Emotional Contagion and Emotional Mimicry in Individuals with Schizophrenia: A Systematic Review
Abstract
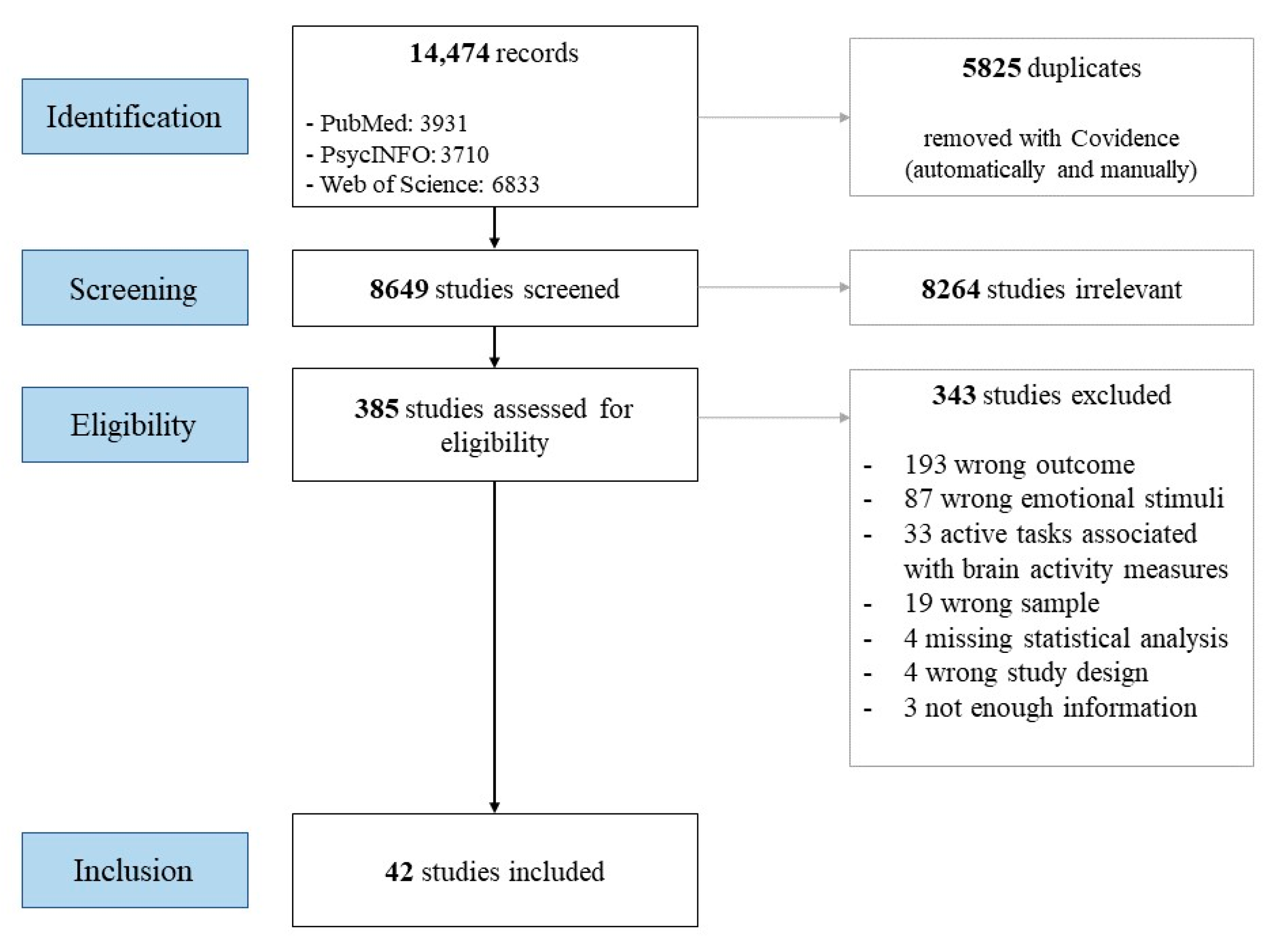
1. Introduction
2. Method
2.1. Inclusion and Exclusion Criteria
- -
- Concerning the emotional stimulus: (1) Studies not using emotional expressions as stimuli (e.g., the International Affective Picture System, IAPS [24]). (2) Studies that used oral presentations of semantically emotional words without specifying an emotional intonation in the voice. (3) Studies that used a stimulus in which the emotion component acts as a distraction (e.g., for a memory task) or incongruent emotional stimuli (e.g., happy face with crying sounds).
- -
- Concerning the task: (4) Emotional processing studies (i.e., brain activation), in which the participants must complete a task while viewing the emotional stimulus other than rating their emotional experience (e.g., gender discrimination, age discrimination). We chose to exclude these studies, as neural responses are modulated by task instructions [25]. (5) Studies using passive oddball paradigms, as neurological measures can be modulated by the novelty of the deviant stimulus rather than its significance [26]. (6) Studies about imitation, in which researchers explicitly asked the participants to imitate or copy the emotional expression.
2.2. Search Strategy
2.3. Filtering of Documents
2.4. Data Extraction
2.5. Risk of Bias
2.6. Synthesis
3. Results
3.1. Risk of Bias
3.2. Emotional Mimicry
3.3. Emotional Contagion through Brain Activity
3.4. Emotional Contagion through Psychophysiological Reactions
3.5. Emotional Contagion through Self-Reported Emotion
3.6. Emotional Contagion through Self-Reported Susceptibility
3.7. Associations with Symptomatology and Medications
4. Discussion
4.1. Emotional Mimicry
4.2. Emotional Contagion through Brain Activity
4.3. Emotional Contagion through Psychophysiological Activity
4.4. Emotional Contagion through Self-Reported Emotional State and Self-Reported Susceptibility
4.5. Discordance between Measures of Emotional Contagion
4.6. Clinical Implications
4.7. Limitations of the Existing Research
4.8. Future Studies
4.9. Limitation of Our Review
4.10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Burns, T.; Patrick, D. Social functioning as an outcome measure in schizophrenia studies. Acta Psychiatr. Scand. 2007, 116, 403–418. [Google Scholar] [CrossRef] [PubMed]
- Hooley, J.M. Social Factors in Schizophrenia. Curr. Dir. Psychol. Sci. 2010, 19, 238–242. [Google Scholar] [CrossRef]
- Fischer, A.H.; Manstead, A.S.R. Social functions of emotion. In Handbook of Emotions, 3rd ed; The Guilford Press: New York, NY, USA, 2008; pp. 456–468. ISBN 978-1-59385-650-2. [Google Scholar]
- Kring, A.M.; Elis, O. Emotion Deficits in People with Schizophrenia. Annu. Rev. Clin. Psychol. 2013, 9, 409–433. [Google Scholar] [CrossRef]
- Trémeau, F. A review of emotion deficits in schizophrenia. Dialogues Clin. Neurosci. 2006, 8, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Hess, U.; Fischer, A. Emotional Mimicry: Why and When We Mimic Emotions. Soc. Personal. Psychol. Compass 2014, 8, 45–57. [Google Scholar] [CrossRef]
- Barsade, S.G.; Coutifaris, C.G.V.; Pillemer, J. Emotional contagion in organizational life. Res. Organ. Behav. 2018, 38, 137–151. [Google Scholar] [CrossRef]
- Hatfield, E.; Cacioppo, J.T.; Rapson, R.L. Emotional Contagion; Cambridge University Press: Cambridge, UK, 1994. [Google Scholar]
- Corbera, S.; Wexler, B.; Bell, M.; Pearlson, G.; Mayer, S.; Pittman, B.; Belamkar, V.; Assaf, M. Predictors of Social Functioning and Quality of Life in Schizophrenia and Autism Spectrum Disorder. Psychiatry Res. 2021, 303, 114087. [Google Scholar] [CrossRef]
- Rum, Y.; Perry, A. Empathic Accuracy in Clinical Populations. Front. Psychiatry 2020, 11, 457. [Google Scholar] [CrossRef]
- Hess, U.; Fischer, A. Emotional mimicry as social regulator: Theoretical considerations. Cogn. Emot. 2022, 36, 785–793. [Google Scholar] [CrossRef]
- Mauersberger, H.; Hess, U. When smiling back helps and scowling back hurts: Individual differences in emotional mimicry are associated with self-reported interaction quality during conflict interactions. Motiv. Emot. 2019, 43, 471–482. [Google Scholar] [CrossRef]
- Stel, M.; Blascovich, J.; Mccall, C.; Mastop, J.; Baaren, R.B.V.; Vonk, R. Mimicking disliked others: Effects of a priori liking on the mimicry-liking link. Eur. J. Soc. Psychol. 2010, 40, 867–880. [Google Scholar] [CrossRef]
- Herrando, C.; Constantinides, E. Emotional Contagion: A Brief Overview and Future Directions. Front. Psychol. 2021, 12, 712606. [Google Scholar] [CrossRef] [PubMed]
- Blairy, S.; Herrera, P.; Hess, U. Mimicry and the Judgment of Emotional Facial Expressions. J. Nonverbal Behav. 1999, 23, 5–41. [Google Scholar] [CrossRef]
- Hess, U.; Blairy, S. Facial mimicry and emotional contagion to dynamic emotional facial expressions and their influence on decoding accuracy. Int. J. Psychophysiol. 2001, 40, 129–141. [Google Scholar] [CrossRef]
- McIntosh, D.N. Spontaneous facial mimicry, liking and emotional contagion. Emot. Contag. 2006, 37, 31. [Google Scholar]
- Olszanowski, M.; Wrobel, M.; Hess, U. Mimicking and sharing emotions: A re-examination of the link between facial mimicry and emotional contagion. Cogn. Emot. 2019, 34, 367–376. [Google Scholar] [CrossRef]
- Sato, W.; Fujimura, T.; Kochiyama, T.; Suzuki, N. Relationships among facial mimicry, emotional experience, and emotion recognition. PLoS ONE 2013, 8, e57889. [Google Scholar] [CrossRef]
- Parkinson, B. Interpersonal Emotion Transfer: Contagion and Social Appraisal. Soc. Personal. Psychol. Compass 2011, 5, 428–439. [Google Scholar] [CrossRef]
- Epstude, K.; Mussweiler, T. What you feel is how you compare: How comparisons influence the social induction of affect. Emotion 2009, 9, 1–14. [Google Scholar] [CrossRef]
- Hatfield, E.; Bensman, L.; Thornton, P.D.; Rapson, R.L. New Perspectives on Emotional Contagion: A Review of Classic and Recent Research on Facial Mimicry and Contagion. Interpersona Int. J. Pers. Relatsh. 2014, 8, 159–179. [Google Scholar] [CrossRef]
- Doherty, R.W. The Emotional Contagion Scale: A Measure of Individual Differences. J. Nonverbal Behav. 1997, 21, 131–154. [Google Scholar] [CrossRef]
- Bradley, M.M.; Lang, P.J. The International Affective Picture System (IAPS) in the study of emotion and attention. In Handbook of Emotion Elicitation and Assessment; Series in Affective Science; Oxford University Press: New York, NY, USA, 2007; pp. 29–46. ISBN 978-0-19-516915-7. [Google Scholar]
