Left Atrial Wall Thickness Estimated by Cardiac CT: Implications for Catheter Ablation of Atrial Fibrillation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Design
2.2. Contrast-Enhanced Multi-Detector Computed Tomography
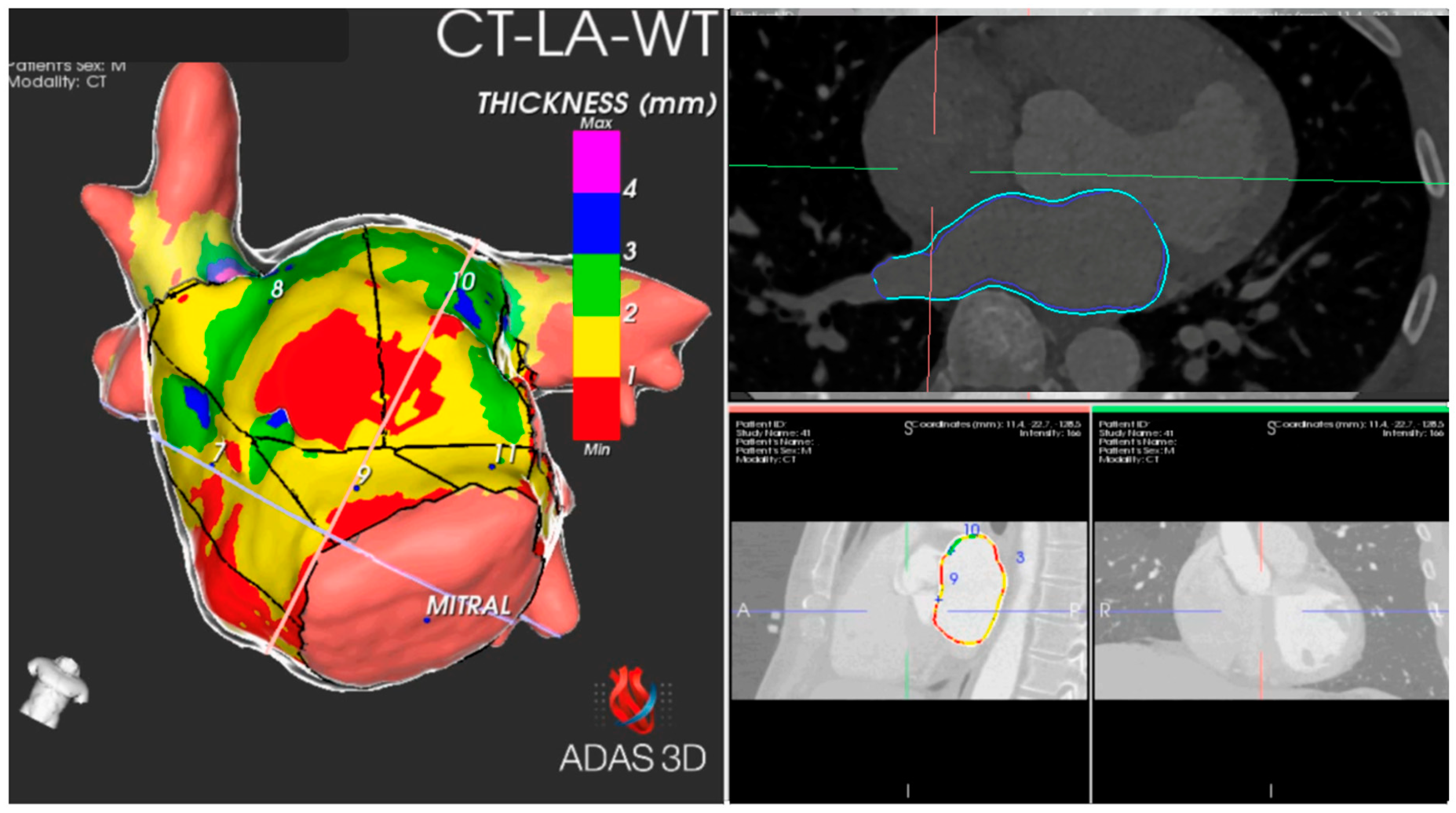
2.3. Image Post-Processing
2.4. Ablation Procedure
2.5. Post-Ablation Evaluation
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Descriptive Analysis
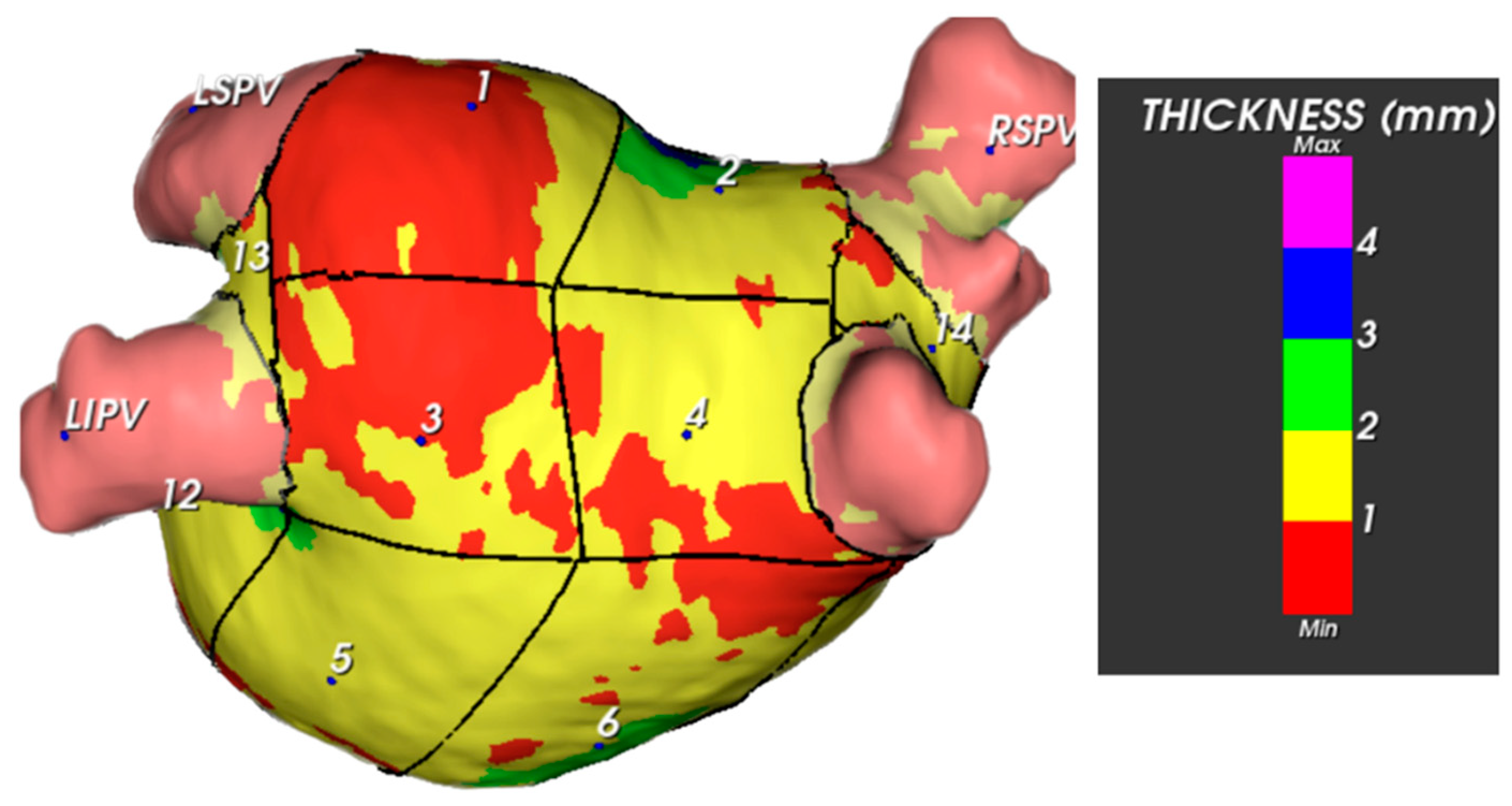
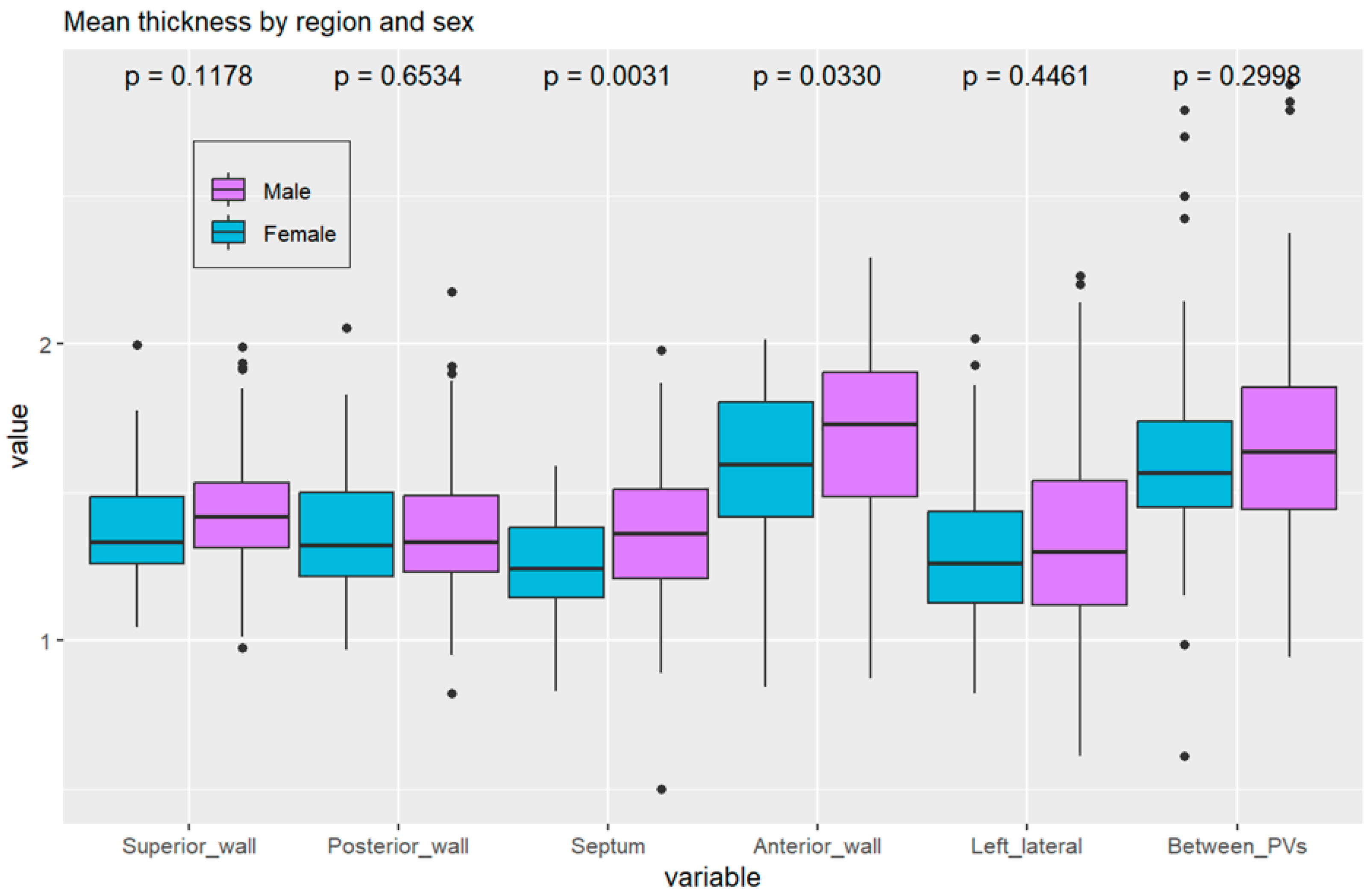
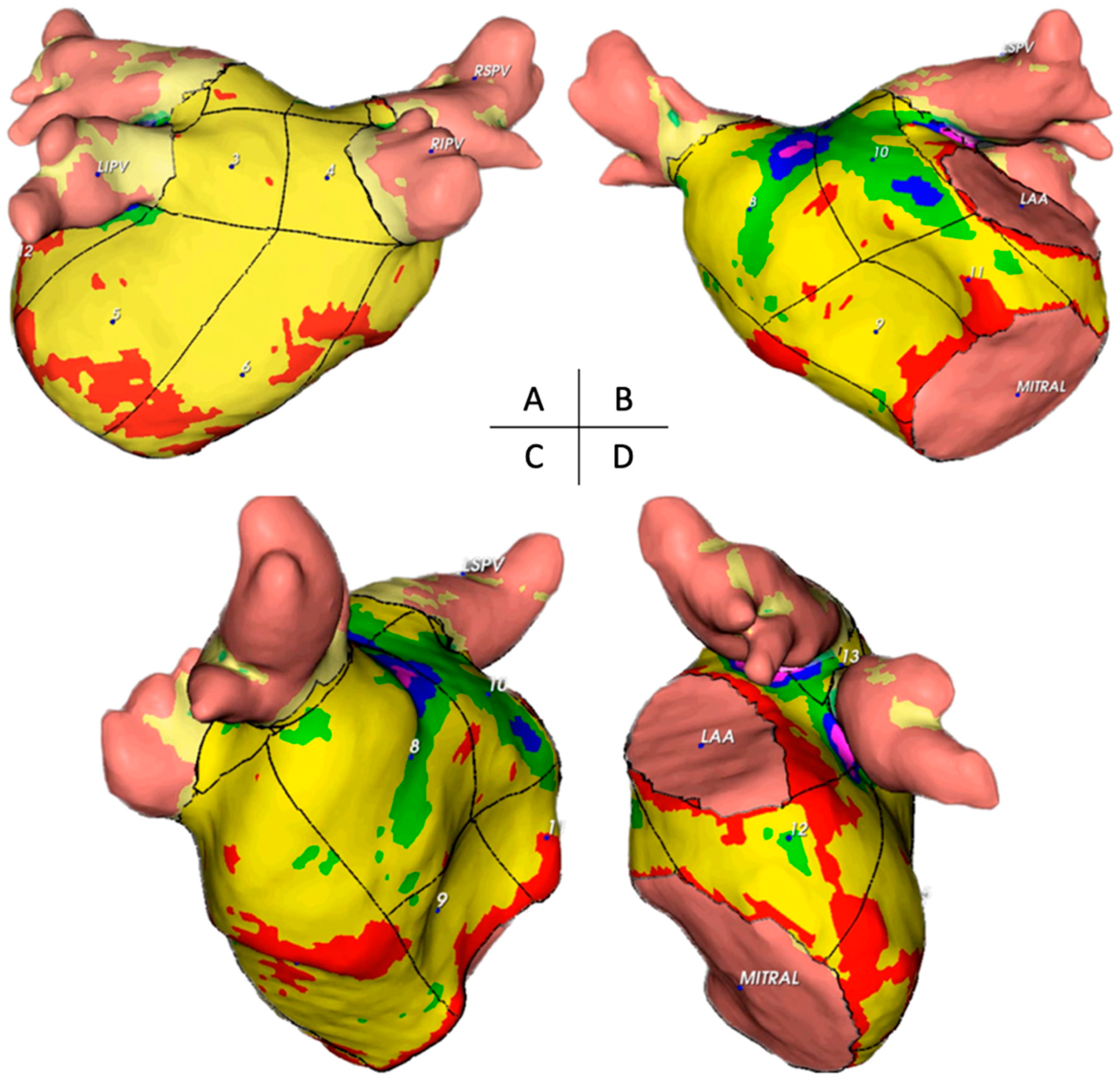
3.2.1. Thickness of Segments and Walls
3.2.2. Mass of Segments and Walls
3.2.3. Data Analysis
3.2.4. Correlations with BMI, Left Atrial Characteristics, and Comorbidities
3.3. Survival Free of AF Analysis
3.3.1. Anterior Wall Thickness
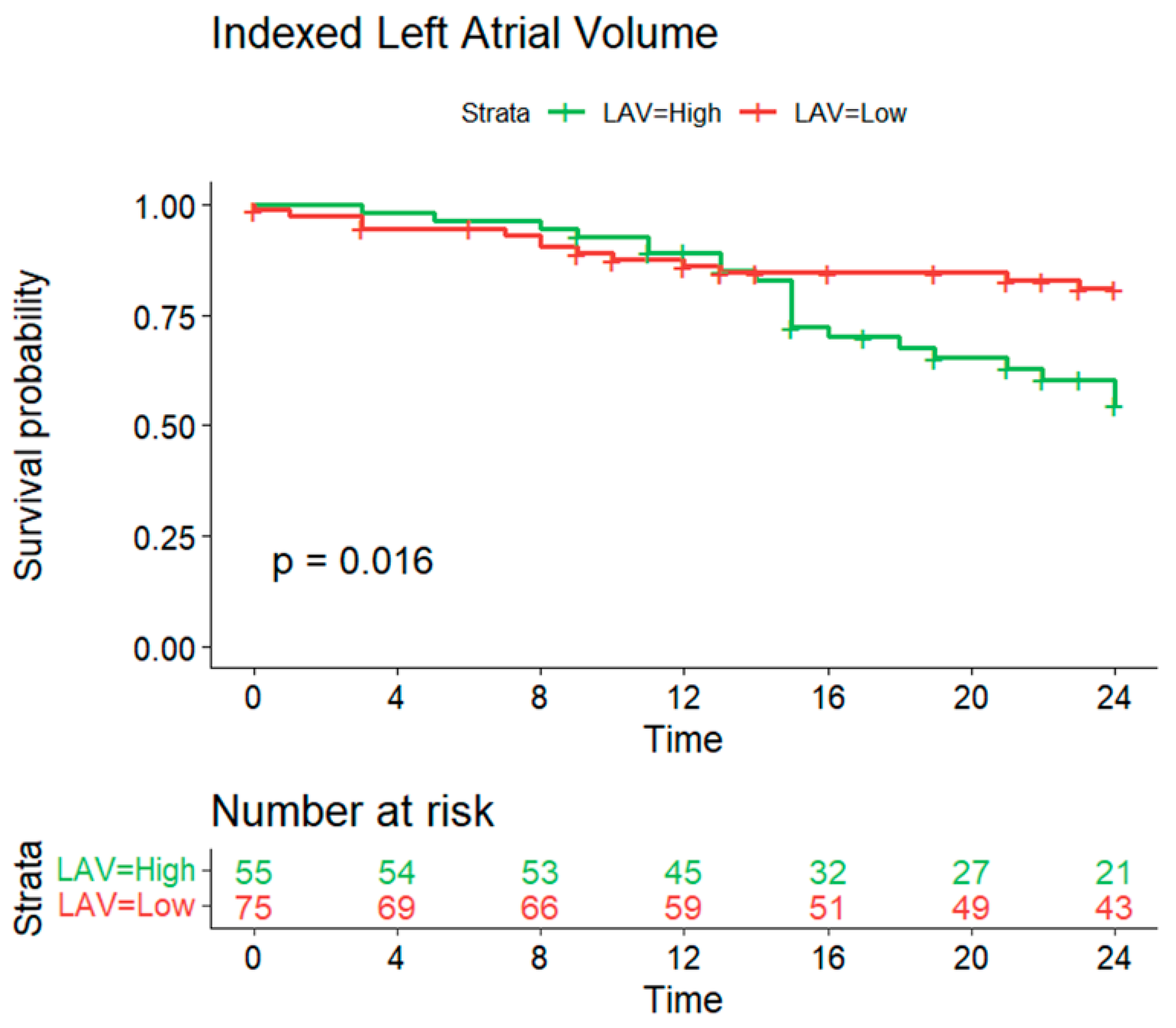
3.3.2. Indexed Left Atrial Volume
4. Discussion
4.1. Main Findings
4.1.1. Left Atrial Volume Index, Atrial Wall Thickness, and AF Recurrence
