Functional Connectome Controllability in Patients with Mild Cognitive Impairment after Repetitive Transcranial Magnetic Stimulation of the Dorsolateral Prefrontal Cortex
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Controllability Metrics
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dubois, B.; Feldman, H.H.; Jacova, C.; Hampel, H.; Molinuevo, J.L.; Blennow, K.; DeKosky, S.T.; Gauthier, S.; Selkoe, D.; Bateman, R.; et al. Advancing Research Diagnostic Criteria for Alzheimer’s Disease: The IWG-2 Criteria. Lancet Neurol. 2014, 13, 614–629. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Zhang, Y.; Shi, H.; Jiao, Z.; Wang, S.-H.; Wang, C. Constructing Dynamic Brain Functional Networks via Hyper-Graph Manifold Regularization for Mild Cognitive Impairment Classification. Front. Neurosci. 2021, 15, 669345. [Google Scholar] [CrossRef] [PubMed]
- Albert, M.S.; DeKosky, S.T.; Dickson, D.; Dubois, B.; Feldman, H.H.; Fox, N.C.; Gamst, A.; Holtzman, D.M.; Jagust, W.J.; Petersen, R.C.; et al. The Diagnosis of Mild Cognitive Impairment Due to Alzheimer’s Disease: Recommendations from the National Institute on Aging-Alzheimer’s Association Workgroups on Diagnostic Guidelines for Alzheimer’s Disease. Alzheimer’s Dement. 2011, 7, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Reisberg, B.; Ferris, S.H.; de Leon, M.J.; Franssen, E.S.E.; Kluger, A.; Mir, P.; Borenstein, J.; George, A.E.; Shulman, E.; Steinberg, G.; et al. Stage-Specific Behavioral, Cognitive, and in Vivo Changes in Community Residing Subjects with Age-Associated Memory Impairment and Primary Degenerative Dementia of the Alzheimer Type. Drug Dev. Res. 1988, 15, 101–114. [Google Scholar] [CrossRef]
- Anderson, N.D. State of the Science on Mild Cognitive Impairment (MCI). CNS Spectr. 2019, 24, 78–87. [Google Scholar] [CrossRef]
- Jiao, Z.; Gao, P.; Ji, Y.; Shi, H. Integration and Segregation of Dynamic Functional Connectivity States for Mild Cognitive Impairment Revealed by Graph Theory Indicators. Contrast Media Mol. Imaging 2021, 2021, 6890024. [Google Scholar] [CrossRef]
- Misra, C.; Fan, Y.; Davatzikos, C. Baseline and Longitudinal Patterns of Brain Atrophy in MCI Patients, and Their Use in Prediction of Short-Term Conversion to AD: Results from ADNI. Neuroimage 2009, 44, 1415–1422. [Google Scholar] [CrossRef]
- Plassman, B.L.; Langa, K.M.; Fisher, G.G.; Heeringa, S.G.; Weir, D.R.; Ofstedal, M.B.; Burke, J.R.; Hurd, M.D.; Potter, G.G.; Rodgers, W.L.; et al. Prevalence of Dementia in the United States: The Aging, Demographics, and Memory Study. Neuroepidemiology 2007, 29, 125–132. [Google Scholar] [CrossRef]
- Mitchell, A.J.; Shiri-Feshki, M. Rate of Progression of Mild Cognitive Impairment to Dementia--Meta-Analysis of 41 Robust Inception Cohort Studies. Acta Psychiatr. Scand. 2009, 119, 252–265. [Google Scholar] [CrossRef]
- Chen, G.; Chen, G.; Xie, C.; Li, S.-J. Negative Functional Connectivity and Its Dependence on the Shortest Path Length of Positive Network in the Resting-State Human Brain. Brain Connect. 2011, 1, 195–206. [Google Scholar] [CrossRef]
- Diogo, V.S.; Ferreira, H.A.; Prata, D.; The Alzheimer’s Disease Neuroimaging Initiative. Early Diagnosis of Alzheimer’s Disease Using Machine Learning: A Multi-Diagnostic, Generalizable Approach. Alzheimer’s Res. Ther. 2022, 14, 107. [Google Scholar] [CrossRef]
- Lissek, V.; Suchan, B. Preventing Dementia? Interventional Approaches in Mild Cognitive Impairment. Neurosci. Biobehav. Rev. 2021, 122, 143–164. [Google Scholar] [CrossRef] [PubMed]
- Sharbafshaaer, M.; Gigi, I.; Lavorgna, L.; Esposito, S.; Bonavita, S.; Tedeschi, G.; Esposito, F.; Trojsi, F. Repetitive Transcranial Magnetic Stimulation (rTMS) in Mild Cognitive Impairment: Effects on Cognitive Functions—A Systematic Review. J. Clin. Med. 2023, 12, 6190. [Google Scholar] [CrossRef] [PubMed]
- Hallett, M. Transcranial Magnetic Stimulation: A Primer. Neuron 2007, 55, 187–199. [Google Scholar] [CrossRef]
- Dichter, G.S.; Gibbs, D.; Smoski, M.J. A Systematic Review of Relations between Resting-State Functional-MRI and Treatment Response in Major Depressive Disorder. J. Affect. Disord. 2015, 172, 8–17. [Google Scholar] [CrossRef]
- Cirillo, G.; Di Pino, G.; Capone, F.; Ranieri, F.; Florio, L.; Todisco, V.; Tedeschi, G.; Funke, K.; Di Lazzaro, V. Neurobiological After-Effects of Non-Invasive Brain Stimulation. Brain Stimul. 2017, 10, 1–18. [Google Scholar] [CrossRef]
- Esposito, S.; Trojsi, F.; Cirillo, G.; de Stefano, M.; Di Nardo, F.; Siciliano, M.; Caiazzo, G.; Ippolito, D.; Ricciardi, D.; Buonanno, D.; et al. Repetitive Transcranial Magnetic Stimulation (rTMS) of Dorsolateral Prefrontal Cortex May Influence Semantic Fluency and Functional Connectivity in Fronto-Parietal Network in Mild Cognitive Impairment (MCI). Biomedicines 2022, 10, 994. [Google Scholar] [CrossRef]
- Ponteri, M. RBANS Repeatable Battery for the Assessment of Neuropsychological Status; Giunti: Florence, Italy, 2007. [Google Scholar]
- Pasqualetti, F.; Zampieri, S.; Bullo, F. Controllability Metrics, Limitations and Algorithms for Complex Networks. IEEE Trans. Control Netw. Syst. 2014, 1, 40–52. [Google Scholar] [CrossRef]
- Beynel, L.; Deng, L.; Crowell, C.A.; Dannhauer, M.; Palmer, H.; Hilbig, S.; Peterchev, A.V.; Luber, B.; Lisanby, S.H.; Cabeza, R.; et al. Structural Controllability Predicts Functional Patterns and Brain Stimulation Benefits Associated with Working Memory. J. Neurosci. 2020, 40, 6770–6778. [Google Scholar] [CrossRef]
- Alizadeh Darbandi, S.S.; Fornito, A.; Ghasemi, A. The Impact of Input Node Placement in the Controllability of Structural Brain Networks. Sci. Rep. 2024, 14, 6902. [Google Scholar] [CrossRef]
- Gu, S.; Betzel, R.F.; Mattar, M.G.; Cieslak, M.; Delio, P.R.; Grafton, S.T.; Pasqualetti, F.; Bassett, D.S. Optimal Trajectories of Brain State Transitions. NeuroImage 2017, 148, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Medaglia, J.D.; Pasqualetti, F.; Hamilton, R.H.; Thompson-Schill, S.L.; Bassett, D.S. Brain and Cognitive Reserve: Translation via Network Control Theory. Neurosci. Biobehav. Rev. 2017, 75, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhang, S.; Yu, X.; Niu, Y.; Niu, J.; Li, D.; Zhang, S.; Xiang, J.; Yan, T.; Yang, J.; et al. Alterations in White Matter Network Dynamics in Patients with Schizophrenia and Bipolar Disorder. Hum. Brain Mapp. 2022, 43, 3909–3922. [Google Scholar] [CrossRef] [PubMed]
- Petrides, M. Lateral Prefrontal Cortex: Architectonic and Functional Organization. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2005, 360, 781–795. [Google Scholar] [CrossRef] [PubMed]
- Jimura, K.; Chushak, M.S.; Westbrook, A.; Braver, T.S. Intertemporal Decision-Making Involves Prefrontal Control Mechanisms Associated with Working Memory. Cereb. Cortex 2018, 28, 1105–1116. [Google Scholar] [CrossRef]
- Kim, K.; Sherwood, M.S.; McIntire, L.K.; McKinley, R.A.; Ranganath, C. Transcranial Direct Current Stimulation Modulates Connectivity of Left Dorsolateral Prefrontal Cortex with Distributed Cortical Networks. J. Cogn. Neurosci. 2021, 33, 1381–1395. [Google Scholar] [CrossRef]
- Tang, R.; Elman, J.A.; Franz, C.E.; Dale, A.M.; Eyler, L.T.; Fennema-Notestine, C.; Hagler, D.J.; Lyons, M.J.; Panizzon, M.S.; Puckett, O.K.; et al. Longitudinal Association of Executive Function and Structural Network Controllability in the Aging Brain. GeroScience 2023, 45, 837–849. [Google Scholar] [CrossRef] [PubMed]
- O’Neil-Pirozzi, T.M.; Doruk, D.; Thomson, J.M.; Fregni, F. Immediate Memory and Electrophysiologic Effects of Prefrontal Cortex Transcranial Direct Current Stimulation on Neurotypical Individuals and Individuals with Chronic Traumatic Brain Injury: A Pilot Study. Int. J. Neurosci. 2017, 127, 592–600. [Google Scholar] [CrossRef]
- Schaefer, A.; Kong, R.; Gordon, E.M.; Laumann, T.O.; Zuo, X.-N.; Holmes, A.J.; Eickhoff, S.B.; Yeo, B.T.T. Local-Global Parcellation of the Human Cerebral Cortex from Intrinsic Functional Connectivity MRI. Cereb. Cortex 2018, 28, 3095–3114. [Google Scholar] [CrossRef]
- Thomas Yeo, B.T.; Krienen, F.M.; Sepulcre, J.; Sabuncu, M.R.; Lashkari, D.; Hollinshead, M.; Roffman, J.L.; Smoller, J.W.; Zöllei, L.; Polimeni, J.R.; et al. The Organization of the Human Cerebral Cortex Estimated by Intrinsic Functional Connectivity. J. Neurophysiol. 2011, 106, 1125–1165. [Google Scholar] [CrossRef]
- Randolph, C.; Tierney, M.C.; Mohr, E.; Chase, T.N. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): Preliminary Clinical Validity. J. Clin. Exp. Neuropsychol. 1998, 20, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Li, J.; Thomas Yeo, B.T.; Gu, S. Control Theory Illustrates the Energy Efficiency in the Dynamic Reconfiguration of Functional Connectivity. Commun. Biol. 2022, 5, 295. [Google Scholar] [CrossRef]