- Lange, K.; Williams, L.M.; Young, A.W.; Bullmore, E.T.; Brammer, M.J.; Williams, S.C.R.; Gray, J.A.; Phillips, M.L. Task instructions modulate neural responses to fearful facial expressions. Biol. Psychiatry 2003, 53, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, V.; Bradley, M.M.; Codispoti, M.; Lang, P.J. Detecting Novelty and Significance. J. Cogn. Neurosci. 2010, 22, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.A.; Shea, B.; O’Connell, D.; Robertson, J.; Peterson, J.; Welch, V.; Losos, M.; Tugwel, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses; Ottawa Hospital Research Institute: Ottawa, ON, Canada, 2011; Available online: https://www.evidencebasedpublichealth.de/download/Newcastle_Ottowa_Scale_Pope_Bruce.pdf (accessed on 5 February 2013).
- Bekele, E.; Bian, D.; Peterman, J.; Park, S.; Sarkar, N. Design of a Virtual Reality System for Affect Analysis in Facial Expressions (VR-SAAFE); Application to Schizophrenia. IEEE Trans. Neural Syst. Rehabil. Eng. 2017, 25, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Berger, P.; Bitsch, F.; Jakobi, B.; Nagels, A.; Straube, B.; Falkenberg, I. Cognitive and emotional empathy in patients with schizophrenia spectrum disorders: A replication and extension study. Psychiatry Res. 2019, 276, 56–59. [Google Scholar] [CrossRef]
- Culbreth, A.; Foti, D.; Barch, D.; Hajcak, G.; Kotov, R. Electrocortical Responses to Emotional Stimuli in Psychotic Disorders: Comparing Schizophrenia Spectrum Disorders and Affective Psychosis. Front. Psychiatry 2018, 9, 586. [Google Scholar] [CrossRef]
- Das, P.; Kemp, A.H.; Flynn, G.; Harris, A.W.F.; Liddell, B.J.; Whitford, T.J.; Peduto, A.; Gordon, E.; Williams, L.M. Functional disconnections in the direct and indirect amygdala pathways for fear processing in schizophrenia. Schizophr. Res. 2007, 90, 284–294. [Google Scholar] [CrossRef]
- Dyck, M.; Loughead, J.; Gur, R.; Schneider, F.; Mathiak, K. Hyperactivation balances sensory processing deficits during mood induction in schizophrenia. Soc. Cogn. Affect. Neurosci. 2014, 9, 167–175. [Google Scholar] [CrossRef]
- Escarti, M.; de la Iglesia-Vaya, M.; Marti-Bonmati, L.; Robles, M.; Carbonell, J.; Lull, J.; Garcia-Marti, G.; Manjon, J.; Aguilar, E.; Aleman, A.; et al. Increased amygdala and parahippocampal gyrus activation in schizophrenic patients with auditory hallucinations: An fMRI study using independent component analysis. Schizophr. Res. 2010, 117, 31–41. [Google Scholar] [CrossRef]
- Falkenberg, I.; Bartels, M.; Wild, B. Keep smiling! Facial reactions to emotional stimuli and their relationship to emotional contagion in patients with schizophrenia. Eur. Arch. Psychiatry Clin. Neurosci. 2008, 258, 245–253. [Google Scholar] [CrossRef]
- Ferri, F.; Costantini, M.; Salone, A.; Ebisch, S.; De Berardis, D.; Mazzola, V.; Arciero, G.; Ferro, F.; Di Giannantonio, M.; Romani, G.; et al. Binding Action and Emotion in First-Episode Schizophrenia. Psychopathology 2014, 47, 394–407. [Google Scholar] [CrossRef]
- Habel, U.; Gur, R.; Mandal, M.; Salloum, J.; Gur, R.; Schneider, F. Emotional processing in schizophrenia across cultures: Standardized measures of discrimination and experience. Schizophr. Res. 2000, 42, 57–66. [Google Scholar] [CrossRef]
- Habel, U.; Klein, M.; Shah, N.; Toni, I.; Zilles, K.; Falkai, P.; Schneider, F. Genetic load on amygdala hypofunction during sadness in nonaffected brothers of schizophrenia patients. Am. J. Psychiatry 2004, 161, 1806–1813. [Google Scholar] [CrossRef]
- Haker, H.; Rossler, W. Empathy in schizophrenia: Impaired resonance. Eur. Arch. Psychiatry Clin. Neurosci. 2009, 259, 352–361. [Google Scholar] [CrossRef]
- Holt, D.; Kunkel, L.; Weiss, A.; Goff, D.; Wright, C.; Shin, L.; Rauch, S.; Hootnick, J.; Heckers, S. Increased medial temporal lobe activation during the passive viewing of emotional and neutral facial expressions in schizophrenia. Schizophr. Res. 2006, 82, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Horan, W.; Iacoboni, M.; Cross, K.; Korb, A.; Lee, J.; Nori, P.; Quintana, J.; Wynn, J.; Green, M. Self-reported empathy and neural activity during action imitation and observation in schizophrenia. Neuroimage Clin. 2014, 5, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Horan, W.; Reise, S.; Kern, R.; Lee, J.; Penn, D.; Green, M. Structure and correlates of self-reported empathy in schizophrenia. J. Psychiatr. Res. 2015, 66–67, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Horan, W.; Jimenez, A.; Lee, J.; Wynn, J.; Eisenberger, N.; Green, M. Pain empathy in schizophrenia: An fMRI study. Soc. Cogn. Affect. Neurosci. 2016, 11, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Horley, K.; Gonsalvez, C.; Williams, L.; Lazzaro, I.; Bahramali, H.; Gordon, E. Event-related potentials to threat-related faces in schizophrenia. Int. J. Neurosci. 2001, 107, 113–130. [Google Scholar] [CrossRef]
- Hyatt, C.; Wexler, B.; Pittman, B.; Nicholson, A.; Pearlson, G.; Corbera, S.; Bell, M.; Pelphrey, K.; Calhoun, V.; Assaf, M. Atypical Dynamic Functional Network Connectivity State Engagement during Social-Emotional Processing in Schizophrenia and Autism. Cereb. Cortex 2022, 32, 3406–3422. [Google Scholar] [CrossRef]
- Jetha, M.K.; Zheng, X.; Goldberg, J.O.; Segalowitz, S.J.; Schmidt, L.A. Shyness and emotional face processing in schizophrenia: An ERP study. Biol. Psychol. 2013, 94, 562–574. [Google Scholar] [CrossRef]
- Koevoets, M.; Prikken, M.; Hagenaar, D.; Kahn, R.; van Haren, N. The Association Between Emotion Recognition, Affective Empathy, and Structural Connectivity in Schizophrenia Patients. Front. Psychiatry 2022, 13, 910985. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, A.; Bahcesular, K.; Brockmann, E.; Biederbick, S.; Dziobek, I.; Gallinat, J.; Montag, C. Subjective experience of emotions and emotional empathy in paranoid schizophrenia. Psychiatry Res. 2014, 220, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Yang, H.; Zhang, Y.; Cai, X.; Wang, Y.; Ni, K.; Pu, C.; Zhou, S.; Ma, Y.; Lui, S.; et al. Validation of the Questionnaire of Cognitive and Affective Empathy in patients with schizophrenia, major depressive disorder and bipolar disorder. Cognit. Neuropsychiatry 2020, 25, 466–479. [Google Scholar] [CrossRef] [PubMed]
- Lindner, C.; Dannlowski, U.; Walhofer, K.; Rodiger, M.; Maisch, B.; Bauer, J.; Ohrmann, P.; Lencer, R.; Zwitserlood, P.; Kersting, A.; et al. Social Alienation in Schizophrenia Patients: Association with Insula Responsiveness to Facial Expressions of Disgust. PLoS ONE 2014, 9, e85014. [Google Scholar] [CrossRef]
