Non-Conventional Prognostic Markers in Life-Threatening COVID-19 Cases—When Less Is More
Abstract
:1. Introduction
2. Materials and Methods
2.1. Inclusion/Exclusion Criteria
2.2. Location and Design
2.3. Laboratory Analysis
2.4. Patient Demographics
2.5. Treatment
- -
- All patients were subcutaneously given a preventive dose of low-molecular-weight heparin (LMWH) in enoxaparin.
- -
- Dexamethasone (12 mg) was administered intravenously to all patients upon admission to the intensive care unit.
- -
- Preventive antibiotic treatment was not employed.
- -
- Each patient received remdesivir intravenously, with a loading dose of 200 mg on Day 1, followed by 100 mg for the subsequent four days.
- -
- Biological therapy was not utilised in any of the patients.
- -
- The preventative administration of platelet aggregation inhibitors was not part of the treatment strategy.
- -
- Organ support therapy was performed as required (IRRT, CRRT, mechanical ventilation, and so on).
2.6. Ethics Approval and Consent
2.7. Statistical Analysis
3. Results
3.1. Data Analysis
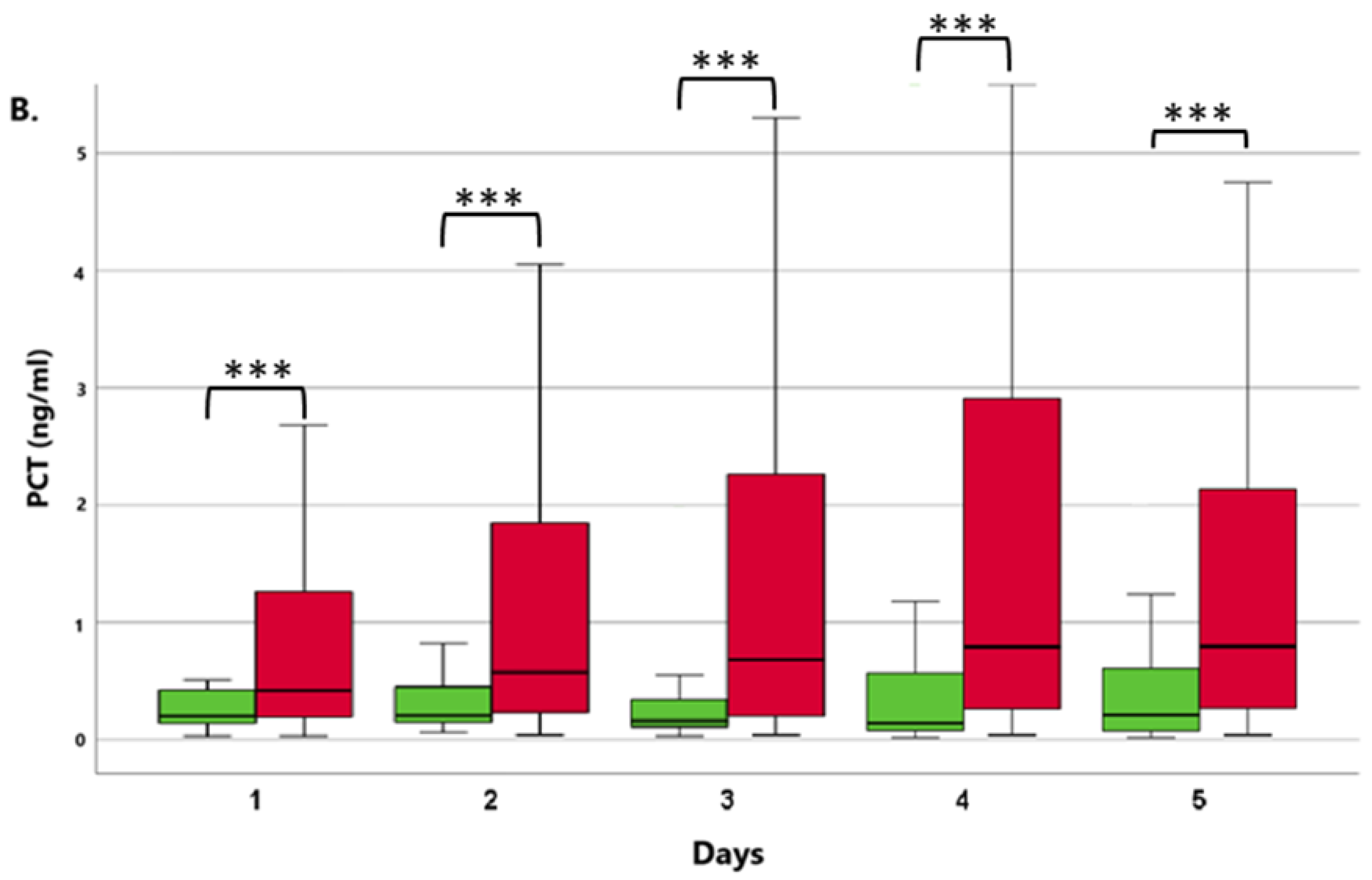
3.1.1. Comparison of ICU Survivor and Non-Survivor Groups
3.1.2. Patients with or without the Need for Renal Replacement Therapy (RRT)
3.1.3. Multivariate Regression Analysis
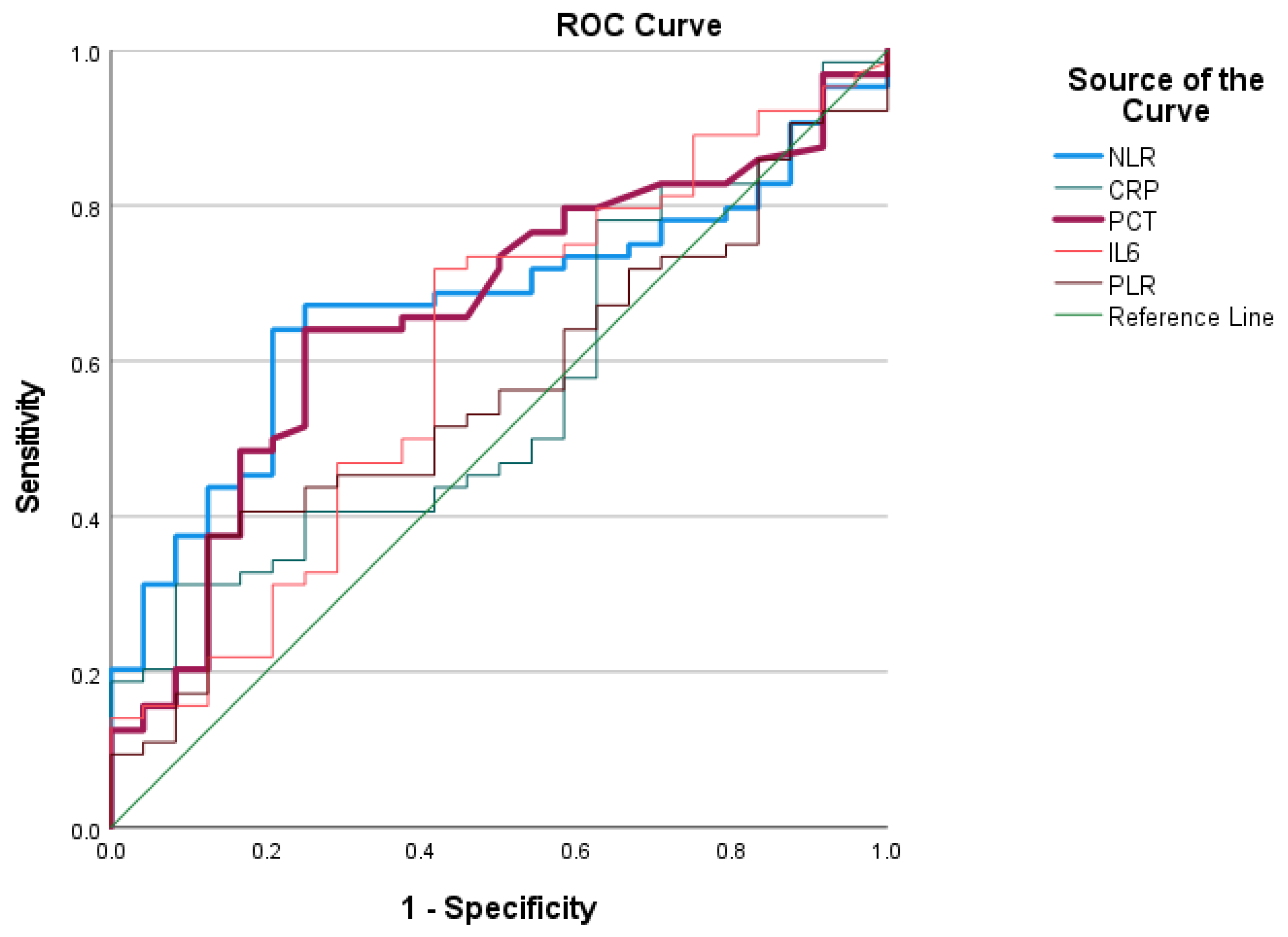
3.1.4. ROC Analysis
4. Discussion
5. Study Limitations
6. Conclusions
7. Future Utilisation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Q.; Guan, X.; Wu, P.; Wang, X.; Zhou, L.; Tong, Y.; Ren, R.; Leung, K.S.M.; Lau, E.H.Y.; Wong, J.Y.; et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus–Infected Pneumonia. N. Engl. J. Med. 2020, 382, 1199–1207. [Google Scholar] [CrossRef]
- Yin, Y.; Wunderink, R.G. MERS, SARS and other coronaviruses as causes of pneumonia. Respirology 2018, 23, 130–137. [Google Scholar] [CrossRef]
- Temperton, N.J.; Chan, P.K.; Simmons, G.; Zambon, M.C.; Tedder, R.S.; Takeuchi, Y.; Weiss, R.A. Longitudinally profiling neutralizing antibody response to SARS coronavirus with pseudotypes. Emerg. Infect. Dis. 2005, 11, 411–416. [Google Scholar] [CrossRef]
- Kang, D.H.; Kim, G.H.J.; Park, S.; Lee, S.; Koh, J.S.; Brown, M.S.; Abtin, F.; Mcnitt-gray, M.F.; Goldin, J.G.; Lee, J.S. Quantitative Computed Tomography Lung COVID Scores with Laboratory Markers: Utilization to Predict Rapid Progression and Monitor Longitudinal Changes in Patients with. Biomedicines 2024, 12, 120. [Google Scholar] [CrossRef]
- Yang, L.; Liu, S.; Liu, J.; Zhang, Z.; Wan, X.; Huang, B.; Chen, Y.; Zhang, Y. COVID-19: Immunopathogenesis and Immunotherapeutics. Signal Transduct. Target. Ther. 2020, 5, 128. [Google Scholar] [CrossRef]
- Hu, B.; Huang, S.; Yin, L. The cytokine storm and COVID-19. J. Med. Virol. 2021, 93, 250–256. [Google Scholar] [CrossRef]
- Chen, G.; Wu, D.; Guo, W.; Cao, Y.; Huang, D.; Wang, H.; Wang, T.; Zhang, X.; Chen, H.; Yu, H.; et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Investig. 2020, 130, 2620–2629. [Google Scholar] [CrossRef]
- Liu, J.; Li, S.; Liu, J.; Liang, B.; Wang, X.; Wang, H.; Li, W.; Tong, Q.; Yi, J.; Zhao, L.; et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine 2020, 55, 102763. [Google Scholar] [CrossRef]
- Wang, C.; Li, W.; Drabek, D.; Okba, N.M.A.; van Haperen, R.; Osterhaus, A.D.M.E.; van Kuppeveld, F.J.M.; Haagmans, B.L.; Grosveld, F.; Bosch, B.J. A human monoclonal antibody blocking SARS-CoV-2 infection. Nat. Commun. 2020, 11, 2251. [Google Scholar] [CrossRef]
- Agresti, N.; Lalezari, J.P.; Amodeo, P.P.; Mody, K.; Mosher, S.F.; Seethamraju, H.; Kelly, S.A.; Pourhassan, N.Z.; Sudduth, C.D.; Bovinet, C.; et al. Disruption of CCR5 signaling to treat COVID-19-associated cytokine storm: Case series of four critically ill patients treated with leronlimab. J. Transl. Autoimmun. 2021, 4, 100083. [Google Scholar] [CrossRef]