- Karrer, T.M.; Kim, J.Z.; Stiso, J.; Kahn, A.E.; Pasqualetti, F.; Habel, U.; Bassett, D.S. A Practical Guide to Methodological Considerations in the Controllability of Structural Brain Networks. J. Neural Eng. 2020, 17, 026031. [Google Scholar] [CrossRef]
- Tang, E.; Bassett, D.S. Colloquium: Control of Dynamics in Brain Networks. Rev. Mod. Phys. 2018, 90, 031003. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B (Methodol.) 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Wilmskoetter, J.; He, X.; Caciagli, L.; Jensen, J.H.; Marebwa, B.; Davis, K.A.; Fridriksson, J.; Basilakos, A.; Johnson, L.P.; Rorden, C.; et al. Language Recovery after Brain Injury: A Structural Network Control Theory Study. J. Neurosci. 2022, 42, 657–669. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Chen, K.; Li, Y. Aerobic Exercise, an Effective Prevention and Treatment for Mild Cognitive Impairment. Front. Aging Neurosci. 2023, 15, 1194559. [Google Scholar] [CrossRef]
- Hötting, K.; Röder, B. Beneficial Effects of Physical Exercise on Neuroplasticity and Cognition. Neurosci. Biobehav. Rev. 2013, 37, 2243–2257. [Google Scholar] [CrossRef] [PubMed]
- Biesbroek, J.M.; Lim, J.-S.; Weaver, N.A.; Arikan, G.; Kang, Y.; Kim, B.J.; Kuijf, H.J.; Postma, A.; Lee, B.-C.; Lee, K.-J.; et al. Anatomy of Phonemic and Semantic Fluency: A Lesion and Disconnectome Study in 1231 Stroke Patients. Cortex 2021, 143, 148–163. [Google Scholar] [CrossRef]
- Cowan, N. Working Memory Underpins Cognitive Development, Learning, and Education. Educ. Psychol. Rev. 2014, 26, 197–223. [Google Scholar] [CrossRef]
- Persson, J.; Lustig, C.; Nelson, J.K.; Reuter-Lorenz, P.A. Age Differences in Deactivation: A Link to Cognitive Control? J. Cogn. Neurosci. 2007, 19, 1021–1032. [Google Scholar] [CrossRef] [PubMed]
- Menardi, A.; Momi, D.; Vallesi, A.; Barabási, A.-L.; Towlson, E.K.; Santarnecchi, E. Maximizing Brain Networks Engagement via Individualized Connectome-Wide Target Search. Brain Stimul. 2022, 15, 1418–1431. [Google Scholar] [CrossRef] [PubMed]
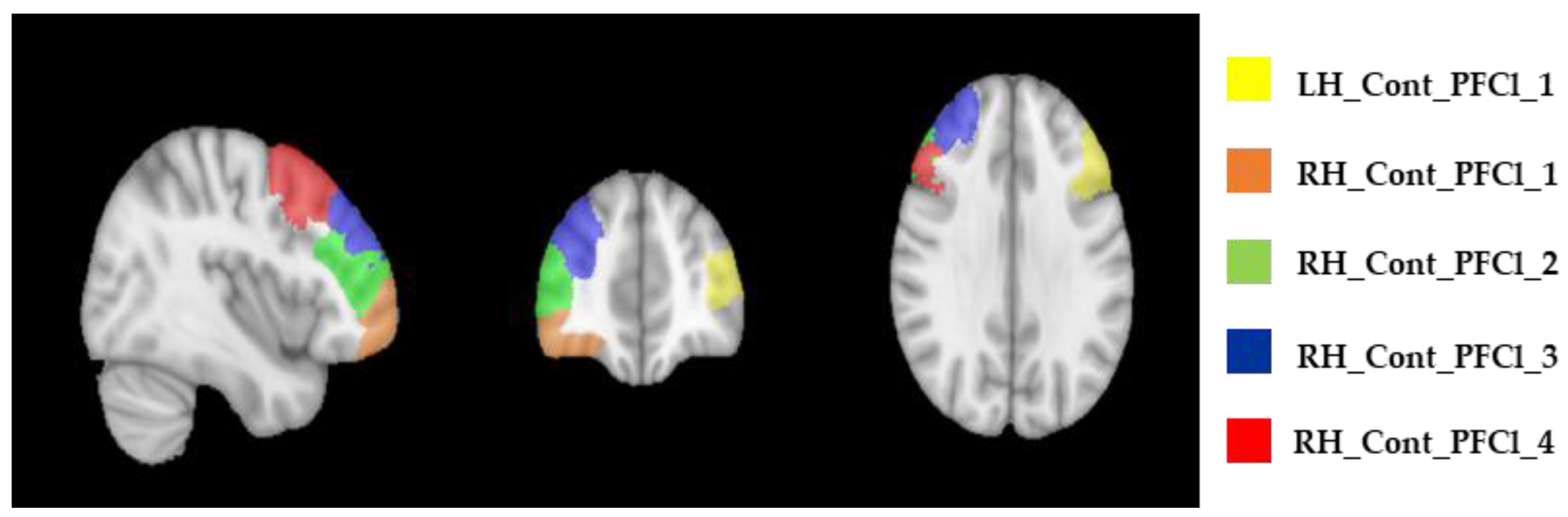

| Variable | MCI-TMS (n = 10) | MCI-C (n = 10) | a MWU Tests b χ2 test | p-Value |
|---|---|---|---|---|
| Age, years | 64.0 (60.8, 71.2) | 70.5 (60.5, 73.8) | a 93.00 | 0.383 |