4.1.2. Mechanisms of Recurrence
4.2. Clinical Implications
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, H.; Song, X.; Liang, Y.; Bai, X.; Liu-Huo, W.-S.; Tang, C.; Chen, W.; Zhao, L. Global, regional, and national burden of disease study of atrial fibrillation/flutter, 1990–2019: Results from a global burden of disease study, 2019. BMC Public Health 2022, 22, 2015. [Google Scholar] [CrossRef] [PubMed]
- Nattel, S.; Harada, M. Atrial Remodeling and Atrial Fibrillation: Recent advances and translational perspectives. J. Am. Coll. Cardiol. 2014, 63, 2335–2345. [Google Scholar] [CrossRef] [PubMed]
- Marino, P.N. Left atrial conduit function: A short review. Physiol. Rep. 2021, 9, e15053. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kottkamp, H. Fibrotic Atrial Cardiomyopathy: A Specific Disease/Syndrome Supplying Substrates for Atrial Fibrillation, Atrial Tachycardia, Sinus Node Disease, AV Node Disease, and Thromboembolic Complications. J. Cardiovasc. Electrophysiol. 2012, 23, 797–799. [Google Scholar] [CrossRef] [PubMed]
- Platonov, P.G.; Mitrofanova, L.B.; Orshanskaya, V.; Ho, S.Y. Structural Abnormalities in Atrial Walls Are Associated with Presence and Persistency of Atrial Fibrillation but Not with Age. J. Am. Coll. Cardiol. 2011, 58, 2225–2232. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, A.F.; Kühl, J.T.; Kofoed, K.F.; Fuchs, A.; Udholm, P.M. Left Atrial Wall Thickness and Pulmonary Vein Size are Increased in Patients with Atrial Fibrillation Compared to Healthy Controls—A Multidetector Computed Tomography Study. Int. J. Clin. Cardiol. 2017, 4, 098. [Google Scholar] [CrossRef]
- Di Biase, L.; Santangeli, P.; Anselmino, M.; Mohanty, P.; Salvetti, I.; Gili, S.; Horton, R.; Sanchez, J.E.; Bai, R.; Mohanty, S.; et al. Does the left atrial appendage morphology correlate with the risk of stroke in patients with atrial fibrillation? Results from a multicenter study. J. Am. Coll. Cardiol. 