- Salton, F.; Confalonieri, P.; Campisciano, G.; Cifaldi, R.; Rizzardi, C.; Generali, D.; Pozzan, R.; Tavano, S.; Bozzi, C.; Lapadula, G.; et al. Cytokine Profiles as Potential Prognostic and Therapeutic Markers in SARS-CoV-2-Induced ARDS. J. Clin. Med. 2022, 11, 2951. [Google Scholar] [CrossRef]
- Zhu, Z.; Chakraborti, S.; He, Y.; Roberts, A.; Sheahan, T.; Xiao, D.; Hensley, L.E.; Prabakaran, P.; Rockx, B.; Sidorov, I.A.; et al. Potent cross-reactive neutralization of SARS coronavirus isolates by human monoclonal antibodies. Proc. Natl. Acad. Sci. USA 2007, 104, 12123–12128. [Google Scholar] [CrossRef]
- Yang, L.; Xie, X.; Tu, Z.; Fu, J.; Xu, D.; Zhou, Y. The signal pathways and treatment of cytokine storm in COVID-19. Signal Transduct. Target. Ther. 2021, 6, 255. [Google Scholar] [CrossRef]
- Russell, C.D.; Parajuli, A.; Gale, H.J.; Bulteel, N.S.; Schuetz, P.; de Jager, C.P.C.; Loonen, A.J.M.; Merekoulias, G.I.; Baillie, J.K. The utility of peripheral blood leucocyte ratios as biomarkers in infectious diseases: A systematic review and meta-analysis. J. Infect. 2019, 78, 339–348. [Google Scholar] [CrossRef]
- Curbelo, J.; Rajas, O.; Arnalich, B.; Galván-Román, J.M.; Luquero-Bueno, S.; Ortega-Gómez, M.; Lancho, A.; Roy, E.; Sánchez Azofra, A.; Mateo Jiménez, G.; et al. Neutrophil Count Percentage and Neutrophil–Lymphocyte Ratio as Prognostic Markers in Patients Hospitalized for Community-Acquired Pneumonia. Arch. Bronconeumol. 2019, 55, 472–477. [Google Scholar] [CrossRef]
- Peng, J.; Qi, D.; Yuan, G.; Deng, X.; Mei, Y.; Feng, L.; Wang, D. Diagnostic value of peripheral hematologic markers for coronavirus disease 2019 (COVID-19): A multicenter, cross-sectional study. J. Clin. Lab. Anal. 2020, 34, e23475. [Google Scholar] [CrossRef]
- Lagunas-Rangel, F.A. Neutrophil-to-lymphocyte ratio and lymphocyte-to-C-reactive protein ratio in patients with severe coronavirus disease 2019 (COVID-19): A meta-analysis. J. Med. Virol. 2020, 92, 1733–1734. [Google Scholar] [CrossRef]
- Qu, R.; Ling, Y.; Zhang, Y.; Wei, L.; Chen, X.; Li, X.; Liu, X.; Liu, H.; Guo, Z.; Ren, H.; et al. Platelet-to-lymphocyte ratio is associated with prognosis in patients with coronavirus disease-19. J. Med. Virol. 2020, 92, 1533–1541. [Google Scholar] [CrossRef]
- Freund, O.; Weiss, T.E.; Tau, L.; Meidan, R.; Liron, Y.; Tchebiner, Z.; Bornstein, G. Safety and outcomes of an early discharge strategy with oxygen home therapy in stable severe COVID-19 patients. Infect. Dis. 2023, 55, 292–298. [Google Scholar] [CrossRef]
- Li, X.; Liu, C.; Mao, Z.; Xiao, M.; Wang, L.; Qi, S.; Zhou, F. Predictive values of neutrophil-to-lymphocyte ratio on disease severity and mortality in COVID-19 patients: A systematic review and meta-analysis. Crit. Care 2020, 24, 647. [Google Scholar] [CrossRef]
- Liu, F.; Li, L.; Da, X.M.; Wu, J.; Luo, D.; Zhu, Y.S.; Li, B.X.; Song, X.Y.; Zhou, X. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J. Clin. Virol. 2020, 127, 104370. [Google Scholar] [CrossRef] [PubMed]
- Damar Çakırca, T.; Torun, A.; Çakırca, G.; Portakal, R.D. Role of NLR, PLR, ELR and CLR in differentiating COVID-19 patients with and without pneumonia. Int. J. Clin. Pract. 2021, 75, 2–7. [Google Scholar] [CrossRef]
- Ponti, G.; Maccaferri, M.; Ruini, C.; Tomasi, A.; Ozben, T. Biomarkers associated with COVID-19 disease progression. Crit. Rev. Clin. Lab. Sci. 2020, 57, 389–399. [Google Scholar] [CrossRef]
- Seitz, T.; Holbik, J.; Grieb, A.; Karolyi, M.; Hind, J.; Gibas, G.; Neuhold, S.; Zoufaly, A.; Wenisch, C. The Role of Bacterial and Fungal Superinfection in Critical COVID-19. Viruses 2022, 14, 2785. [Google Scholar] [CrossRef]