| Sex, male | 4 (40%) | 5 (50%) | b 0.22 | 0.639 |
| Story memory-IR at T0 | 15 (15, 16.5) | 13 (10.8, 15) | 33 | 0.201 |
| Line orientation at T0 | 14 (11.2, 16.8) | 13 (11, 16.2) | 44.5 | 0.704 |
| Semantic fluency at T0 | 13 (12, 14.5) | 16 (13.5, 17) | 73 | 0.0836 |
| ROI Labels | Baseline—T0 Median (IQR) | After 4 Weeks—T1 Median (IQR) | After 6 Months—T2 Median (IQR) |
|---|---|---|---|
| LH_Cont_PFCl_1 | 1.30 (1.19–1.32) | 1.26 (1.19–1.31) | 1.24 (1.17–1.34) |
| RH_Cont_PFCl_1 | 1.01 (1.00–1.03) | 1.03 (1.01–1.07) | 1.02 (1.01–1.15) |
| RH_Cont_PFCl_2 | 1.06 (1.03–1.11) | 1.07 (1.05–1.17) | 1.06 (1.02–1.15) |
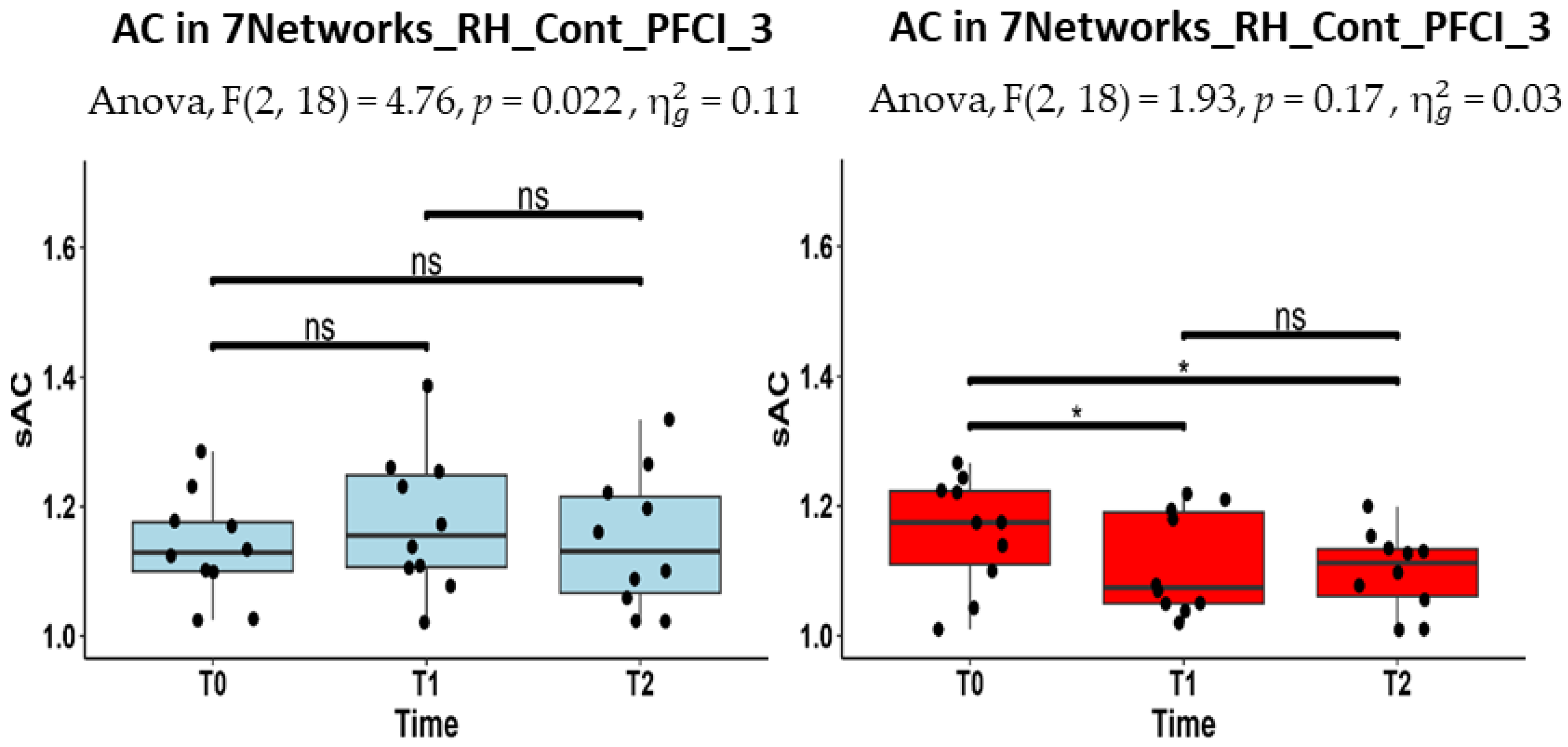
| RH_Cont_PFCl_3 | 1.13 (1.10–1.18) | 1.16 (1.11–1.25) | 1.13 (1.07–1.22) |
| RH_Cont_PFCl_4 | 1.19 (1.12–1.22) | 1.21 (1.14–1.25) | 1.21 (1.07–1.30) |
| ROI Labels | Baseline—T0 Median (IQR) | After 4 Weeks—T1 Median (IQR) | After 6 Months—T2 Median (IQR) |
|---|---|---|---|
| LH_Cont_PFCl_1 | 1.18 (1.10–1.22) | 1.17 (1.14–1.25) | 1.17 (1.12–1.27) |
| RH_Cont_PFCl_1 | 1.02 (1.00–1.08) | 1.02 (1.00–1.03) | 1.05 (1.02–1.10) |
| RH_Cont_PFCl_2 | 1.10 (1.03–1.19) | 1.03 (1.02–1.15) | 1.08 (1.02–1.11) |
| RH_Cont_PFCl_3 | 1.17 (1.11- 1.22) | 1.07 (1.05–1.19) | 1.11 (1.06–1.13) |
| RH_Cont_PFCl_4 | 1.17 (1.11–1.24) | 1.10 (1.04–1.15) | 1.10 (1.04–1.20) |
| ROI Labels | Baseline—T0 Median (IQR) | After 4 Weeks—T1 Median (IQR) | After 6 Months—T2 Median (IQR) |
|---|---|---|---|
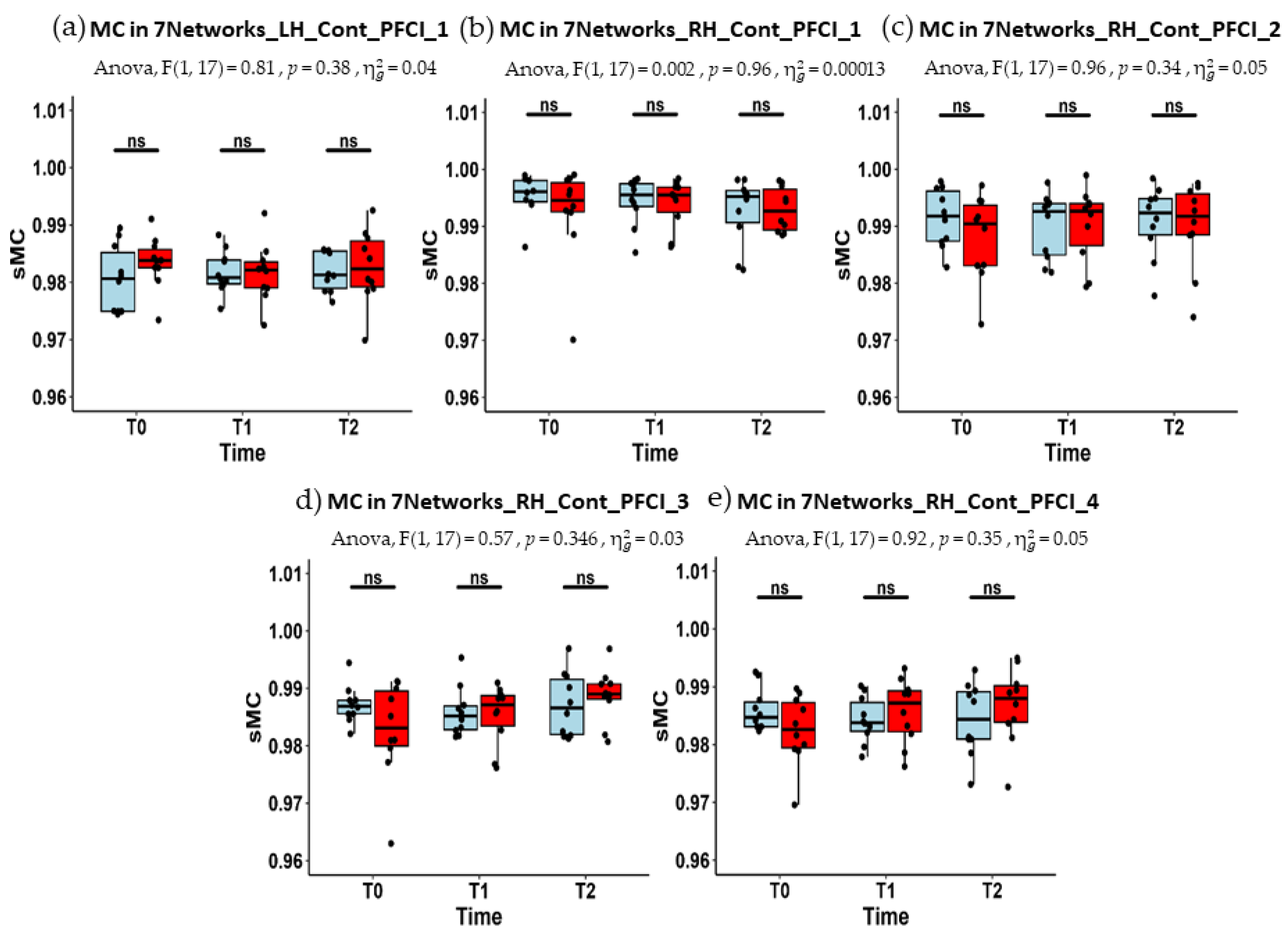
| LH_Cont_PFCl_1 | 0.981 (0.975–0.985) | 0.981 (0.980–0.984) | 0.981 (0.979–0.985) |
| RH_Cont_PFCl_1 | 0.996 (0.994–0.998) | 0.996 (0.993–0.997) | 0.995 (0.991–0.996) |
| RH_Cont_PFCl_2 | 0.992 (0.987–0.996) | 0.993 (0.985–0.994) | 0.992 (0.988–0.995) |
| RH_Cont_PFCl_3 | 0.987 (0.986–0.988) | 0.985 (0.983–0.987) | 0.987 (0.982–0.992) |
| RH_Cont_PFCl_4 | 0.985 (0.983–0.987) | 0.984 (0.982–0.987) | 0.984 (0.981–0.989) |
| ROI Labels | Baseline—T0 Median (IQR) | After 4 Weeks—T1 Median (IQR) | After 6 Months—T2 Median (IQR) |
|---|---|---|---|
| LH_Cont_PFCl_1 | 0.984 (0.983–0.986) | 0.982 (0.979–0.984) | 0.982 (0.979–0.984) |
| RH_Cont_PFCl_1 | 0.995 (0.993–0.998) | 0.995 (0.992–0.997) | 0.993 (0.989–0.997) |
