.; Hsu, J. Quantitative optical coherence tomography angiography features and visual function in eyes with branch retinal vein occlusion. Am. J. Ophthalmol. 2016, 166, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Parodi, M.B.; Vinstin, F.; Rupe, P.D.; Ravalico, G. Foveal Avascular Zone Macular Branch Retin Vein Occlusion. Int. Ophthalmol. 1995, 19, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, T.; Sato, T.; Hara-Ueno, C.; Fukushima, Y.; Sayanagi, K.; Shiraki, N.; Sawa, M.; Ikuno, Y.; Sakaguchi, H.; Nishida, K. Retinal microvasculature and visual acuity in eyes with branch retinal vein occlusion: Imaging analysis by optical coherence tomography angiography. Investig. Ophthalmol. Vis. Sci. 2017, 58, 2087–2094. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.W.; Yoo, R.; Jo, Y.H.; Kim, H.C. Correlation of microvascular structures on optical coherence tomography angiography with visual acuity in retinal vein occlusion. Retina 2017, 37, 1700–1709. [Google Scholar] [CrossRef] [PubMed]
- Ouederni, M.; Khalifa, M.B.H.; Sassi, H.; Nefaa, F.; Ayed, O.; Cheour, M. Quantitative Analysis of Microvascular Network with Optical Coherence Tomography Angiography and its Correlation with Visual Acuity in Retinal Vein Occlusion. J. Curr. Ophthalmol. 2021, 33, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Winegarner, A.; Wakabayashi, T.; Fukushima, Y.; Sato, T.; Hara-Ueno, C.; Busch, C.; Nishiyama, I.; Shiraki, N.; Sayanagi, K.; Nishida, K.; et al. Changes in retinal microvasculature and visual acuity after antivascular endothelial growth factor therapy in retinal vein occlusion. Investig. Ophthalmol. Vis. Sci. 2018, 59, 2708–2716. [Google Scholar] [CrossRef] [PubMed]
- Ting, D.S.W.; Cheung, C.Y.L.; Lim, G.; Tan, G.S.W.; Quang, N.D.; Gan, A.; Hamzah, H.; Garcia-Franco, R.; San Yeo, I.Y.; Lee, S.Y.; et al. Development and validation of a deep learning system for diabetic retinopathy and related eye diseases using retinal images from multiethnic populations with diabetes. JAMA 2017, 318, 2211–2223. [Google Scholar] [CrossRef] [PubMed]
- Prahs, P.; Radeck, V.; Mayer, C.; Cvetkov, Y.; Cvetkova, N.; Helbig, H.; Märker, D. OCT-based deep learning algorithm for the evaluation of treatment indication with anti-vascular endothelial growth factor medications. Graefes Arch. Clin. Exp. Ophthalmol. 2018, 256, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Treder, M.; Lauermann, J.L.; Eter, N. Automated detection of exudative age-related macular degeneration in spectral domain optical coherence tomography using deep learning. Graefes Arch. Clin. Exp. Ophthalmol. 2018, 256, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Rohm, M.; Tresp, V.; Müller, M.; Kern, C.; Manakov, I.; Weiss, M.; Sim, D.A.; Priglinger, S.; Keane, P.A.; Kortuem, K. Predicting visual acuity by using machine learning in patients treated for neovascular age-related macular degeneration. Ophthalmology 2018, 125, 1028–1036. [Google Scholar] [CrossRef] [PubMed]