- Lindner, C.; Dannlowski, U.; Bauer, J.; Ohrmann, P.; Lencer, R.; Zwitserlood, P.; Kugel, H.; Suslow, T. Affective Flattening in Patients with Schizophrenia: Differential Association with Amygdala Response to Threat-Related Facial Expression under Automatic and Controlled Processing Conditions. Psychiatry Investig. 2016, 13, 102–111. [Google Scholar] [CrossRef]
- Mathews, J.R.L.; Bach, D.M. Emotional processing, social cognition, and social functioning deficits in schizophrenia. ProQuest Inf. Learn. 2010, 119, 50–59. [Google Scholar] [CrossRef]
- Michaels, T.; Horan, W.; Ginger, E.; Martinovich, Z.; Pinkham, A.; Smith, M. Cognitive empathy contributes to poor social functioning in schizophrenia: Evidence from a new self-report measure of cognitive and affective empathy. Psychiatry Res. 2014, 220, 803–810. [Google Scholar] [CrossRef]
- Mitchell, R.; Elliott, R.; Barry, M.; Cruttenden, A.; Woodruff, P. Neural response to emotional prosody in schizophrenia and in bipolar affective disorder. Br. J. Psychiatry 2004, 184, 223–230. [Google Scholar] [CrossRef]
- Mothersill, O.; Morris, D.; Kelly, S.; Rose, E.; Bokde, A.; Reilly, R.; Gill, M.; Corvin, A.; Donohoe, G. Altered medial prefrontal activity during dynamic face processing in schizophrenia spectrum patients. Schizophr. Res. 2014, 157, 225–230. [Google Scholar] [CrossRef]
- Popov, T.; Rockstroh, B.; Popova, P.; Carolus, A.; Miller, G. Dynamics of alpha oscillations elucidate facial affect recognition in schizophrenia. Cogn. Affect. Behav. Neurosci. 2014, 14, 364–377. [Google Scholar] [CrossRef][Green Version]
- Regenbogen, C.; Kellermann, T.; Seubert, J.; Schneider, D.; Gur, R.; Derntl, B.; Schneider, F.; Habel, U. Neural responses to dynamic multimodal stimuli and pathology-specific impairments of social cognition in schizophrenia and depression. Br. J. Psychiatry 2015, 206, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Reske, M.; Kellermann, T.; Habel, U.; Shah, N.; Backes, V.; von Wilmsdorff, M.; Stocker, T.; Gaebel, W.; Schneider, F. Stability of emotional dysfunctions? A long-term fMRI study in first-episode schizophrenia. J. Psychiatr. Res. 2007, 41, 918–927. [Google Scholar] [CrossRef]
- Riehle, M.; Lincoln, T. Investigating the Social Costs of Schizophrenia: Facial Expressions in Dyadic Interactions of People With and Without Schizophrenia. J. Abnorm. Psychol. 2018, 127, 202–215. [Google Scholar] [CrossRef] [PubMed]
- Schneider, F.; Gur, R.C.; Gur, R.E.; Shtasel, D.L. Emotional processing in schizophrenia: Neurobehavioral probes in relation to psychopathology. Schizophr. Res. 1995, 17, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Schneider, F.; Weiss, U.; Kessler, C.; Salloum, J.; Posse, S.; Grodd, W.; Muller-Gartner, H. Differential amygdala activation in schizophrenia during sadness. Schizophr. Res. 1998, 34, 133–142. [Google Scholar] [CrossRef]
- Sestito, M.; Umilta, M.; De Paola, G.; Fortunati, R.; Raballo, A.; Leuci, E.; Maffei, S.; Tonna, M.; Amore, M.; Maggini, C.; et al. Facial reactions in response to dynamic emotional stimuli in different modalities in patients suffering from schizophrenia: A behavioral and EMG study. Front. Hum. Neurosci. 2013, 7, 368. [Google Scholar] [CrossRef]
- Spilka, M.; Arnold, A.; Goghari, V. Functional activation abnormalities during facial emotion perception in schizophrenia patients and nonpsychotic relatives. Schizophr. Res. 2015, 168, 330–337. [Google Scholar] [CrossRef]
- Suslow, T.; Roestel, C.; Arolt, V. Affective priming in schizophrenia and without affective negative symptoms. Eur. Arch. Psychiatry Clin. Neurosci. 2003, 253, 292–300. [Google Scholar] [CrossRef]
- Torregrossa, L.; Bian, D.; Wade, J.; Adery, L.; Ichinose, M.; Nichols, H.; Bekele, E.; Sarkar, N.; Park, S. Decoupling of spontaneous facial mimicry from emotion recognition in schizophrenia. Psychiatry Res. 2019, 275, 169–176. [Google Scholar] [CrossRef]
- Varcin, K.; Bailey, P.; Henry, J. Empathic deficits in schizophrenia: The potential role of rapid facial mimicry. J. Int. Neuropsychol. Soc. 2010, 16, 621–629. [Google Scholar] [CrossRef]
- Varcin, K.; Nangle, M.; Henry, J.; Bailey, P.; Richmond, J. Intact spontaneous emotional expressivity to non-facial but not facial stimuli in schizophrenia: An electromyographic study. Schizophr. Res. 2019, 206, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.; Das, P.; Harris, A.; Liddell, B.; Brammer, M.; Olivieri, G.; Skerrett, D.; Phillips, M.; David, A.; Peduto, A.; et al. Dysregulation of arousal and amygdala-prefrontal systems in paranoid schizophrenia. Am. J. Psychiatry 2004, 161, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.; Das, P.; Liddell, B.; Olivieri, G.; Peduto, A.; David, A.; Gordon, E.; Harris, A. Fronto-limbic and autonomic disjunctions to negative emotion distinguish schizophrenia subtypes. Psychiatry Res. Neuroimaging 2007, 155, 29–44. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.; Whitford, T.; Nagy, M.; Flynn, G.; Harris, A.; Silverstein, S.; Gordon, E. Emotion-elicited gamma synchrony in patients with first-episode schizophrenia: A neural correlate of social cognition outcomes. J. Psychiatry Neurosci. 2009, 34, 303–313. [Google Scholar] [PubMed]
- Fusar-Poli, P.; Placentino, A.; Carletti, F.; Landi, P.; Allen, P.; Surguladze, S.; Benedetti, F.; Abbamonte, M.; Gasparotti, R.; Barale, F.; et al. Functional atlas of emotional faces processing: A voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. J. Psychiatry Neurosci. 2009, 34, 418–432. [Google Scholar]
- Bradley, M.M.; Lang, P.J. Measuring emotion: The self-assessment manikin and the semantic differential. J. Behav. Ther. Exp. Psychiatry 1994, 25, 49–59. [Google Scholar] [CrossRef]
- Watson, D.; Clark, L.; Tellegen, A. Development and Validation of Brief Measures of Positive and Negative Affect—The Panas Scales. J. Pers. Soc. Psychol. 1988, 54, 1063–1070. [Google Scholar] [CrossRef]
- Schneider, F.; Gur, R.C.; Gur, R.E.; Muenz, L.R. Standardized mood induction with happy and sad facial expressions. Psychiatry Res. 1994, 51, 19–31. [Google Scholar] [CrossRef]
- Payne, K.; Lundberg, K. The Affect Misattribution Procedure: Ten Years of Evidence on Reliability, Validity, and Mechanisms. Soc. Personal. Psychol. Compass 2014, 8, 672–686. [Google Scholar] [CrossRef]
- Reniers, R.L.E.P.; Corcoran, R.; Drake, R.; Shryane, N.M.; Völlm, B.A. The QCAE: A Questionnaire of Cognitive and Affective Empathy. J. Pers. Assess. 2011, 93, 84–95. [Google Scholar] [CrossRef]
