Advancements in Modern Treatment Approaches for Central Post-Stroke Pain: A Narrative Review
Abstract
1. Introduction
2. Mechanisms Underlying CPSP
2.1. Pharmacologic Treatments for Central Post-Stroke Pain
2.1.1. Antidepressants
2.1.2. Anticonvulsants
2.1.3. Opioids
2.1.4. Alternative Pharmacotherapy
2.2. Non-Pharmacologic Treatments
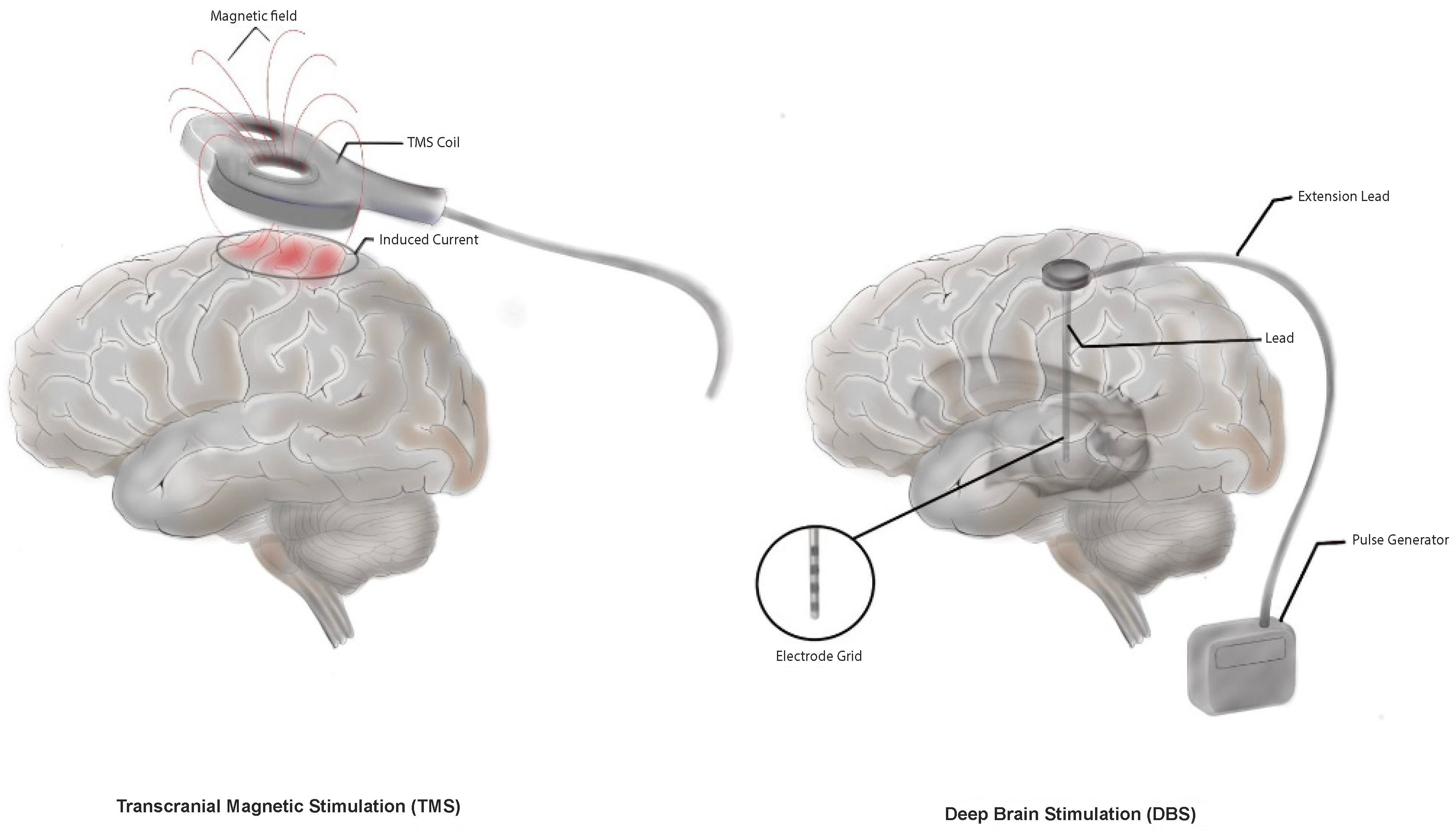
2.2.1. Deep Brain Stimulation
2.2.2. Motor Cortex Stimulation
2.2.3. Transcranial Magnetic Stimulation
2.2.4. Transcutaneous Electrical Nerve Stimulation
2.2.5. Cognitive Behavioral Therapy
2.2.6. Virtual Reality
2.2.7. Desensitization Therapy
3. Outcome and Outlook
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AEDs | Antiepileptic drugs |
| CBT | Cognitive behavioral therapy |
| CPSP | Central post-stroke pain |
| DBS | Deep brain stimulation |
| DLPFC | Dorsolateral prefrontal cortex |
| DTI | Diffusion tensor imaging |
| fMRI | Functional MRI |
| MCS | Motor cortex stimulation |
| M1 | Primary motor cortex |
| PET | Positron emission tomography |
| SNRIs | Serotonin-norepinephrine reuptake inhibitors |
| S2 | Secondary Somatosensory Cortex |
| TCA | Tricyclic Antidepressant |
| TENS | Transcutaneous electrical nerve stimulation |
| TMS | Transcranial magnetic stimulation |
| VAS | Visual Analog Scale |
References
- Chen, K.-Y.; Li, R.-Y. Efficacy and safety of different antidepressants and anticonvulsants in central poststroke pain: A network meta-analysis and systematic review. PLoS ONE 2022, 17, e0276012. [Google Scholar] [CrossRef] [PubMed]
- Harrison, R.A.; Field, T.S. Post Stroke Pain: Identification, Assessment, and Therapy. Cerebrovasc. Dis. 2015, 39, 190–201. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, M.J.; Diener, H.-C.; Sacco, R.L.; Panju, A.A.; Vinisko, R.; Yusuf, S. Chronic Pain Syndromes After Ischemic Stroke. Stroke 2013, 44, 1238–1243. [Google Scholar] [CrossRef] [PubMed]
- Andersen, G.; Vestergaard, K.; Ingeman-Nielsen, M.; Jensen, T.S. Incidence of central post-stroke pain. Pain 1995, 61, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Mohanan, A.T.; Nithya, S.; Nomier, Y.; Hassan, D.A.; Jali, A.M.; Qadri, M.; Machanchery, S. Stroke-Induced Central Pain: Overview of the Mechanisms, Management, and Emerging Targets of Central Post-Stroke Pain. Pharmaceuticals 2023, 16, 1103. [Google Scholar] [CrossRef]
- Attal, N.; Fermanian, C.; Fermanian, J.; Lanteri-Minet, M.; Alchaar, H.; Bouhassira, D. Neuropathic pain: Are there distinct subtypes depending on the aetiology or anatomical lesion? Pain 2008, 138, 343–353. [Google Scholar] [CrossRef]
- Svendsen, K.B.; Jensen, T.S.; Hansen, H.J.; Bach, F.W. Sensory function and quality of life in patients with multiple sclerosis and pain. Pain 2005, 114, 473–481. [Google Scholar] [CrossRef]
- Klit, H.; Finnerup, N.B.; Jensen, T.S. Central post-stroke pain: Clinical characteristics, pathophysiology, and management. Lancet Neurol. 2009, 8, 857–868. [Google Scholar] [CrossRef]
- Boivie, J.; Leijon, G.; Johansson, I. Central post-stroke pain—A study of the mechanisms through analyses of the sensory abnormalities. Pain 1989, 37, 173–185. [Google Scholar] [CrossRef]
- Bowsher, D. Central pain: Clinical and physiological characteristics. J. Neurol. Neurosurg. Psychiatry 1996, 61, 62–69. [Google Scholar] [CrossRef]