| RH_Cont_PFCl_2 | 0.990 (0.983–0.994) | 0.993 (0.987–0.994) | 0.992 (0.988–0.996) |
| RH_Cont_PFCl_3 | 0.983 (0.980–0.990) | 0.987 (0.983–0.989) | 0.989 (0.988–0.991) |
| RH_Cont_PFCl_4 | 0.983 (0.979–0.987) | 0.987 (0.982–0.989) | 0.988 (0.984–0.990) |
| ROI Labels And Time Point | Story Memory-IR ρ | Line Orientation ρ | Semantic Fluency ρ |
|---|---|---|---|
| LH_Cont_PFCl_1 at T1 | −0.2857 | 0.1273 | −0.0303 |
| RH_Cont_PFCl_1 at T1 | 0.2917 | 0.4788 | 0.3576 |
| RH_Cont_PFCl_2 at T1 | 0.3890 | 0.4667 | 0.5636 |
| RH_Cont_PFCl_3 at T1 | 0.2188 | 0.5030 | 0.4788 |
| RH_Cont_PFCl_4 at T1 | 0.0364 | 0.4303 | 0.3939 |
| LH_Cont_PFCl_1 at T2 | −0.0121 | 0.0061 | −0.1951 |
| RH_Cont_PFCl_1 at T2 | −0.1763 | 0.3526 | 0.3659 |
| RH_Cont_PFCl_2 at T2 | 0.1702 | 0.3465 | 0.3720 |
| RH_Cont_PFCl_3 at T2 | −0.0364 | −0.0973 | −0.2805 |
| RH_Cont_PFCl_4 at T2 | −0.1641 | −0.0486 | 0.3903 |
| ROI Labels and Time Point | Story Memory-IR ρ | Line Orientation ρ | Semantic Fluency ρ |
|---|---|---|---|
| LH_Cont_PFCl_1 at T1 | −0.5653 | 0.2364 | −0.4182 |
| RH_Cont_PFCl_1 at T1 | 0.3890 | −0.1152 | −0.7939 |
| RH_Cont_PFCl_2 at T1 | 0.0243 | −0.1879 | −0.4788 |
| RH_Cont_PFCl_3 at T1 | 0.0243 | −0.2000 | −0.2364 |
| RH_Cont_PFCl_4 at T1 | 0.0851 | −0.4182 | −0.4424 |
| LH_Cont_PFCl_1 at T2 | 0.5653 | 0.3161 | 0.1030 |
| RH_Cont_PFCl_1 at T2 | −0.5288 | 0.1885 | −0.1636 |
| RH_Cont_PFCl_2 at T2 | −0.2067 | −0.0608 | 0.1152 |
| RH_Cont_PFCl_3 at T2 | −0.1094 | −0.0912 | −0.1273 |
| RH_Cont_PFCl_4 at T2 | 0.0182 | −0.3100 | −0.1879 |
| ROI Labels And Time point | Story Memory-IR ρ | Line Orientation ρ | Semantic Fluency ρ |
|---|---|---|---|
| LH_Cont_PFCl_1 at T1 | 0.7234 | 0.4182 | 0.5636 |
| RH_Cont_PFCl_1 at T1 | 0.5167 | −0.3576 | 0.3212 |
| RH_Cont_PFCl_2 at T1 | −0.3829 | −0.3091 | −0.5879 |
| RH_Cont_PFCl_3 at T1 | 0.0668 | −0.2242 | −0.2606 |
| RH_Cont_PFCl_4 at T1 | 0.3222 | −0.1758 | −0.1152 |
| LH_Cont_PFCl_1 at T2 | 0.4194 | 0.1824 | 0.5000 |
| RH_Cont_PFCl_1 at T2 | 0.4741 | 0.3222 | 0.0549 |
| RH_Cont_PFCl_2 at T2 | 0.1823 | −0.3283 | −0.5366 |
| RH_Cont_PFCl_3 at T2 | 0.7112 | 0.4620 | 0.6829 |
| RH_Cont_PFCl_4 at T2 | 0.3282 | 0.2492 | −0.1341 |
| ROI Labels and Time Point | Story Memory-IR ρ | Line Orientation ρ | Semantic Fluency ρ |
|---|---|---|---|
| LH_Cont_PFCl_1 at T1 | 0.6018 | −0.2727 | −0.7212 |
| RH_Cont_PFCl_1 at T1 | −0.5654 | −0.2000 | 0.6242 |
| RH_Cont_PFCl_2 at T1 | −0.0729 | −0.1030 | 0.5879 |
| RH_Cont_PFCl_3 at T1 | 0.0304 | −0.1758 | 0.2485 |
| RH_Cont_PFCl_4 at T1 | 0.1216 | 0.4061 | 0.3333 |
| LH_Cont_PFCl_1 at T2 | −0.1520 | 0.0851 | 0.2121 |
| RH_Cont_PFCl_1 at T2 | 0.7234 | 0.1824 | 0.3697 |
| RH_Cont_PFCl_2 at T2 | 0.2736 | 0.4924 | −0.0909 |
| RH_Cont_PFCl_3 at T2 | 0.7416 | 0.4134 | 0.4061 |
| RH_Cont_PFCl_4 at T2 | 0.5228 | 0.5957 | 0.3576 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papallo, S.; Di Nardo, F.; Siciliano, M.; Esposito, S.; Canale, F.; Cirillo, G.; Cirillo, M.; Trojsi, F.; Esposito, F. Functional Connectome Controllability in Patients with Mild Cognitive Impairment after Repetitive Transcranial Magnetic Stimulation of the Dorsolateral Prefrontal Cortex. J. Clin. Med. 2024, 13, 5367. https://doi.org/10.3390/jcm13185367
Papallo S, Di Nardo F, Siciliano M, Esposito S, Canale F, Cirillo G, Cirillo M, Trojsi F, Esposito F. Functional Connectome Controllability in Patients with Mild Cognitive Impairment after Repetitive Transcranial Magnetic Stimulation of the Dorsolateral Prefrontal Cortex. Journal of Clinical Medicine. 2024; 13(18):5367. https://doi.org/10.3390/jcm13185367
Chicago/Turabian StylePapallo, Simone, Federica Di Nardo, Mattia Siciliano, Sabrina Esposito, Fabrizio Canale, Giovanni Cirillo, Mario Cirillo, Francesca Trojsi, and Fabrizio Esposito. 2024. "Functional Connectome Controllability in Patients with Mild Cognitive Impairment after Repetitive Transcranial Magnetic Stimulation of the Dorsolateral Prefrontal Cortex" Journal of Clinical Medicine 13, no. 18: 5367. https://doi.org/10.3390/jcm13185367
APA StylePapallo, S., Di Nardo, F., Siciliano, M., Esposito, S., Canale, F., Cirillo, G., Cirillo, M., Trojsi, F., & Esposito, F. (2024). Functional Connectome Controllability in Patients with Mild Cognitive Impairment after Repetitive Transcranial Magnetic Stimulation of the Dorsolateral Prefrontal Cortex. Journal of Clinical Medicine, 13(18), 5367. https://doi.org/10.3390/jcm13185367