- Bersani, G.; Bersani, F.S.; Valeriani, G.; Robiony, M.; Anastasia, A.; Colletti, C.; Liberati, D.; Capra, E.; Quartini, A.; Polli, E. Comparison of facial expression in patients with obsessive-compulsive disorder and schizophrenia using the Facial Action Coding System: A preliminary study. Neuropsychiatr. Dis. Treat. 2012, 8, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Mattes, R.M.; Schneider, F.; Heimann, H.; Birbaumer, N. Reduced emotional response of schizophrenic patients in remission during social interaction. Schizophr. Res. 1995, 17, 249–255. [Google Scholar] [CrossRef] [PubMed]
- McTeague, L.M.; Rosenberg, B.M.; Lopez, J.W.; Carreon, D.M.; Huemer, J.; Jiang, Y.; Chick, C.F.; Eickhoff, S.B.; Etkin, A. Identification of Common Neural Circuit Disruptions in Emotional Processing Across Psychiatric Disorders. Am. J. Psychiatry 2020, 177, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Knable, M.B.; Weinberger, D.R. Dopamine, the prefrontal cortex and schizophrenia. J. Psychopharmacol. Oxf. Engl. 1997, 11, 123–131. [Google Scholar] [CrossRef]
- Kapur, S. Psychosis as a state of aberrant salience: A framework linking biology, phenomenology, and pharmacology in schizophrenia. Am. J. Psychiatry 2003, 160, 13–23. [Google Scholar] [CrossRef]
- Kapur, S.; Mizrahi, R.; Li, M. From dopamine to salience to psychosis--linking biology, pharmacology and phenomenology of psychosis. Schizophr. Res. 2005, 79, 59–68. [Google Scholar] [CrossRef]
- Mier, D.; Kirsch, P. Social-Cognitive Deficits in Schizophrenia. Curr. Top. Behav. Neurosci. 2017, 30, 397–409. [Google Scholar] [CrossRef]
- Dugre, J.R.; Bitar, N.; Dumais, A.; Potvin, S. Limbic Hyperactivity in Response to Emotionally Neutral Stimuli in Schizophrenia: A Neuroimaging Meta-Analysis of the Hypervigilant Mind. Am. J. Psychiatry 2019, 176, 1021–1029. [Google Scholar] [CrossRef]
- Belge, J.-B.; Maurage, P.; Mangelinckx, C.; Leleux, D.; Delatte, B.; Constant, E. Facial decoding in schizophrenia is underpinned by basic visual processing impairments. Psychiatry Res. 2017, 255, 167–172. [Google Scholar] [CrossRef]
- Hempel, R.; Tulen, J.; Beveren, N.; Steenis, H.; Mulder, P.; Hengeveld, M.W. Physiological responsivity to emotional pictures in schizophrenia. J. Psychiatr. Res. 2005, 39, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Schlenker, R.; Cohen, R.; Hopmann, G. Affective modulation of the startle reflex in schizophrenic patients. Eur. Arch. Psychiatry Clin. Neurosci. 1995, 245, 309–318. [Google Scholar] [CrossRef]
- Cohen, A.S.; Minor, K.S. Emotional experience in patients with schizophrenia revisited: Meta-analysis of laboratory studies. Schizophr. Bull. 2010, 36, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Kring, A.M.; Earnst, K.S. Stability of emotional responding in schizophrenia. Behav. Ther. 1999, 30, 373–388. [Google Scholar] [CrossRef]
- Deci, E.L. The Psychology of Self-Determination; Lexington Books: Lexington, MA, USA, 1980; ISBN 978-0-669-04045-6. [Google Scholar]
- Wrobel, M.; Imbir, K. Broadening the Perspective on Emotional Contagion and Emotional Mimicry: The Correction Hypothesis. Perspect. Psychol. Sci. 2019, 14, 437–451. [Google Scholar] [CrossRef]
- Asgharpour, M.; Tehrani-Doost, M.; Ahmadi, M.; Moshki, H. Visual Attention to Emotional Face in Schizophrenia: An Eye Tracking Study. Iran. J. Psychiatry 2015, 10, 13–18. [Google Scholar]
- Simpson, C.; Pinkham, A.E.; Kelsven, S.; Sasson, N.J. Emotion recognition abilities across stimulus modalities in schizophrenia and the role of visual attention. Schizophr. Res. 2013, 151, 102–106. [Google Scholar] [CrossRef]
- Yabar, Y.; Hess, U. Display of empathy and perception of out-group members. N. Z. J. Psychol. 2007, 36, 42–49. [Google Scholar]
- Isern-Mas, C.; Gomila, A. Making sense of emotional contagion. Humana. Mente J. Philos. Stud. 2019, 12, 71–100. [Google Scholar]
- d’Arma, A.; Isernia, S.; Di Tella, S.; Rovaris, M.; Valle, A.; Baglio, F.; Marchetti, A. Social Cognition Training for Enhancing Affective and Cognitive Theory of Mind in Schizophrenia: A Systematic Review and a Meta-Analysis. J. Psychol. 2021, 155, 26–58. [Google Scholar] [CrossRef]
- Wrobel, M.; Olszanowski, M. Emotional reactions to dynamic morphed facial expressions: A new method to induce emotional contagion. Rocz. Psychol. 2019, 22, 91–102. [Google Scholar] [CrossRef]
- Freeman, D.; Loe, B.S.; Kingdon, D.; Startup, H.; Molodynski, A.; Rosebrock, L.; Brown, P.; Sheaves, B.; Waite, F.; Bird, J.C. The revised Green et al., Paranoid Thoughts Scale (R-GPTS): Psychometric properties, severity ranges, and clinical cut-offs. Psychol. Med. 2021, 51, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Kring, A.M.; Gur, R.E.; Blanchard, J.J.; Horan, W.P.; Reise, S.P. The Clinical Assessment Interview for Negative Symptoms (CAINS): Final development and validation. Am. J. Psychiatry 2013, 170, 165–172. [Google Scholar] [CrossRef] [PubMed]

| Study | Selection | Comparability | Exposure | Power-Analysis |
|---|---|---|---|---|
| Bekele et al., 2017 [28] | ◊◊◊ | ◊◊ | ◊◊◊ | |
| Berger et al., 2019 [29] | ◊◊◊ | ◊◊ | ◊◊◊ | |
| Culbreth et al., 2018 [30] | ◊◊◊◊ | ◊◊ | ◊◊ | ◊ |
| Das et al., 2017 [31] | ◊◊◊ | ◊◊ | ◊◊◊ | |
| Dyck et al., 2014 [32] | ◊◊◊ | ◊◊ | ◊ | |
| Escarti et al., 2010 [33] | ◊◊ | ◊ | ◊◊ | |
| Falkenberg et al., 2008 [34] | ◊◊◊◊ | ◊◊ | ◊◊◊ | |
| Ferri et al., 2014 [35] | ◊◊◊ | ◊◊ | ◊◊ | |
| Habel et al., 2000 [36] | ◊◊◊ | ◊ | ◊◊ | |
| Habel et al., 2004 [37] | ◊◊ | ◊◊ | ◊ | |
| Haker and Rossler (2009) [38] | ◊◊◊ | ◊◊ | ◊◊ | |
| Holt et al., 2006 [39] | ◊◊◊ | ◊◊ | ◊◊◊ | |
| Horan et al., 2014 [40] | ◊◊◊◊ | ◊◊ | ◊◊ | |
| Horan et al., 2015 [41] | ◊◊◊◊ | ◊◊ | ◊◊◊ | |
| Horan et al., 2016 [42] | ◊◊◊◊ | ◊◊ | ◊◊ | |
| Horley et al., 2001 [43] | ◊◊◊ | ◊◊ | ◊ | |
| Hyatt et al., 2022 [44] | ◊◊◊◊ | ◊◊ | ||
| Jetha et al., 2013 [45] | ◊◊◊◊ | ◊◊ | ◊◊◊ | |
| Koevoets et al., 2022 [46] | ◊◊◊◊ | ◊◊ | ◊◊ | |
| Lehmann et al., 2014 [47] | ◊◊◊◊ | ◊◊ | ◊◊◊ | |
| Liang et al., 2020 [48] | ◊◊◊◊ | ◊ | ◊◊◊ | |
| Lindner et al., 2014 [49] | ◊◊◊ | ◊◊ | ◊◊ | |
| Lindner et al., 2016 [50] | ◊◊◊ | ◊◊ | ◊ | |
| Mathews and Bach, 2010 [51] | ◊◊◊◊ | ◊◊ | ◊◊ | |
| Michaels et al., 2014 [52] | ◊◊◊◊ | ◊◊ | ◊◊◊ | |
| Mitchell et al., 2004 [53] | ◊◊◊ | ◊ | ◊◊ | |
| Mothersill et al., 2014 [54] | ◊◊ | ◊◊ | ◊◊ | |
| Popov et al., 2014 [55] | ◊◊◊ | ◊◊ | ◊◊◊ | |
| Regenbogen et al., 2015 [56] | ◊◊ | ◊ | ◊◊◊ | |
| Reske et al., 2007 [57] | ◊◊◊◊ | ◊◊ | ◊◊ | |
| Riehle and Lincoln, 2018 [58] | ◊◊◊◊ | ◊◊ | ◊ | |
| Schneider et al., 1995 [59] | ◊◊◊ | ◊ | ◊◊ | |
| Schneider et al., 1998 [60] | ◊◊ | ◊◊ | ◊◊ | |
| Sestito et al., 2013 [61] | ◊◊◊◊ | ◊◊ | ◊◊◊ | |
| Spilka et al., 2015 [62] | ◊◊◊◊ | ◊◊ | ◊ | |
| Suslow et al., 2003 [63] | ◊◊◊◊ | ◊◊ | ◊◊◊ | |
| Torregrossa et al., 2019 [64] | ◊◊◊◊ | ◊◊ | ◊◊◊ | |