- Greenspan, D.J.; Ohara, S.; Sarlani, E.; Lenz, A.F. Allodynia in patients with post-stroke central pain (CPSP) studied by statistical quantitative sensory testing within individuals. Pain 2004, 109, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Widar, M.; Samuelsson, L.; Karlsson-Tivenius, S.; Ahlström, G. Long-term pain conditions after a stroke. J. Rehabil. Med. 2002, 34, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Lundström, E.; Smits, A.; Terént, A.; Borg, J. Risk factors for stroke-related pain 1 year after first-ever stroke. Eur. J. Neurol. 2009, 16, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, A.-C. Prevalence and intensity of pain after stroke: A population based study focusing on patients’ perspectives. J. Neurol. Neurosurg. Psychiatry 2006, 77, 590–595. [Google Scholar] [CrossRef] [PubMed]
- Kong, K.-H.; Woon, V.-C.; Yang, S.-Y. Prevalence of chronic pain and its impact on health-related quality of life in stroke survivors. Arch. Phys. Med. Rehabil. 2004, 85, 35–40. [Google Scholar] [CrossRef]
- Treister, A.K.; Hatch, M.N.; Cramer, S.C.; Chang, E.Y. Demystifying Poststroke Pain: From Etiology to Treatment. PM&R. 2017, 9, 63–75. [Google Scholar]
- Şahin-Onat, Ş.; Ünsal-Delialioğlu, S.; Kulaklı, F.; Özel, S. The effects of central post-stroke pain on quality of life and depression in patients with stroke. J. Phys. Ther. Sci. 2016, 28, 96–101. [Google Scholar] [CrossRef]
- Betancur, D.F.A.; Tarragó, M.d.G.L.; Torres, I.L.d.S.; Fregni, F.; Caumo, W. Central Post-Stroke Pain: An Integrative Review of Somatotopic Damage, Clinical Symptoms, and Neurophysiological Measures. Front. Neurol. 2021, 12, 678198. [Google Scholar] [CrossRef]
- Bello, C.; Andereggen, L.; Luedi, M.M.; Beilstein, C.M. Postcraniotomy Headache: Etiologies and Treatments. Curr. Pain. Headache Rep. 2022, 26, 357–364. [Google Scholar] [CrossRef]
- Bello, C.M.; Mackert, S.; Harnik, M.A.; Filipovic, M.G.; Urman, R.D.; Luedi, M.M. Shared Decision-Making in Acute Pain Services. Curr. Pain Headache Rep. 2023, 27, 193–202. [Google Scholar] [CrossRef]
- Asadauskas, A.; Luedi, M.M.; Urman, R.D.; Andereggen, L. Modern Approaches to the Treatment of Acute Facial Pain. Curr. Pain Headache Rep. 2024, 28, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Tamasauskas, A.; Marshall, A.; Silva-Passadouro, B.; Fallon, N.; Frank, B.; Laurinaviciute, S.; Keller, S. Management of Central Post-Stroke Pain: Systematic Review and Meta-Analysis. medRxiv 2024. [Google Scholar] [CrossRef]
- Widyadharma, I.P.E.; Tertia, C.; Wijayanti, I.S.; Barus, J.F. Central post stroke pain: What are the new insights? Rom. J. Neurol. 2021, 20, 28–34. [Google Scholar] [CrossRef]
- Head, H.; Holmes, G. Sensory disturbances from cerebral lesions. Brain 1911, 34, 102–254. [Google Scholar] [CrossRef]
- Craig, A.D.; Bowsher, D.; Tasker, R.R.; Lenz, F.A.; Dougherty, P.M.; Wiesenfeld-Hallin, Z. A new version of the thalamic disinhibition hypothesis of central pain. Pain. Forum 1998, 7, 1–28. [Google Scholar] [CrossRef]
- Vartiainen, N.; Perchet, C.; Magnin, M.; Creac’h, C.; Convers, P.; Nighoghossian, N.; Garcia-Larrea, L. Thalamic pain: Anatomical and physiological indices of prediction. Brain 2016, 139, 708–722. [Google Scholar] [CrossRef]
- Kuan, Y.-H.; Shih, H.-C.; Shyu, B.-C. Involvement of P2X7 Receptors and BDNF in the Pathogenesis of Central Poststroke Pain. In Advances in Experimental Medicine and Biology; Springer: Singapore, 2018; pp. 211–227. [Google Scholar]
- Bazzari, A.H.; Bazzari, F.H. Advances in targeting central sensitization and brain plasticity in chronic pain. Egypt. J. Neurol. Psychiatr. Neurosurg. 2022, 58, 38. [Google Scholar] [CrossRef]
- Hassan, I.; Kotrotsou, A.; Bakhtiari, A.S.; Thomas, G.A.; Weinberg, J.S.; Kumar, A.J.; Colen, R.R. Radiomic Texture Analysis Mapping Predicts Areas of True Functional MRI Activity. Sci. Rep. 2016, 6, 25295. [Google Scholar] [CrossRef]