2012, 60, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Mahnkopf, C.; Badger, T.J.; Burgon, N.S.; Daccarett, M.; Haslam, T.S.; Badger, C.T.; McGann, C.J.; Akoum, N.; Kholmovski, E.; Macleod, R.S.; et al. Evaluation of the left atrial substrate in patients with lone atrial fibrillation using delayed-enhanced MRI: Implications for disease progression and response to catheter ablation. Hearth Rhythm. 2010, 7, 1475–1481. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rebecchi, M.; Panattoni, G.; Edoardo, B.; de Ruvo, E.; Sciarra, L.; Politano, A.; Sgueglia, M.; Ricagni, C.; Verbena, S.; Crescenzi, C.; et al. Atrial fibrillation and autonomic nervous system: A translational approach to guide therapeutic goals. J. Arrhythmia 2021, 37, 320–330. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kostin, S.; Klein, G.; Szalay, Z.; Hein, S.; Bauer, E.P.; Schaper, J. Structural correlate of atrial fibrillation in human patients. Cardiovasc. Res. 2002, 54, 361–379. [Google Scholar] [CrossRef] [PubMed]
- Whitaker, J.; Rajani, R.; Chubb, H.; Gabrawi, M.; Varela, M.; Wright, M.; Niederer, S.; O’Neill, M.D. The role of myocardial wall thickness in atrial arrhythmogenesis. Europace 2016, 18, 1758–1772. [Google Scholar] [CrossRef] [PubMed]
- Marrouche, N.F.; Wilber, D.; Hindricks, G.; Jais, P.; Akoum, N.; Marchlinski, F.; Kholmovski, E.; Burgon, N.; Hu, N.; Mont, L.; et al. Association of Atrial Tissue Fibrosis Identified by Delayed Enhancement MRI and Atrial Fibrillation Catheter Ablation. JAMA 2014, 311, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Nicol, E.D.; Norgaard, B.L.; Blanke, P.; Ahmadi, A.; Weir-McCall, J.; Horvat, P.M.; Han, K.; Bax, J.J.; Leipsic, J. The Future of Cardiovascular Computed Tomography. JACC Cardiovasc. Imaging 2019, 12, 1058–1072. [Google Scholar] [CrossRef] [PubMed]