| Varcin et al., 2010 [65] | ◊◊◊◊ | ◊◊ | ◊ | |
| Varcin et al., 2019 [66] | ◊◊◊◊ | ◊◊ | ◊ | |
| Williams et al., 2004 [67] | ◊◊◊ | ◊◊ | ◊◊◊ | |
| Williams et al., 2007 [68] | ◊◊◊ | ◊◊ | ◊◊ | |
| Williams et al., 2009 [69] | ◊◊◊ | ◊◊ | ◊◊ |
| Citation | Sample Characteristics | Variable (s) Measured | Measurement Tool | Stimuli | Main Outcomes Measures of Emotional Contagion or Emotional Mimicry | Additional Outcomes Psychotics Symptomatology | Additional Outcomes Medication |
|---|---|---|---|---|---|---|---|
| Bekele et al., 2016 [28] | ISZ: n = 12 (4W, 8M) mean age: 45.7 (9.4) HC: n = 12 (SD = 6,6) mean age: 44.9 (SD = 9.9) Matched in age and sex. | EC through psychophysiological reactions. | Skin conductance response rate, mean skin conductance level, breathing rate, mean skin temperature. | Avatars in virtual reality settings narrate an emotional memory and produce emotional expressions (enjoyment, surprise, sadness, disgust, anger) | ISZ showed significantly different psychophysiological reactions in response to positive and negative emotions. | Not reported. | Not reported. |
| Berger et al., 2019 [29] | ISZ: n = 35 (12W, 23M) mean age: 34.84 (SD = 11.0) HC: n = 18 (10W, 8M) mean age: 29.47 (SD = 5.21) Matched in age and sex. | Self-reported susceptibility to EC. | ECS | - | Higher susceptibility to emotional contagion of fear for ISZ compared to HC. No difference between groups for other emotions (happiness, love, fear, and sadness). | Not reported. | Not reported. |
| Culbreth et al., 2018 [30] | ISZ: n = 37 (16W, 21M) mean age: 44.9 (SD = 7.8) AP (Affective disorder, MDD and BD): n = 37 (15W, 22M) mean age: 44.3 (SD = 9.3) Matched in age and sex. | EC through brain activity. | EEG | Pictures of facial expressions (happy, sad, angry, afraid, and neutral). | No between-group differences for the late positive potential contrast of neutral and emotional expression. | No significant correlation was found. | No significant correlation was found. |
| Das et al., 2007 [31] | FES: 14 (M) mean age: 20.4 (SD = 3.3) HC: 14 (M) mean age: 23.1 (SD = 5.9) Matched in age and sex. | EC through brain activity. | fMRI | Pictures of fear and neutral facial expressions were presented under conscious (500ms) or unconscious conditions (16 ms of emotional expression and 163 ms of neutral expression). | ISZ showed reduced amygdala activity in response to fearful expressions compared to HC. ISZ showed the reversal of the normal pattern of connectivity between the amygdala and the brainstem, visual cortex, and dorsal and ventral divisions of the medial prefrontal cortex. | Not reported. | No significant correlation was found. |
| Dyck et al., 2014 [32] | ISZ: 16 (6W, 10M) mean age: 35.94 (SD = 8.98) HC: 16 (6W, 10M) mean age: 34.25 (8.51) Matched in age and sex. | EC through brain activity. EC through self-reported emotion. | fMRI SAM | Pictures of facial expressions (happiness, sadness, neutral). | ISZ showed decreased activation in the left lingual gyrus compared to HC. ISZ showed increased connectivity between early and late processing areas within the visual cortex compared to HC. No between-group differences for self-reported emotional state. | Not reported. | Not reported. |
| Escarti et al., 2010 [33] | ISZ (H): n = 27 (13W, 14M) mean age: 39.15 (SD = 8.76) ISZ (NH): n = 14 (6W, 8M) mean age: 42.93 (SD = 14.76) HC: 31 (15W, 16M) mean age: 31.34 (10.52) | EC through brain activity. | fMRI | Emotional and neutral words are pronounced in an emotional and neutral tone, respectively. | Different functional connectivity in limbic regions between HC, ISZ (H), and ISZ (NH). ISZ (H) showed increased amygdala and parahippocampal gyrus activation compared to HC and ISZ (NH). | Not reported. | Not reported. |
| Falkenberg et al., 2008 [34] | ISZ: n = 17 (6W, 11M), mean age: 28.2 (SD = 7.4) HC: n = 17 (6W, 11M), mean age: 27.6 (SD = 5.4) Matched in age and sex. | Self-reported susceptibility to EC. | ECS | - | No difference in the overall score between HC and ISZ. No difference in susceptibility to “joy” and “sadness”. Lower susceptibility to “love” in ISZ compared to HC. Stronger susceptibility to “anger” in ISZ compared to HC. | Not reported. | Not reported. |
| Ferri et al., 2014 [35] | ISZ: n = 22 (8W, 14M) mean age: 27.45 (SD = 5) HC:22 (10W, 12M) mean age: 28 (SD = 3.77) Matched in age and sex. | EC through brain activity. | fMRI | Video of an actor performing an action (grasping a bottle) with either a neutral, an angry, or a happy face. | ISZ showed decreased activation in the right anterior insula for angry stimulus compared to HC. No between-group differences for the happy stimulus. | No significant correlation was found. | No significant correlation was found. |
| Habel et al., 2000 [36] | Am ISZ: n = 40 (19W, 21M) mean age: 30.43 (SD = 7.72) AmHC: not specified. mean age: 21.75 (SD = 3.71) Ger ISZ: n = 24 (12W, 12M) GerHC: n = 24 (12W, 12M) mean age: 32.42 (SD = 8.71) In ISZ: n = 29 (male) mean age: 34.69 (SD = 7.41) In HC: n = 29 (19W, 10M) mean age: 28.10 (SD = 1.80) | EC through self-reported emotion. | PANAS | Pictures of facial expressions (sad and happy). | All cultures: ISZ had lower positive and higher negative emotions during happy emotional contagion. Am: no between-group differences. Indian: ISZ showed less positive emotion during happy and sad induction compared to HC. German: ISZ showed more negative emotion following happiness and sadness emotional contagion compared to HC. | Not reported. | Not reported. |
| Habel et al., 2004 [37] | ISZ: n = 13 (males) mean age: 32.8 (SD = 8.5) Relatives: n = 13 (males) mean age: 33.8 (SD = 8.7) HC: n = 26 (males) mean age: 33.4 (SD = 8.1) Matched in age and sex. | EC through brain activity. EC through self-reported emotion. | fMRI PANAS | Pictures of facial expressions (sad and happy). | For sadness stimuli, ISZ and relatives showed hypoactivation of the amygdala compared to HC. ISZ also showed hypoactivation in other brain regions (left orbitofrontal area, left superior temporal cortex, left precuneus). No between-group differences were found for brain activation following happiness stimuli. Emotional contagion through self-reported emotion was effective for both groups. No significant between-group differences were found. | No significant correlation was found. | No significant correlation was found. |
| Haker and Rossler 2009 [38] | ISZ: n = 43 (11W, 32M), mean age: 34 (SD = 10) HC: n = 45 (12W, 33M), 35 (SD = 11) Matched in age and sex. | Emotional mimicry. | Judge (clinical psychiatrists) measuring signs of yawning/sighing or laughing/smiling. | Video sequence of 15 s centered on the face laughing, yawning, or neutral. | ISZ showed less mimicry of both laughing and yawning compared to HC. ISZ produced more incongruent reactions than healthy controls. | Mimicry of laughing correlated negatively with the PANSS negative scale (r = −0.348, p = 0.02). Incongruent mimicry correlated negatively with the PANSS negative scale (r = −0.408, p = 0.007). | Incongruent mimicry correlated negatively with the dosage of antipsychotics (r = −0.33, p = 0.014). |
| Holt et al., 2006 [39] | ISZ: n = 15 (males) mean age: 47.7 (SD = 7.1) HC: n = 16 (males) mean age: 48.2 (SD = 9.6) Matched in age and sex. | EC through brain activity. | fMRI | Pictures of facial expressions (happiness and fear). | ISZ showed increased left hippocampal activation for happy and fearful stimuli compared to HC. ISZ also showed increased right amygdala activation for fearful stimuli compared to HC. | Not reported. | Not reported. |
| Horan et al., 2014 [40] | ISZ: n = 23 (6W, 17M) mean age: 46.5 (SD = 11.1) HC: n = 23 (7W, 16M) mean age: 46.7 (SD = 6.9) Matched in age and sex. | EC through brain activity. | fMRI | Pictures of facial expressions (happiness, sadness, anger, fear). | No between-group differences. ISZ and HC showed similar brain activation. | Not reported. | Not reported. |
| Horan et al., 2015 [41] | ISZ: n = 145 (36W, 109M) mean age: 40.9 (SD = 12.4) HC: n = 45 (13W, 32M) mean age: 43.3 (SD = 10.4) Matched in age and sex. | Self-reported susceptibility to EC. | QCAE | - | Higher susceptibility to emotional contagion for ISZ compared to HC. | No significant correlation was found. | Not reported. |
| Horan et al., 2016 [42] | ISZ: n = 21 (6W, 15M) mean age: 48.2 (SD = 10.4) HC: n = 21 (7W, 14M) mean age: 46.5 (7.1) Matched in age and sex. | EC through brain activity. EC through self-reported emotion. | fMRI Likert scale (from 1 “not painful” to 4 “extremely painful”). | Video of a person listening to a painful sound and showing facial expression from neutral to painful. | No between-group differences. ISZ and HC showed similar brain activation. No between-group differences. ISZ and HC reported similar painful emotions. | Not reported. | Not reported. |
| Horley et al., 2001 [43] | ISZ: n = 25 (gender not specified) mean age: 33.6 (SD = 7.63) HC: n = 25 (gender not specified) mean age: 34.36 (SD = 9.07) Matched in age and sex. | EC through brain activity. | EEG | Pictures of facial expressions (neutral and angry). | ISZ showed reduced amplitude (P200) and delay latency (N100, P200, N200, P300) compared to HC. | Not reported. | No significant correlation was found. |
| Hyatt et al., 2022 [44] | ISZ: n = 41 (12W, 29M) mean age: 30.9 (SD = 3.8) HC: n = 55 (27W, 28M) mean age: 29.1 (3.6) ASD: n = 42 (8W, 34M) mean age: 26.8 (SD = 3.6) | EC through brain activity. EC through self-reported emotion. | fMRI Emotional valence scale from 1 to 9. | Video of an actor narrating an emotional (happy, sad, or neutral) personal story displaying nonverbal emotional expressions. | ISZ showed different functional network connectivity state engagement compared to HC and ASD. No between-group difference in EC through self-reported emotions. | No significant correlation was found. | No significant correlation was found. |
| Jetha et al., 2013 [45] | ISZ: n = 40 (12W, 28M) mean age: 42.2 (SD = 6.4) HC: n = 39 (12W, 27M) mean age: 39.3 (SD = 7.8) Matched in age and sex. | EC through brain activity. | EEG | Pictures of facial expressions (happy, fear, angry, and neutral). | No between-group differences for the P100 amplitude. ISZ showed decreased N170 amplitude compared to HC. | Not reported. | Not reported. |
| Koevoets et al., 2022 [46] | ISZ: n = 47 (7W, 40M) mean age: 35.88 (SD = 8.24) HC: n = 47 (4W, 43M) mean age: 32.88 (SD = 7.91) Matched in age and sex. | EC through self-reported emotion. | Likert scale from 1 to 7 for positive emotions (compassionate, soft-hearted, warm, tender) and negative emotions (worried, distressed, disturbed, upset, troubled, and agitated). | Pictures of facial expressions followed by short clips (10 s) of the same person expressing the same emotion. | ISZ reported higher positive and negative emotions compared to HC. | Not reported. | Not reported. |
| Lehmann et al., 2014 [47] | ISZ: n = 55 (23W, 32M) mean age: 39.8 (SD = 11.9) HC: n = 69 (25W, 30M) mean age: 38.9 (SD = 12.8) Matched in age and sex. | Self-reported susceptibility to EC. | ECS | - | Higher susceptibility in the overall score for ISZ compared to HC. Higher susceptibility to negative emotions (fear, anger, sadness) for ISZ compared to HC. No differences between groups for susceptibility to positive emotions. | Not reported. | Not reported. |
| Liang et al., 2020 [48] | ISZ: n = 158 (91W, 67M) BD: n = 213 (139W, 74M) MDD: n = 163 (92W, 71M) HC: n = 107 (53W, 54M) ISZ:29.82 (7.1) BD:30.47 (6.28) MDD:30.64 (6.14) HC:29.42 (6.25) | Self-reported susceptibility to EC. | QCAE | Lower emotional contagion scores for ISZ and BD compared to MDD. | No significant correlation was found. | Not reported. | |
| Lindner et al., 2014 [49] | ISZ: n = 36 (14W, 22M) mean age: 30.8 (SD = 7.9) HC: n = 40 (13W, 27M) mean age: 29.5 (SD = 8.3) Matched in age and sex. | EC through brain activity. | fMRI | Picture of facial expressions (disgust, and neutral) presented in conscious (533ms) and nonconscious conditions (33 ms of emotional expression followed by 500 ms of a neutral face). | ISZ showed reduced insula activation compared to HC following masked disgust stimuli. No between-group differences for unmasked stimuli. No between-group differences in amygdala activation. | Not reported. | Not reported. |
| Lindner et al., 2016 [50] | ISZ: n = 36 (13W, 23M) mean age: 30.6 (SD = 8) HC: n = 42 (13W, 27M) mean age: 29.5 (SD = 8.3) Matched in age and sex. | EC through brain activity. | fMRI | Pictures of facial expressions (fear and neutral) presented in conscious (533 ms) and nonconscious conditions (33 ms of emotional expression followed by 500 ms of a neutral face). | ISZ showed hyperactivation of the amygdala compared to HC. ISZ with affective flattening showed increased amygdala activation compared to HC and ISZ without affective flattening following masked fear stimuli. | Affective flattening is positively correlated with the amygdala response to masked fearful faces (r = 0.52, p < 0.001) and negatively correlated with the amygdala response to unmasked fearful faces (r = −0.4, p < 0.001). | Not reported. |
| Mathews and Bach, 2010 [51] | ISZ: n = 40 (14W, 26M) mean age: 36.8 (SD = 8.99) HC: 40 (15W, 25M) mean age: 36.30 (SD = 10.47) Matched in age and sex. | EC through self-reported emotion. | Question (“Press 1 if they felt negative, 2 if felt neutral, and 3 if felt positive”) | Pictures of facial expressions (positive, negative, and neutral). | Emotional contagion was effective in both groups. Controls reported experiencing more positive emotions in response to positive stimuli and more negative emotions in response to negative stimuli. | No significant correlation was found. | Not reported. |
| Michaels et al., 2014 [52] | ISZ: n = 52 (12W, 40M) mean age: 35.3 (SD = 8.8) HC: 37 (17W, 20M), mean age: 33.4 (8.9) Matched in age and sex. | Self-reported susceptibility to EC. | QCAE | - | Higher susceptibility to emotional contagion for ISZ compared to HC. | No significant correlation was found. | No significant correlation was found. |
| Mitchell et al., 2004 [53] | ISZ: n = 12 (male) mean age: 45.7 (SD = 2.7) HC: n = 13 (male) mean age: 32.2 (SD = 3.6) BD: n = 11 (male) mean age: 42.8 (1.8) | EC through brain activity. | fMRI | An actor reads an emotional scenario (happy, sad, neutral) with the related emotional intonation (happy, sad, neutral). | ISZ showed a reversal of the normal right-lateralized temporal lobe response compared to HC. ISZ showed hyperactivation of the left insula compared to HC. | Not reported. | No significant correlation was found. |
| Mothersill et al., 2014 [54] | ISZ: n = 25 (5W, 20M) mean age: 42.88 (SD = 10.99) HC: n = 21 (5W, 16M) mean age: 38.24 (SD = 8.62) Matched in age and sex. | EC through brain activity. | fMRI | Short video clips of faces going from a neutral to an angry expression. | ISZ showed weaker deactivation of the medial prefrontal cortex, anterior cingulate cortex, and decreased left cerebellum compared to HC. | Not reported. | Not reported. |
| Popov et al., 2013 [55] | ISZ: n = 44 (13W, 31M) mean age: 32 (SD = 9.4) HC: n = 44 (20W, 24M) mean age: 29.2 (SD = 7.9) Matched in age and sex. | EC through brain activity. | MEG | Morphed images go from a neutral face to a target facial expression (fearful or happy). | ISZ did not show the sequence of alpha power increase and alpha connectivity decrease compared to HC. | Not reported. | Not reported. |
| Regenbogen et al., 2015 [56] | ISZ: n = 20 (gender not specified) mean age: 37.3 (SD = 8.44) HC: n = 24 (gender not specified) mean age: 35.25 (SD = 9.8) MDD: n = 24 (gender not specified) mean age: 36.42 (SD = 12.01). | EC through brain activity. EC through self-reported emotion. | fMRI Seven-point scale from “very negative” to “very positive”. | Video clip of an actor telling stories about disgusting, fearful, happy, sad, or neutral situations with either emotional or neutral prosody and facial expression. | No between-group differences in brain activation for the trimodal congruent emotional stimulus. No between-group differences for self-reported emotional state. | Not reported | Not reported. |
| Reske et al., 2007 [57] | FES: n = 10 (4W, 6M) mean age: 37.4 (SD = 6.06) HC: n = 10 (4W, 6M) mean age: 35.3 (SD = 8.71) Matched in age and sex. | EC through brain activity. EC through self-reported emotion. | fMRI PANAS/ ESR (emotional self-rating scale, unipolar for each emotion). | Pictures of happy and sad facial expressions. | Compared to HC, ISZ showed hypoactivation in the anterior cingulate cortex, orbitofrontal, temporal areas, and hippocampus. No between-group differences for self-reported emotional state. Emotional contagion was effective in both groups according to self-reports. | Therapy and symptom improvement are associated with increased in pre- and postcentral, inferior temporal, and frontal areas for sadness stimuli only. | Not reported. |
| Riehle and Lincoln, 2018 [58] | ISZ: 28 (16W, 12M) mean age: 41.7 (SD = 10.7) HC: 28 (16W, 12M) mean age 43.0 (SD = 12.1) IP: 28 (16W, 12M) mean age: 39.8 (SD = 13.7) Matched in age and sex. | Emotional mimicry. | EMG | Interacting partners describing emotionally positive and negative memories. | No between-group difference in smiling, smiling mimicry, or frowning. | Smiling activity negatively correlated with the CAINS item reduced facial expressiveness (r = −0.49, p < 0.01) and PANSS N1 blunted affect (r = −0.40, p < 0.05). Frowning activity negatively correlated with the CAINS EXP (r = −0.40, p < 0.05) and the PANSS N1 blunted affect (r = −0.46, p < 0.05). Smiling synchrony negatively correlated with the CAINS reduced facial expressiveness (r = −0.41, p < 0.05). | No significant correlation was found. |
| Schneider et al., 1995 [59] | ISZ: n = 40 (19W, 21M) mean age: 30.4 (SD = 7.7) HC: n = 40 (not specified) mean age: not specified. | EC through self-reported emotion. | PANAS Unipolar intensity scale from 1 to 5 for happiness and sadness. | Pictures of facial expressions (sad and happy). | No between-group differences: emotional contagion was effective in both groups. | The hallucination subscale of the SAPS was positively correlated with emotional contagion effectiveness (r = 0.40, p < 0.05). Anhedonia was negatively correlated with emotional contagion effectiveness (r not specified). | No significant correlation was found. |
| Schneider et al., 1998 [60] | ISZ: 13 (males) mean age: 32.46 (SD = 8.03) HC: 13 (males) mean age: 31.69 (SD = 7.65) Matched in age and sex. | EC through brain activity. EC through self-reported emotion. | fMRI PANAS/ ESR (emotional self-rating scale, unipolar for each emotion). | Pictures of facial expressions (sad and happy). | ISZ showed hypoactivation of the amygdala in the sadness induction compared to HC. No between-group differences for self-reported emotional state. | Thought disorder of the SAPS was positively correlated with the activity of the amygdala during happiness contagion (r = 0.58, p < 0.04). Hallucination and delusion subscales of the SAPS negatively were correlated with the emotional contagion of sadness through self-reported emotion (r = −0.56, p < 0.04, r = −0.60, p < 0.03). | No significant correlation was found. |
| Sestito et al., 2013 [61] | ISZ: 15 (5W, 10M), mean age: 32.8 (SD = 1.7) HC: 15 (5W, 10M), mean age: 35.8 (SD = 2.3) Matched in age and sex. | Emotional mimicry. | EMG | 2 s videos of professional actors showing positive, negative, and neutral expressions. Clips included vocalization and facial expressions. Stimuli were presented in different categories (visual, audio, audio–visual congruent). | No difference between groups for the corrugator. HC and ISZ reacted in a similar way to negative emotional stimuli. In the audio-only modality, ISZ showed no activation in reaction to positive stimuli compared to HC. In the audiovisual and visual modalities, ISZ exhibited a nonspecific response (i.e., a similar activation of the zygomaticus for the negative and the positive emotions). | Not reported. | Not reported. |