- Kamat, P.; Nath, C. Okadaic acid: A tool to study regulatory mechanisms for neurodegeneration and regeneration in Alzheimer′s disease. Neural Regen. Res. 2015, 10, 365. [Google Scholar] [CrossRef]
- Gilmore, C.A.; Kapural, L.; McGee, M.J.; Boggs, J.W. Percutaneous Peripheral Nerve Stimulation for Chronic Low Back Pain: Prospective Case Series With 1 Year of Sustained Relief Following Short-Term Implant. Pain Pract. 2020, 20, 310–320. [Google Scholar] [CrossRef]
- Finnerup, N.B.; Haroutounian, S.; Kamerman, P.; Baron, R.; Bennett, D.L.; Bouhassira, D.; Jensen, T.S. Neuropathic pain: An updated grading system for research and clinical practice. Pain 2016, 157, 1599–1606. [Google Scholar] [CrossRef] [PubMed]
- Gilron, I.; Baron, R.; Jensen, T. Neuropathic Pain: Principles of Diagnosis and Treatment. Mayo Clin. Proc. 2015, 90, 532–545. [Google Scholar] [CrossRef] [PubMed]
- Shyu, B.C.; He, A.B.; Yu, Y.H.; Huang, A.C.W. Tricyclic antidepressants and selective serotonin reuptake inhibitors but not anticonvulsants ameliorate pain, anxiety, and depression symptoms in an animal model of central post-stroke pain. Mol. Pain. 2021, 17, 174480692110633. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.R.; Aktas, A.; Bottros, M.M. Pharmacotherapy to Manage Central Post-Stroke Pain. CNS Drugs 2021, 35, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Flaster, M.; Meresh, E.; Rao, M.; Biller, J. Central Poststroke Pain: Current Diagnosis and Treatment. Top. Stroke Rehabil. 2013, 20, 116–123. [Google Scholar] [CrossRef]
- Kim, N.Y.; Lee, S.C.; Kim, Y.W. Effect of Duloxetine for the Treatment of Chronic Central Poststroke Pain. Clin. Neuropharmacol. 2019, 42, 73–76. [Google Scholar] [CrossRef]
- Bo, Z.; Jian, Y.; Yan, L.; Gangfeng, G.; Xiaojing, L.; Xiaolan, L.; Jian, W. Pharmacotherapies for Central Post-Stroke Pain: A Systematic Review and Network Meta-Analysis. Oxid. Med. Cell Longev. 2022, 2022, 3511385. [Google Scholar] [CrossRef]
- Kalita, J.; Chandra, S.; Misra, U. Pregabalin and lamotrigine in central poststroke pain: A pilot study. Neurol. India 2017, 65, 506. [Google Scholar]
- Vranken, J.H.; Dijkgraaf, M.G.W.; Kruis, M.R.; van der Vegt, M.H.; Hollmann, M.W.; Heesen, M. Pregabalin in patients with central neuropathic pain: A randomized, double-blind, placebo-controlled trial of a flexible-dose regimen. Pain 2008, 136, 150–157. [Google Scholar] [CrossRef]
- Alles, S.R.A.; Cain, S.M.; Snutch, T.P. Pregabalin as a Pain Therapeutic: Beyond Calcium Channels. Front. Cell Neurosci. 2020, 14, 83. [Google Scholar] [CrossRef]
- Kim, J.S.; Bashford, G.; Murphy, K.T.; Martin, A.; Dror, V.; Cheung, R. Safety and efficacy of pregabalin in patients with central post-stroke pain. Pain 2011, 152, 1018–1023. [Google Scholar] [CrossRef] [PubMed]
- Kattan, M.; Moulin, D.E. Central post-stroke pain. In Neuropathic Pain; Cambridge University Press: Cambridge, UK, 2013; pp. 170–176. [Google Scholar]
- Kato, H.; Miyazaki, M.; Takeuchi, M.; Tsukuura, H.; Sugishita, M.; Noda, Y.; Yamada, K. A retrospective study to identify risk factors for somnolence and dizziness in patients treated with pregabalin. J. Pharm. Health Care Sci. 2015, 1, 22. [Google Scholar] [CrossRef] [PubMed]
- Vestergaard, K.; Andersen, G.; Gottrup, H.; Kristensen, B.T.; Jensen, T.S. Lamotrigine for central poststroke pain. Neurology 2001, 56, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S. Pharmacological Management of Central Post-Stroke Pain: A Practical Guide. CNS Drugs 2014, 28, 787–797. [Google Scholar] [CrossRef] [PubMed]