- Omodaka, K.; An, G.; Tsuda, S.; Shiga, Y.; Takada, N.; Kikawa, T.; Takahashi, H.; Yokota, H.; Akiba, M.; Nakazawa, T. Classification of optic disc shape in glaucoma using machine learning based on quantified ocular parameters. PLoS ONE 2017, 12, e0190012. [Google Scholar] [CrossRef]
- Fukutsu, K.; Saito, M.; Noda, K.; Murata, M.; Kase, S.; Shiba, R.; Isogai, N.; Asano, Y.; Hanawa, N.; Dohke, M.; et al. A deep learning architecture for vascular area measurement in fundus images. Ophthalmol. Sci. 2021, 1, 100004. [Google Scholar] [CrossRef] [PubMed]
- Fukutsu, K.; Saito, M.; Noda, K.; Murata, M.; Kase, S.; Shiba, R.; Isogai, N.; Asano, Y.; Hanawa, N.; Dohke, M.; et al. Relationship between brachial-ankle pulse wave velocity and fundus arteriolar area calculated using a deep-learning algorithm. Curr. Eye Res. 2022, 47, 1534–1537. [Google Scholar] [CrossRef] [PubMed]
- Selvaraju, R.R.; Cogswell, M.; Das, A.; Vedantam, R.; Parikh, D.; Batra, D. Visual Explanations from Deep Networks via Gradient-Based Localization: Grad-CAM. Int. J. Comput. Vis. 2017, 128, 618–626. [Google Scholar] [CrossRef]
- He, K.; Zhang, X.; Ren, S.; Sun, J. Deep Residual Learning for Image Recognition. In Proceedings of the 2016 IEEE Conference on Computer Vision and Pattern Recognition (CVPR), Las Vegas, NV, USA, 27–30 June 2016; Volume 1, p. 770. [Google Scholar] [CrossRef]
- Poplin, R.; Varadarajan, A.V.; Blumer, K.; Liu, Y.; McConnell, M.V.; Corrado, G.S.; Peng, L.; Webster, D.R. Prediction of cardiovascular risk factors from retinal fundus photographs via deep learning. Nat. Biomed. Eng. 2018, 2, 158–164. [Google Scholar] [CrossRef] [PubMed]

| Questionnaire Item | Selectivity | Kappa Coefficient |
|---|---|---|
| (a) Foveal avascular zone (FAZ) | 62.2% | 89.8% |
| (b) FAZ enlargement | 19.1% | 81.5% |
| (c) Normal retinal vessels adjacent to FAZ | 60.0% | 75.2% |
| (d) Abnormal retinal vessels adjacent to FAZ | 57.2% | 85.4% |
| (e) Normal retinal vessels away from FAZ | 39.5% | 89.0% |
| (f) Abnormal retinal vessels away from FAZ | 24.2% | 80.4% |
| (g) Non-perfusion area (NPA) adjacent to FAZ | 12.9% | 78.8% |
| (h) NPA away from FAZ | 9.1% | 88.4% |
| (i) Unknown | 22.0% | 77.7% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saito, M.; Mitamura, M.; Kimura, M.; Ito, Y.; Endo, H.; Katsuta, S.; Kase, M.; Ishida, S. Grad-CAM-Based Investigation into Acute-Stage Fluorescein Angiography Images to Predict Long-Term Visual Prognosis of Branch Retinal Vein Occlusion. J. Clin. Med. 2024, 13, 5271. https://doi.org/10.3390/jcm13175271
Saito M, Mitamura M, Kimura M, Ito Y, Endo H, Katsuta S, Kase M, Ishida S. Grad-CAM-Based Investigation into Acute-Stage Fluorescein Angiography Images to Predict Long-Term Visual Prognosis of Branch Retinal Vein Occlusion. Journal of Clinical Medicine. 2024; 13(17):5271. https://doi.org/10.3390/jcm13175271
Chicago/Turabian StyleSaito, Michiyuki, Mizuho Mitamura, Mayuko Kimura, Yuki Ito, Hiroaki Endo, Satoshi Katsuta, Manabu Kase, and Susumu Ishida. 2024. "Grad-CAM-Based Investigation into Acute-Stage Fluorescein Angiography Images to Predict Long-Term Visual Prognosis of Branch Retinal Vein Occlusion" Journal of Clinical Medicine 13, no. 17: 5271. https://doi.org/10.3390/jcm13175271