| Survivors N = 39 (%) | Non-Survivors N = 128 (%) | All Patients N = 167 (%) | p-Value | |
|---|---|---|---|---|
| Age | 65 [30–80] | 71 [47.35–85.55] | 69 [46–85] | 0.667 |
| Male | 29 (74) | 75 (59) | 104 (62) | 0.112 |
| Female | 10 (26) | 53 (41) | 63 (38) | 0.017 |
| BMI > 30 | 21 (54) | 53 (41) | 74 (44) | 0.236 |
| Hypertension | 34 (87) | 57 (45) | 91(54) | <0.001 |
| Diabetes mellitus | 15 (38) | 31 (24) | 46 (28) | 0.124 |
| COPD | 2 (5) | 19 (15) | 21 (13) | 0.185 |
| RRT | 0 | 29 (23) | 29 (17) | <0.001 |
| Day | Marker | Sig. (p) | OR | 95% CI for OR |
|---|---|---|---|---|
| 1 | NLR | 0.152 | 1.037 | 0.987–1.089 |
| PCT | 0.047 | 3.267 | 1.014–10.528 | |
| 2 | NLR | 0.021 | 1.058 | 1.009–1.110 |
| 3 | NLR | 0.073 | 1.040 | 0.996–1.085 |
| CRP | 0.143 | 1.013 | 0.999–1.007 | |
| 4 | CRP | 0.040 | 1.013 | 1.001–1.026 |
| IL-6 | 0.041 | 1.013 | 1.001–1.025 | |
| 5 | NLR | 0.007 | 1.140 | 1.036–1.255 |
| IL-6 | 0.025 | 1.008 | 1.001–1.014 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rozanovic, M.; Várady-Szabó, K.; Domokos, K.; Kiss, T.; Loibl, C.; Márovics, G.; Rendeki, S.; Csontos, C. Non-Conventional Prognostic Markers in Life-Threatening COVID-19 Cases—When Less Is More. J. Clin. Med. 2024, 13, 5369. https://doi.org/10.3390/jcm13185369
Rozanovic M, Várady-Szabó K, Domokos K, Kiss T, Loibl C, Márovics G, Rendeki S, Csontos C. Non-Conventional Prognostic Markers in Life-Threatening COVID-19 Cases—When Less Is More. Journal of Clinical Medicine. 2024; 13(18):5369. https://doi.org/10.3390/jcm13185369
Chicago/Turabian StyleRozanovic, Martin, Kata Várady-Szabó, Kamilla Domokos, Tamás Kiss, Csaba Loibl, Gergely Márovics, Szilárd Rendeki, and Csaba Csontos. 2024. "Non-Conventional Prognostic Markers in Life-Threatening COVID-19 Cases—When Less Is More" Journal of Clinical Medicine 13, no. 18: 5369. https://doi.org/10.3390/jcm13185369