- Agnew, D.C.; Goldberg, V.D. A brief trial of phenytoin therapy for thalamic pain. Bull. Los. Angeles Neurol. Soc. 1976, 41, 9–12. [Google Scholar]
- Dworkin, R.H.; O’connor, A.B.; Backonja, M.; Farrar, J.T.; Finnerup, N.B.; Jensen, T.S.; Wallace, M.S. Pharmacologic management of neuropathic pain: Evidence-based recommendations. Pain 2007, 132, 237–251. [Google Scholar] [CrossRef]
- Scuteri, D.; Mantovani, E.; Tamburin, S.; Sandrini, G.; Corasaniti, M.T.; Bagetta, G.; Tonin, P. Opioids in Post-Stroke Pain: A Systematic Review and Meta-Analysis. Front. Pharmacol. 2020, 11, 587050. [Google Scholar] [CrossRef]
- Rowbotham, M.C.; Twilling, L.; Davies, P.S.; Reisner, L.; Taylor, K.; Mohr, D. Oral Opioid Therapy for Chronic Peripheral and Central Neuropathic Pain. New Engl. J. Med. 2003, 348, 1223–1232. [Google Scholar] [CrossRef]
- Attal, N.; Guirimand, F.; Brasseur, L.; Gaude, V.; Chauvin, M.; Bouhassira, D. Effects of IV morphine in central pain. Neurology 2002, 58, 554–563. [Google Scholar] [CrossRef]
- Willoch, F.; Schindler, F.; Wester, H.J.; Empl, M.; Straube, A.; Schwaiger, M.; Tölle, T.R. Central poststroke pain and reduced opioid receptor binding within pain processing circuitries: A [11C]diprenorphine PET study. Pain 2004, 108, 213–220. [Google Scholar] [CrossRef]
- Bainton, T.; Fox, M.; Bowsher, D.; Wells, C. A double-blind trial of naloxone in central post-stroke pain. Pain 1992, 48, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Budd, K. The use of the opiate antagonist, naloxone, in the treatment of intractable pain. Neuropeptides 1985, 5, 419–422. [Google Scholar] [CrossRef] [PubMed]
- Baumann, L.; Bello, C.; Georg, F.M.; Urman, R.D.; Luedi, M.M.; Andereggen, L. Acute Pain and Development of Opioid Use Disorder: Patient Risk Factors. Curr. Pain Headache Rep. 2023, 27, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Botea, M.O.; Andereggen, L.; Urman, R.D.; Luedi, M.M.; Romero, C.S. Cannabinoids for Acute Pain Management: Approaches and Rationale. Curr. Pain. Headache Rep. 2024, 28, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Kummer, I.; Lüthi, A.; Klingler, G.; Andereggen, L.; Urman, R.D.; Luedi, M.M.; Stieger, A. Adjuvant Analgesics in Acute Pain—Evaluation of Efficacy. Curr. Pain Headache Rep. 2024, 1–10. [Google Scholar] [CrossRef]
- Vranken, J.H.; Dijkgraaf, M.G.W.; Kruis, M.R.; van Dasselaar, N.T.; van der Vegt, M.H. Iontophoretic administration of S(+)-ketamine in patients with intractable central pain: A placebo-controlled trial. Pain 2005, 118, 224–231. [Google Scholar] [CrossRef]
- Vick, P.G.; Lamer, T.J. Treatment of central post-stroke pain with oral ketamine. Pain 2001, 92, 311–313. [Google Scholar] [CrossRef]
- Angstadt, R.; Esperti, S.; Mangano, A.; Meyer, S. Palliative ketamine: The use of ketamine in central post-stroke pain syndrome—A case report. Ann. Palliat. Med. 2021, 10, 6974–6978. [Google Scholar] [CrossRef]
- Mücke, M.; Phillips, T.; Radbruch, L.; Petzke, F.; Häuser, W. Cannabis-based medicines for chronic neuropathic pain in adults. Cochrane Database Syst. Rev. 2018, 3, CD012182. [Google Scholar] [CrossRef]
- Morishita, T.; Inoue, T. Brain Stimulation Therapy for Central Post-Stroke Pain from a Perspective of Interhemispheric Neural Network Remodeling. Front. Hum. Neurosci. 2016, 10, 166. [Google Scholar] [CrossRef]
- Kumar, B.; Kalita, J.; Kumar, G.; Misra, U.K. Central Poststroke Pain: A Review of Pathophysiology and Treatment. Anesth. Analg. 2009, 108, 1645–1657. [Google Scholar] [CrossRef] [PubMed]
- Wipplinger, F.; Holthof, N.; Andereggen, L.; Urman, R.D.; Luedi, M.M.; Bello, C. Meditation as an Adjunct to the Management of Acute Pain. Curr. Pain. Headache Rep. 2023, 27, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Rogger, R.; Bello, C.; Romero, C.S.; Urman, R.D.; Luedi, M.M.; Filipovic, M.G. Cultural Framing and the Impact On Acute Pain and Pain Services. Curr. Pain. Headache Rep. 2023, 27, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Frickmann, F.C.S.; Urman, R.D.; Siercks, K.; Burgermeister, G.; Luedi, M.M.; Lersch, F.E. The Effect of Perioperative Auditory Stimulation with Music on Procedural Pain: A Narrative Review. Curr. Pain. Headache Rep. 2023, 27, 217–226. [Google Scholar] [CrossRef]
- Lersch, F.E.; Frickmann, F.C.; Urman, R.D.; Burgermeister, G.; Siercks, K.; Luedi, M.M.; Straumann, S. Analgesia for the Bayesian Brain: How Predictive Coding Offers Insights Into the Subjectivity of Pain. Curr. Pain. Headache Rep. 2023, 27, 631–638. [Google Scholar] [CrossRef]
- Nowacki, A.; Zhang, D.; Barlatey, S.; Ai-Schläppi, J.; Rosner, J.; Arnold, M.; Pollo, C. Deep Brain Stimulation of the Central Lateral and Ventral Posterior Thalamus for Central Poststroke Pain Syndrome: Preliminary Experience. Neuromodulation Technol. Neural Interface 2023, 26, 1747–1756. [Google Scholar] [CrossRef]
- Johnson, M.D.; Lim, H.H.; Netoff, T.I.; Connolly, A.T.; Johnson, N.; Roy, A.; He, B. Neuromodulation for Brain Disorders: Challenges and Opportunities. IEEE Trans. Biomed. Eng. 2013, 60, 610–624. [Google Scholar] [CrossRef]
- Rasche, D.; Rinaldi, P.C.; Young, R.F.; Tronnier, V.M. Deep brain stimulation for the treatment of various chronic pain syndromes. Neurosurg. Focus. 2006, 21, 1–8. [Google Scholar] [CrossRef]
- Owen, S.L.F.; Green, A.L.; Stein, J.F.; Aziz, T.Z. Deep brain stimulation for the alleviation of post-stroke neuropathic pain. Pain 2006, 120, 202–206. [Google Scholar] [CrossRef]
- Katayama, Y.; Yamamoto, T.; Kobayashi, K.; Kasai, M.; Oshima, H.; Fukaya, C. Motor Cortex Stimulation for Post-Stroke Pain: Comparison of Spinal Cord and Thalamic Stimulation. Stereotact. Funct. Neurosurg. 2001, 77, 183–186. [Google Scholar] [CrossRef]
- Coffey, R.J. Deep brain stimulation for chronic pain: Results of two multicenter trials and a structured review. Pain Med. 2001, 2, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, N.; Shaheen, A.; Elgendy, A.; Bezchlibnyk, Y.B.; Zesiewicz, T.; Dalm, B.; Flouty, O. Deep brain stimulation for chronic pain: A systematic review and meta-analysis. Front. Hum. Neurosci. 2023, 17, 1297894. [Google Scholar] [CrossRef] [PubMed]
- Gray, A.M.; Pounds-Cornish, E.; Eccles, F.J.R.; Aziz, T.Z.; Green, A.L.; Scott, R.B. Deep brain stimulation as a treatment for neuropathic pain: A longitudinal study addressing neuropsychological outcomes. J. Pain 2014, 15, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Lempka, S.F.; Malone, D.A., Jr.; Hu, B.; Baker, K.B.; Wyant, A.; Ozinga, J.G., IV; Machado, A.G. Randomized clinical trial of deep brain stimulation for poststroke pain. Ann. Neurol. 2017, 81, 653–663. [Google Scholar] [CrossRef]
- Vieira de Almeida, M.; Sobreira Neto, A.A.; Cavalcante, G.M.; Oliveira Moreira, A.P.; Paes de Andrade Saraiva, G.; Nobre Nogueira, G.; Barbosa de Sousa Santos Filho, L.; Fernandes Gonçalves, R.; Bezerra de Araújo, M.; Lopes Santos, D.; et al. Headache Medicine Deep Brain Stimulation for Treatment of Central Post-Stroke Pain: A Systematic Review of Case Reports. Headache Med. 2023, 14. Available online: https://headachemedicine.com.br/index.php/hm/article/view/957 (accessed on 5 September 2024).
- Volkers, R.; Giesen, E.; van der Heiden, M.; Kerperien, M.; Lange, S.; Kurt, E.; Henssen, D. Invasive Motor Cortex Stimulation Influences Intracerebral Structures in Patients With Neuropathic Pain: An Activation Likelihood Estimation Meta-Analysis of Imaging Data. Neuromodulation Technol. Neural Interface 2020, 23, 436–443. [Google Scholar] [CrossRef]
- Kishima, H.; Saitoh, Y.; Osaki, Y.; Nishimura, H.; Kato, A.; Hatazawa, J.; Yoshimine, T. Motor cortex stimulation in patients with deafferentation pain: Activation of the posterior insula and thalamus. J. Neurosurg. 2007, 107, 43–48. [Google Scholar] [CrossRef]
- Saitoh, Y.; Osaki, Y.; Nishimura, H.; Hirano, S.I.; Kato, A.; Hashikawa, K.; Yoshimine, T. Increased regional cerebral blood flow in the contralateral thalamus after successful motor cortex stimulation in a patient with poststroke pain. J. Neurosurg. 2004, 100, 935–939. [Google Scholar] [CrossRef]
- Subedi, M.; Bajaj, S.; Kumar, M.S.; Mayur, Y.C. An overview of tramadol and its usage in pain management and future perspective. Biomed. Pharmacother. 2019, 111, 443–451. [Google Scholar] [CrossRef]
- Fontaine, D.; Hamani, C.; Lozano, A. Efficacy and safety of motor cortex stimulation for chronic neuropathic pain: Critical review of the literature. J. Neurosurg. 2009, 110, 251–256. [Google Scholar] [CrossRef]
- Cruccu, G.; Aziz, T.Z.; Garcia-Larrea, L.; Hansson, P.; Jensen, T.S.; Lefaucheur, J.P.; Taylor, R.S. EFNS guidelines on neurostimulation therapy for neuropathic pain. Eur. J. Neurol. 2007, 14, 952–970. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhu, H.; Tao, W.; Li, Y.; Hu, Y. Motor Cortex Stimulation Therapy for Relief of Central Post-Stroke Pain: A Retrospective Study with Neuropathic Pain Symptom Inventory. Stereotact. Funct. Neurosurg. 2018, 96, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Rasche, D.; Ruppolt, M.; Stippich, C.; Unterberg, A.; Tronnier, V.M. Motor cortex stimulation for long-term relief of chronic neuropathic pain: A 10 year experience. Pain 2006, 121, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Gurdiel-Álvarez, F.; Navarro-López, V.; Varela-Rodríguez, S.; Juárez-Vela, R.; Cobos-Rincón, A.; Sánchez-González, J.L. Transcranial magnetic stimulation therapy for central post-stroke pain: Systematic review and meta-analysis. Front. Neurosci. 2024, 18, 1345128. [Google Scholar] [CrossRef]
- Radiansyah, R.S.; Hadi, D.W. Repetitive transcranial magnetic stimulation in central post-stroke pain: Current status and future perspective. Korean J. Pain 2023, 36, 408–424. [Google Scholar] [CrossRef]
- Migita, K.; Uozumi, T.; Arita, K.; Monden, S. Transcranial Magnetic Coil Stimulation of Motor Cortex in Patients with Central Pain. Neurosurgery 1995, 36, 1037–1040. [Google Scholar] [CrossRef]
- Wu, L.-N.; Zheng, H.-Y.; Xue, S.-A.; Chen, K.-Y.; Li, R.-Y. The Efficacy and Safety of Different Noninvasive Therapies in the Treatment of Central Poststroke Pain (CPSP): A Network Meta-Analysis and Systematic Review. J. Integr. Neurosci. 2023, 22, 102. [Google Scholar] [CrossRef]
- Ramger, B.C.; Bader, K.A.; Davies, S.P.; Stewart, D.A.; Ledbetter, L.S.; Simon, C.B.; Feld, J.A. Effects of Non-Invasive Brain Stimulation on Clinical Pain Intensity and Experimental Pain Sensitivity Among Individuals with Central Post-Stroke Pain: A Systematic Review. J. Pain. Res. 2019, 12, 3319–3329. [Google Scholar] [CrossRef]
- de Oliveira, R.A.A.; de Andrade, D.C.; Mendonça, M.; Barros, R.; Luvisoto, T.; Myczkowski, M.L.; Teixeira, M.J. Repetitive Transcranial Magnetic Stimulation of the Left Premotor/Dorsolateral Prefrontal Cortex Does Not Have Analgesic Effect on Central Poststroke Pain. J. Pain 2014, 15, 1271–1281. [Google Scholar] [CrossRef]
- Yokoe, M.; Mano, T.; Maruo, T.; Hosomi, K.; Shimokawa, T.; Kishima, H.; Saitoh, Y. The optimal stimulation site for high-frequency repetitive transcranial magnetic stimulation in Parkinson’s disease: A double-blind crossover pilot study. J. Clin. Neurosci. 2018, 47, 72–78. [Google Scholar] [CrossRef]
- Ojala, J.; Vanhanen, J.; Harno, H.; Lioumis, P.; Vaalto, S.; Kaunisto, M.A.; Kalso, E. A Randomized, Sham-Controlled Trial of Repetitive Transcranial Magnetic Stimulation Targeting M1 and S2 in Central Poststroke Pain: A Pilot Trial. Neuromodulation Technol. Neural Interface 2022, 25, 538–548. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-C.; Chuang, Y.-F.; Huang, A.C.-W.; Chen, C.-K.; Chang, Y.-J. The antalgic effects of non-invasive physical modalities on central post-stroke pain: A systematic review. J. Phys. Ther. Sci. 2016, 28, 1368–1373. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.I.; Bjordal, J.M. Transcutaneous electrical nerve stimulation for the management of painful conditions: Focus on neuropathic pain. Expert. Rev. Neurother. 2011, 11, 735–753. [Google Scholar] [CrossRef] [PubMed]
- Leandri, M.; Parodi, C.I.; Corrieri, N.; Rigardo, S. Comparison of TENS treatments in hemiplegic shoulder pain. Scand. J. Rehabil. Med. 1990, 22, 69–71. [Google Scholar] [CrossRef] [PubMed]
- Robbins, S.M.; Houghton, P.E.; Woodbury, M.G.; Brown, J.L. The Therapeutic Effect of Functional and Transcutaneous Electric Stimulation on Improving Gait Speed in Stroke Patients: A Meta-Analysis. Arch. Phys. Med. Rehabil. 2006, 87, 853–859. [Google Scholar] [CrossRef]
- Leijon, G.; Boivie, J. Central post-stroke pain—The effect of high and low frequency TENS. Pain 1989, 38, 187–191. [Google Scholar] [CrossRef]
- Williams, A.C.d.C.; Eccleston, C.; Morley, S. Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database Syst. Rev. 2012, 14, 11. [Google Scholar] [CrossRef]
- Lim, J.-A.; Choi, S.-H.; Lee, W.J.; Jang, J.H.; Moon, J.Y.; Kim, Y.C.; Kang, D.H. Cognitive-behavioral therapy for patients with chronic pain: Implications of gender differences in empathy. Medicine 2018, 97, e10867. [Google Scholar] [CrossRef]
- Surya Manurung, S.; Glorino, M.; Pandin, R. Cognitive Therapy Approach For Post-Stroke Patients: A Review Of Literature. medRxiv 2023. [Google Scholar] [CrossRef]
- Sanabria-Mazo, J.P.; Colomer-Carbonell, A.; Fernández-Vázquez, Ó.; Noboa-Rocamora, G.; Cardona-Ros, G.; McCracken, L.M.; Luciano, J.V. A systematic review of cognitive behavioral therapy-based interventions for comorbid chronic pain and clinically relevant psychological distress. Front. Psychol. 2023, 14, 1200685. [Google Scholar] [CrossRef]
- Nakao, M.; Shirotsuki, K.; Sugaya, N. Cognitive-behavioral therapy for management of mental health and stress-related disorders: Recent advances in techniques and technologies. Biopsychosoc. Med. 2021, 15, 16. [Google Scholar] [CrossRef] [PubMed]
- Meints, S.M.; Edwards, R.R. Evaluating psychosocial contributions to chronic pain outcomes. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 87 (Pt B), 168–182. [Google Scholar] [CrossRef]
- Hofer, D.M.; Lehmann, T.; Zaslansky, R.; Harnik, M.; Meissner, W.; Stüber, F.; Stamer, U.M. Rethinking the definition of chronic postsurgical pain: Composites of patient-reported pain-related outcomes vs. pain intensities alone. Pain 2022, 163, 2457–2465. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.C.d.C.; Fisher, E.; Hearn, L.; Eccleston, C. Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database Syst. Rev. 2020, 8, CD007407. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, P.M. The 7th International Conference on Disability, Virtual Reality and Associated Technologies with ArtAbilitation: Proceedings, Maia, Portugal, 8–11 September 2008; ICDVRAT; University of Reading: Reading, UK, 2008. [Google Scholar]
- Ali, F.; Suleman, R.; Noor, A.; Ahmad, I.; Shakeel, M.; Aqeel, M. Effectiveness of a virtual reality-based rehabilitation program versus conventional physical therapy in improving motor function and balance in stroke survivors: A randomized controlled trial. J. Health Rehabil. Res. 2023, 3, 817–821. [Google Scholar] [CrossRef]
- Viderman, D.; Tapinova, K.; Dossov, M.; Seitenov, S.; Abdildin, Y.G. Virtual reality for pain management: An Umbrella Review. Front. Med. 2023, 10, 1203670. [Google Scholar] [CrossRef]
- Luque-Moreno, C.; Kiper, P.; Solís-Marcos, I.; Agostini, M.; Polli, A.; Turolla, A.; Oliva-Pascual-Vaca, A. Virtual reality and physiotherapy in post-stroke functional re-education of the lower extremity: A controlled clinical trial on a new approach. J. Pers. Med. 2021, 11, 1210. [Google Scholar] [CrossRef]
- Funao, H.; Tsujikawa, M.; Momosaki, R.; Shimaoka, M. Virtual reality applied to home-visit rehabilitation for hemiplegic shoulder pain in a stroke patient: A case report. J. Rural. Med. 2021, 16, 174–178. [Google Scholar] [CrossRef]
- Souchet, A.D.; Lourdeaux, D.; Burkhardt, J.M.; Hancock, P.A. Design guidelines for limiting and eliminating virtual reality-induced symptoms and effects at work: A comprehensive, factor-oriented review. Front. Psychol. 2023, 14, 1161932. [Google Scholar] [CrossRef]
- Aderinto, N.; Olatunji, G.; Abdulbasit, M.O.; Edun, M.; Aboderin, G.; Egbunu, E. Exploring the efficacy of virtual reality-based rehabilitation in stroke: A narrative review of current evidence. Ann. Med. 2023, 55, 2285907. [Google Scholar] [CrossRef]
- Dydyk, A.M.; Munakomi, S. Thalamic Pain Syndrome. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Phillips, C.; Blakey, G., 3rd; Essick, G.K. Sensory retraining: A cognitive behavioral therapy for altered sensation. Atlas Oral. Maxillofac. Surg. Clin. N. Am. 2011, 19, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Tejasri, B.P.; Arunachalam, R.; Kumaresan Kiruthika, S. Desensitisation therapy in post-stroke pain syndrome: A case study. Int. J. Physiother. Res. 2017, 5, 2541–2544. [Google Scholar] [CrossRef][Green Version]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asadauskas, A.; Stieger, A.; Luedi, M.M.; Andereggen, L. Advancements in Modern Treatment Approaches for Central Post-Stroke Pain: A Narrative Review. J. Clin. Med. 2024, 13, 5377. https://doi.org/10.3390/jcm13185377
Asadauskas A, Stieger A, Luedi MM, Andereggen L. Advancements in Modern Treatment Approaches for Central Post-Stroke Pain: A Narrative Review. Journal of Clinical Medicine. 2024; 13(18):5377. https://doi.org/10.3390/jcm13185377
Chicago/Turabian StyleAsadauskas, Auste, Andrea Stieger, Markus M. Luedi, and Lukas Andereggen. 2024. "Advancements in Modern Treatment Approaches for Central Post-Stroke Pain: A Narrative Review" Journal of Clinical Medicine 13, no. 18: 5377. https://doi.org/10.3390/jcm13185377
APA StyleAsadauskas, A., Stieger, A., Luedi, M. M., & Andereggen, L. (2024). Advancements in Modern Treatment Approaches for Central Post-Stroke Pain: A Narrative Review. Journal of Clinical Medicine, 13(18), 5377. https://doi.org/10.3390/jcm13185377