- Wongcharoen, W.; Tsao, H.; Wu, M.; Tai, C.; Chang, S.; Lin, Y.; Lo, L.; Chen, Y.; Sheu, M.; Chang, C.; et al. Morphologic Characteristics of the Left Atrial Appendage, Roof, and Septum: Implications for the Ablation of Atrial Fibrillation. J. Cardiovasc. Electrophysiol. 2006, 17, 951–956. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, J.A.; Ho, S.Y.; Climent, V.; Sánchez-Quintana, D. The architecture of the left lateral atrial wall: A particular anatomic region with implications for ablation of atrial fibrillation. Eur. Hearth J. 2008, 29, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Cunha, P.S.; Portugal, G.; Laranjo, S.; Alves, M.; Papoila, A.L.; Valente, B.; Delgado, A.S.; Lousinha, A.; Paulo, M.; Brás, M.; et al. The atrial fibrillation burden during the blanking period is predictive of time to recurrence after catheter ablation. IJC Hearth Vasc. 2022, 43, 101138. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Taghji, P.; El Haddad, M.; Phlips, T.; Wolf, M.; Knecht, S.; Vandekerckhove, Y.; Tavernier, R.; Nakagawa, H.; Duytschaever, M. Evaluation of a Strategy Aiming to Enclose the Pulmonary Veins with Contiguous and Optimized Radiofrequency Lesions in Paroxysmal Atrial Fibrillation. JACC Clin. Electrophysiol. 2018, 4, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Stavrakis, S.; Po, S. Ganglionated Plexi Ablation: Physiology and Clinical Applications. Arrhythmia Electrophysiol. Rev. 2017, 6, 186–190. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Njoku, A.; Kannabhiran, M.; Arora, R.; Reddy, P.; Gopinathannair, R.; Lakkireddy, D.; Dominic, P. Left atrial volume predicts atrial fibrillation recurrence after radiofrequency ablation: A meta-analysis. Europace 2018, 20, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Mayyas, F.; Niebauer, M.; