The Effectiveness of the Surgical Correction of Vesicoureteral Reflux on Febrile Urinary Tract Infections after a Kidney Transplant: A Single-Center Retrospective Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Methods
2.2. Statistical Analysis
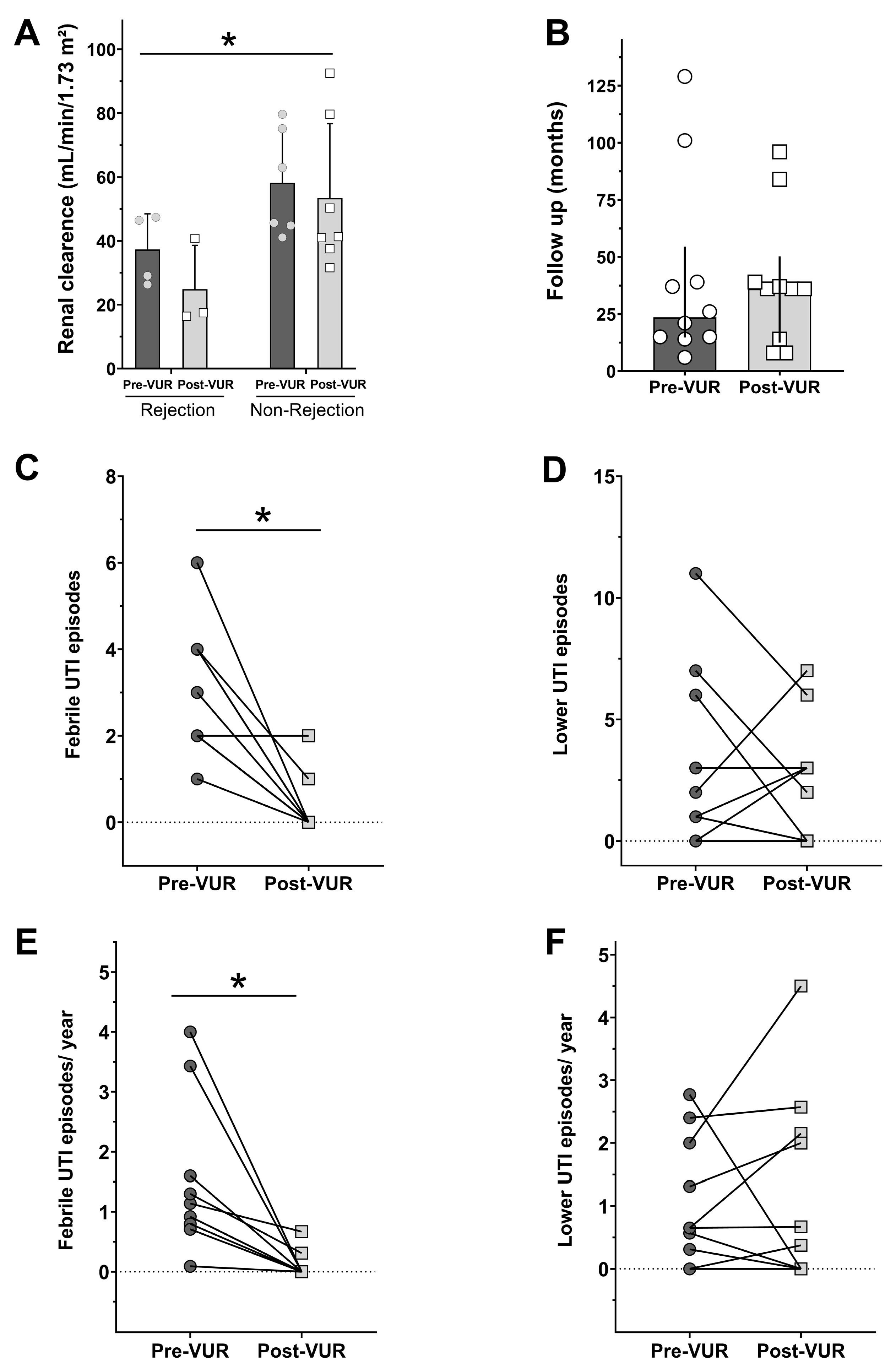
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abecassis, M.; Bartlett, S.T.; Collins, A.J.; Davis, C.L.; Delmonico, F.L.; Friedewald, J.J.; Hays, R.; Howard, A.; Jones, E.; Leichtman, A.B.; et al. Kidney transplantation as primary therapy for end-stage renal disease: A National Kidney Foundation/Kidney Disease Outcomes Quality Initiative (NKF/KDOQITM) conference. Clin. J. Am. Soc. Nephrol. 2008, 3, 471–480. [Google Scholar] [CrossRef]
- Kostro, J.; Hellmann, A.; Kobiela, J.; Skóra, I.; Lichodziejewska-Niemierko, M.; Dębska-Ślizień, A.; Śledziński, Z. Quality of life after kidney transplantation: A prospective study. Transplant. Proc. 2016, 48, 50–54. [Google Scholar] [CrossRef]
- Memikoğlu, K.; Keven, K.; Şengül, S.; Soypaçaci, Z.; Ertürk, S.; Erbay, B. Urinary tract infections following renal transplantation: A single-center experience. Transplant. Proc. 2007, 39, 3131–3134. [Google Scholar] [CrossRef]
- Chuang, P.; Parikh, C.R.; Langone, A. Urinary tract infections after renal transplantation: A retrospective review at two US transplant centers. Clin. Transplant. 2005, 19, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Meena, P.; Bhargava, V.; Rana, D.S.; Bhalla, A.K. Urinary tract infection in renal transplant recipient: A clinical comprehensive review. Saudi J. Kidney Dis. Transplant. 2021, 32, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Brescacin, A.; Iesari, S.; Guzzo, S.; Alfieri, C.M.; Darisi, R.; Perego, M.; Puliatti, C.; Ferraresso, M.; Favi, E. Allograft vesicoureteral reflux after kidney transplantation. Medicina 2022, 58, 81. [Google Scholar] [CrossRef]
- Capozza, N.; Caione, P. Vesicoureteral reflux: Surgical and endoscopic treatment. Pediatr. Nephrol. 2007, 22, 1261–1265. [Google Scholar] [CrossRef][Green Version]
- Dogan, H.S.; Bozaci, A.C.; Ozdemir, B.; Tonyali, S.; Tekgul, S. Ureteroneocystostomy in primary vesicoureteral reflux: Critical retrospective analysis of factors affecting the postoperative urinary tract infection rates. Int. Urol 2014, 40, 539–545. [Google Scholar] [CrossRef]
- Khoury, A.E.; Bägli, D.J. Vesicoureteral Reflux. In Campbell Walsh Urology, 11th ed.; Wein, A.J., Kavoussi, L.R., Novick, A.C., Partin, A.W., Peters, C.A., Eds.; Saunders: Philadelphia, PA, USA, 2016; Chapter 137. [Google Scholar]
- Mastrosimone, S.; Pignata, G.; Maresca, M.C.; Calconi, G.; Rabassini, A.; Butini, R.; Fandella, A.; Di Falco, G.; Chiara, G.; Caldato, C. Clinical significance of vesicoureteral reflux after kidney transplantation. Clin. Nephrol. 1993, 40, 38–45. [Google Scholar]
- Dinckan, A.; Aliosmanoglu, I.; Kocak, H.; Gunseren, F.; Mesci, A.; Ertug, Z.; Yucel, S.; Suleymanlar, G.; Gurkan, A. Surgical correction of vesico-ureteric reflux for recurrent febrile urinary tract infections after kidney transplantation. BJU Int. 2013, 112, E366–E371. [Google Scholar] [CrossRef]
- Abbott, K.C.; Swanson, S.; Richter, E.R.; Bohen, E.M.; Agodoa, L.Y.; Peters, T.G.; Barbour, G.; Lipnick, R.; Cruess, D.F. Late urinary tract infection after renal transplantation in the United States. Am. J. Kidney Dis. 2004, 44, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Roig, M.L.; Kirsch, A.J. Urinary tract infection in the setting of vesicoureteral reflux. F1000Research 2016, 5, 1552. [Google Scholar] [CrossRef] [PubMed]
- Van Batavia, J.P.; Ahn, J.J.; Fast, A.M.; Combs, A.J.; Glassberg, K.I. Prevalence of urinary tract infection and vesicoureteral reflux in children with lower urinary tract dysfunction. J. Urol. 2013, 190, 1495–1500. [Google Scholar] [CrossRef]
- Sui, W.; Lipsky, M.J.; Matulay, J.T.; Robins, D.J.; Onyeji, I.C.; James, M.B.; Theofanides, M.C.; Wenske, S. Timing and predictors of early urologic and infectious complications after renal transplant: An analysis of a New York statewide database. Exp. Clin. Transplant. 2018, 16, 665–670. [Google Scholar] [CrossRef]
- Neuhaus, T.J.; Schwöbel, M.; Schlumpf, R.; Offner, G.; Leumann, E.; Willi, U. Pyelonephritis and vesicoureteral reflux after renal transplantation in young children. J. Urol. 1997, 157, 1400–1403. [Google Scholar] [CrossRef]
- Yucel, S.; Akin, Y.; Celik, O.; Erdogru, T.; Baykara, M. Endoscopic vesicoureteral reflux correction in transplanted kidneys: Does injection technique matter? J. Endourol. 2010, 24, 1661–1664. [Google Scholar] [CrossRef]
- EAU Guidelines. Presented at the EAU Annual Congress Milan March 2023. Available online: https://uroweb.org/eau-guidelines/citing-usage-republication (accessed on 4 September 2024).
- Cash, H.; Slowinski, T.; Buechler, A.; Grimm, A.; Friedersdorff, F.; Schmidt, D.; Miller, K.; Giessing, M.; Fuller, T.F. Impact of surgeon experience on complication rates and functional outcomes of 484 deceased donor renal transplants: A single-centre retrospective study. BJU Int. 2012, 110, E368–E373. [Google Scholar] [CrossRef] [PubMed]
- Nicolle, L.E.; Gupta, K.; Bradley, S.F.; Colgan, R.; DeMuri, G.P.; Drekonja, D.; Eckert, L.O.; Geerlings, S.E.; Köves, B.; Hooton, T.M.; et al. Clinical Practice Guideline for the Management of Asymptomatic Bacteriuria: 2019 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2019, 68, e83–e110. [Google Scholar] [CrossRef]
- Favi, E.; Spagnoletti, G.; Valentini, A.; Tondolo, V.; Nanni, G.; Citterio, F.; Castagneto, M. Long-term clinical impact of vesicoureteral reflux in kidney transplantation. Transplant. Proc. 2009, 41, 1218–1220. [Google Scholar] [CrossRef]
- Gołębiewska, J.; Dębska-Ślizień, A.; Komarnicka, J.; Samet, A.; Rutkowski, B. Urinary tract infections in renal transplant recipients. Transplant. Proc. 2011, 43, 2985–2990. [Google Scholar] [CrossRef]
- Pellé, G.; Vimont, S.; Levy, P.P.; Hertig, A.; Ouali, N.; Chassin, C.; Arlet, G.; Rondeau, E.; Vandewalle, A. Acute pyelonephritis represents a risk factor impairing long-term kidney graft function. Am. J. Transplant. 2007, 7, 899–907. [Google Scholar] [CrossRef] [PubMed]
- Kamath, N.; John, G.; Neelakantan, N.; Kirubakaran, M.; Jacob, C. Acute graft pyelonephritis following renal transplantation. Transpl. Infect. Dis. 2006, 8, 140–147. [Google Scholar] [CrossRef]
- Giral, M.; Pascuariello, G.; Karam, G.; Hourmant, M.; Cantarovich, D.; Dantal, J.; Blancho, G.; Coupel, S.; Josien, R.; Daguin, P.; et al. Acute graft pyelonephritis and long-term kidney allograft outcome. Kidney Int. 2002, 61, 1880–1886. [Google Scholar] [CrossRef] [PubMed]
- Coulthard, M.G.; Keir, M.J. Reflux nephropathy in kidney transplants, demonstrated by dimercaptosuccinic acid scanning. Transplantation 2006, 82, 205–210. [Google Scholar] [CrossRef]
- Muller, V.; Becker, G.; Delfs, M.; Albrecht, K.-H.; Philipp, T.; Heemann, U. Do urinary tract infections trigger chronic kidney transplant rejection in man? J. Urol. 1998, 159, 1826–1829. [Google Scholar] [CrossRef]
- Ranchin, B.; Chapuis, F.; Dawhara, M.; Canterino, I.; Hadj-Aïssa, A.; Saïd, M.; Parchoux, B.; Dubourg, L.; Pouillaude, J.; Floret, D.; et al. Vesicoureteral reflux after kidney transplantation in children. Nephrol. Dial. Transplant. 2000, 15, 1852–1858. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, A.; Swana, H.; Mathias, R.; Baskin, L.S. Redo ureteroneocystostomy using an extravesical approach in pediatric renal transplant patients with reflux: A retrospective analysis and description of technique. J. Urol. 2006, 176, 1582–1587. [Google Scholar] [CrossRef]
- Duty, B.D.; Conlin, M.J.; Fuchs, E.F.; Barry, J.M. The current role of endourologic management of renal transplantation complications. Adv. Urol. 2013, 2013, 246520. [Google Scholar] [CrossRef]
| All | Female | Male | |
|---|---|---|---|
| N (%) | 10 (100) | 8 (80) | 2 (20) |
| Etiology of chronic renal failure (%) | |||
| Polycystic kidney disease | 1 (10) | - | 1 (50) |
| Diabetes mellitus | 3 (30) | 3 (37.5) | - |
| Chronic glomerulonephritis | 4 (40) | 4 (50) | - |
| Nephrosclerosis | 1 (10) | - | 1 (50) |
| Lupus | 1 (10) | 1 (12.5) | - |
| Age at the time of kidney transplantation (years [mean ± SD]) | 40.3 ± 18.5 | 35.4 ± 17.3 | 60.0 ± 2.8 |
| Graft type (%) | |||
| Live | 1 (10) | 1 (12.5) | - |
| Cadaveric | 9 (90) | 7 (87.5) | 2 (100) |
| Ischemia duration | |||
| Live | |||
| Warm ischemia (s) | 230 | 230 | - |
| Cold ischemia (min) | 75 | 75 | - |
| Cadaveric | |||
| Cold ischemia (h [mean ± SD]) | 19.1 ± 5.5 | 17.7 ± 3.1 | 22.6 ± 10.4 |
| Age at VTU correction surgery (years [mean ± SD]) | 43.3 ± 18.2 | 38.8 ± 17.6 | 61.0 ± 2.8 |
| Number of Patients (%) | |
|---|---|
| Immunosuppressive treatment protocol before VUR correction | |
| PRED + TAC + SIR | 1 (10) |
| PRED + TAC + AZA | 1 (10) |
| PRED + TAC + MIC | 8 (80) |
| Immunosuppressive treatment protocol after VUR correction | |
| PRED + TAC + AZA | 1 (10) |
| PRED + TAC + MIC | 9 (90) |
| Rejection before VUR correction (number of episodes) | |
| 0 | 6 (60) |
| 1 | 4 (40) |
| Rejection after VUR correction (number of episodes) | |
| 0 | 7 (70) |
| 1 | 3 (30) |
| Fisher’s test on rejection pre- and post-VUR | |
| Relative Risk for rejection after VUR correction (95% IC) | 0.8077 (0.3483 to 2.101); p = 0.99 |
| NNT | 9.1 |
| Pre-VUR Surgical Correction | Post-VUR Surgical Correction | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient | Follow-Up (months) | VUR Grade | Lower UTI Episodes | Etiologic Agent | Febrile ITU Episodes | Etiologic Agent | Follow-Up (months) | Lower UTI Episodes | Etiologic Agent | Febrile ITU Episodes | Etiologic Agent | Other Information |
| 01 | 21 | N.I. | 1 | Proteus spp. (1×) | 2 | Escherichia coli (ESBL) (1×); Klebsiella pneumoniae (ESBL) (1×) | 36 | 0 | - | 2 | Escherichia coli (ESBL) (1×); Klebsiella pneumoniae (1×) | Graft rejection pre-VUR correction; Death by neurocryptococcosis |
| 02 | 14 | N.I. | 0 | - | 4 | Escherichia coli (ESBL) (2×); N.I. (2×) | 96 | 3 | Proteus spp. (1×); Klebsiella sp. (1×); Citrobacter sp. (1×) | 0 | - | - |
| 03 | 37 | II | 2 | N.I. (2×) | 4 | Escherichia coli (MDR) (2×); N.I. (2×) | 39 | 7 | Klebsiella sp. (1×); Escherichia coli (5×), (MDR) (1×) | 1 | N.I. | - |
| 04 | 26 | N.I. | 6 | Escherichia coli (ESBL) (5×); Klebsiella sp. (1×) | 2 | N.I. (2×) | 84 | 0 | - | 0 | - | Graft rejection pre-and post-VUR correction; Death by COVID-19 |
| 05 | 15 | IV | 3 | Klebsiella sp. (3×) | 1 | N.I. (1×) | 19 | 3 | Escherichia coli (3×) | 0 | - | Death by COVID-19 |
| 06 | 15 | III | 0 | - | 2 | N.I. (2×) | 8 | 0 | - | 0 | - | Graft rejection pre-and post-VUR correction; Graft loss (transplant glomerulopathy)–started hemodialysis |
| 07 | 101 | IV | 11 | Escherichia coli (7×); (MDR) (1×); N.I. (3×) | 6 | Escherichia coli (MDR) (2×); Enterobacter sp. (1×); N.I. (3×) | 36 | 6 | Escherichia coli (ESBL) (3×); Morganella morganii (3×) | 0 | - | - |
| 08 | 6 | IV | 1 | Escherichia coli (1×); | 2 | Klebsiella sp. (ESBL) (2×) | 8 | 3 | Klebsiella pneumoniae (3×) | 0 | - | Graft rejection pre-and post-VUR correction; Graft loss (rejection) started hemodialysis |
| 09 | 39 | V | 1 | Raoultella sp. (MDR) (1×) | 3 | Escherichia coli (1×); N.I. (2×) | 37 | 0 | - | 0 | - | - |
| 10 | 129 | III (right kidney) # | 7 | Escherichia coli (4×) (ESBL) (1×); (MDR) (1×); N.I. (1×) | 1 | Escherichia coli (ESBL) (1×) | 36 | 2 | Escherichia coli (ESBL) (1×), (MDR) (1×) | 0 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varaschin, A.E.; Gomar, G.G.; Rocco, A.M.; Hokazono, S.R.; Garlet, Q.I.; Oliveira, C.S. The Effectiveness of the Surgical Correction of Vesicoureteral Reflux on Febrile Urinary Tract Infections after a Kidney Transplant: A Single-Center Retrospective Study. J. Clin. Med. 2024, 13, 5295. https://doi.org/10.3390/jcm13175295
Varaschin AE, Gomar GG, Rocco AM, Hokazono SR, Garlet QI, Oliveira CS. The Effectiveness of the Surgical Correction of Vesicoureteral Reflux on Febrile Urinary Tract Infections after a Kidney Transplant: A Single-Center Retrospective Study. Journal of Clinical Medicine. 2024; 13(17):5295. https://doi.org/10.3390/jcm13175295
Chicago/Turabian StyleVaraschin, Andre E., Gabriella G. Gomar, Amanda M. Rocco, Silvia R. Hokazono, Quelen I. Garlet, and Cláudia S. Oliveira. 2024. "The Effectiveness of the Surgical Correction of Vesicoureteral Reflux on Febrile Urinary Tract Infections after a Kidney Transplant: A Single-Center Retrospective Study" Journal of Clinical Medicine 13, no. 17: 5295. https://doi.org/10.3390/jcm13175295
APA StyleVaraschin, A. E., Gomar, G. G., Rocco, A. M., Hokazono, S. R., Garlet, Q. I., & Oliveira, C. S. (2024). The Effectiveness of the Surgical Correction of Vesicoureteral Reflux on Febrile Urinary Tract Infections after a Kidney Transplant: A Single-Center Retrospective Study. Journal of Clinical Medicine, 13(17), 5295. https://doi.org/10.3390/jcm13175295