| Spilka et al., 2015 [62] | ISZ: n = 28 (13W, 15M) mean age: 41.07 (SD = 11.15) Relatives: n = 27 (17W, 10M) mean age: 41.19 (SD = 15.46) HC: n = 27 (14W, 13M) mean age: 40.7 (SD = 11.1) Matched in age and sex. | EC through brain activity. | fMRI | Pictures of facial expressions (happy, sad, angry, fearful, and neutral). | ISZ and relatives showed hypoactivation in the bilateral FFA (fusiform face area), OFA (occipital face area), and visual cortex in response to sadness stimuli. | Not reported. | Not reported. |
| Suslow et al., 2003 [63] | Anhedonic ISZ: n = 30 (15W, 15M) mean age: 37.1 (SD = 9.8) Flat affect ISZ: n = 30 (10W, 20M) mean age: 32.9 (SD = 8.4) ISZ: n = 28 (14W, 14M) mean age: 35.7 (SD = 9.4) HC: n = 30 (15W, 15M) mean age: 35.5 (SD = 8.6) Matched in age and sex. | EC through self-reported emotions. | Implicit affective valence measure. | Pictures of facial expressions (happiness, sadness, and neutral). | No between-group differences, except for anhedonic individuals who showed no emotional contagion in response to sadness stimuli. | Anhedonia subscale score of the SANS positively correlated with negative emotional contagion (r = 0.20, p < 0.05) and negatively correlated with positive emotional contagion (r = −0.21, p < 0.05). | No significant correlation was found. |
| Torregrossa et al., 2019 [64] | ISZ: n = 21 (9W, 12M), mean age: 47.9 (SD = 7.83) HC: n = 23 (13W, 10M), mean age: 45.65 (SD = 8.17) Matched in age and sex. | Emotional mimicry | EMG | Different avatars displayed a neutral face for 2 s and an emotional face (joy, surprise, sadness, fear, disgust, contempt, or anger) for 2.5 s at different intensities (low, medium, high). | No differences between HC and ISZ. | Not reported. | Not reported. |
| Varcin et al., 2010 [65] | ISZ: n = 25 (15W, 10M), mean age: 42.9 (SD = 9.43) HC: n = 25 (14W, 11M) 39.2 (SD = 10.85) Matched in age and sex. | Emotional mimicry. | EMG | Black and white pictures of facial expressions (angry and happy). | ISZ showed comparable activity with HC for the zygomaticus in response to happy stimuli and for the corrugator in reaction to the angry stimuli. ISZ showed no increased activation of the zygomaticus in the happy compared to the angry stimuli and no increased activation of the corrugator in the angry compared to the happy stimuli, whereas these activations were observed in the HC. | No significant correlation was found. | No significant correlation was found. |
| Varcin et al., 2019 [66] | ISZ: n = 24 (12W, 12M), mean age: 46.2 (SD = 8.78) HC: n = 21 (13W, 8M), mean age: 44.9 (SD = 13.54) Matched in age and sex. | Emotional mimicry. | EMG | Pictures of happy and angry facial expressions. | ISZ showed less zygomaticus activity while watching the happy stimuli and less corrugator activity while watching the angry stimuli compared to HC. | Zygomaticus activity in response to happy stimuli inversely correlated with levels of negative symptomatology (r = −0.45, p = 0.033) | No significant correlation was found. |
| Williams et al., 2004 [67] | ISZ: n = 27 (10W, 17M) mean age: 27.3 (SD = 9.6) HC: n = 22 (8W, 14M), mean age: 27.2 (SD = 8.1) Matched in age and sex. | EC through psychophysiological reactions. | Skin conductance (number and amplitude). | Pictures of expressions of fear and neutral expression. | ISZ produced more skin conductance (number and amplitude) than HC for fear and neutral expressions. HC showed a significant difference between reactions to neutral and fearful expressions, whereas ISZ did not show a significant difference between fear and neutral stimuli. Paranoid ISZ showed more skin conductance to fear than nonparanoid ISZ (amplitude and number). Paranoid ISZ and nonparanoid ISZ did not differ from neutral. | Not reported. | Not reported. |
| Williams et al., 2007 [68] | PISZ: n = 13 (5W, 8M), mean age: 26.9 (SD = 9.1) NPISZ: n = 14 (5W, 9M), mean age: 27.8 (SD = 10.4) HC: n = 13 (10W, 17M), mean age: 25.1 (SD = 8.1) Matched in age and sex. | EC through psychophysiological reactions. | Skin conductance (number and amplitude). | Pictures of expressions of fear anger and disgust. | PISZ generated a higher frequency and amplitude of SCRs in response to fear and disgust than HC. PISZ generated more SCRs than HC in response to anger. NPISZ elicited greater amplitude of SCRs than HC for disgust. PISZ elicited greater amplitude of SCRs to fear than NPISZ. | Heightened SCR amplitude correlated with higher levels of suspiciousness/persecution for both fear (r = 0.55, p = 0.009) and anger (r = 0.39, p = 0.02). A greater number of SCRs to disgust positively correlated with delusions (r = 0.47, p = 0.01). | Not reported. |
| Williams et al., 2009 [69] | FES: n = 28 (8W, 20M) mean age: 19 (SD = 3) HC: n = 72 (18W, 4M) mean age: 20 (SD = 2.8) Matched in age and sex. | EC through brain activity. | EEG | Pictures of facial expressions (fear and happiness) presented in conscious (500 ms) and nonconscious conditions (10ms of emotional expression, followed by 150 ms of a neutral face). | FES showed absolute and relative gamma synchrony alterations under conscious and nonconscious conditions in response to happy and fearful stimuli. | PANSS-negative symptoms were positively predicted by left temporal synchrony (R2 = 0.18). | No significant correlation was found. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parisi, M.; Marin, L.; Fauviaux, T.; Aigoin, E.; Raffard, S. Emotional Contagion and Emotional Mimicry in Individuals with Schizophrenia: A Systematic Review. J. Clin. Med. 2024, 13, 5296. https://doi.org/10.3390/jcm13175296
Parisi M, Marin L, Fauviaux T, Aigoin E, Raffard S. Emotional Contagion and Emotional Mimicry in Individuals with Schizophrenia: A Systematic Review. Journal of Clinical Medicine. 2024; 13(17):5296. https://doi.org/10.3390/jcm13175296
Chicago/Turabian StyleParisi, Mathilde, Ludovic Marin, Tifenn Fauviaux, Emilie Aigoin, and Stéphane Raffard. 2024. "Emotional Contagion and Emotional Mimicry in Individuals with Schizophrenia: A Systematic Review" Journal of Clinical Medicine 13, no. 17: 5296. https://doi.org/10.3390/jcm13175296
APA StyleParisi, M., Marin, L., Fauviaux, T., Aigoin, E., & Raffard, S. (2024). Emotional Contagion and Emotional Mimicry in Individuals with Schizophrenia: A Systematic Review. Journal of Clinical Medicine, 13(17), 5296. https://doi.org/10.3390/jcm13175296






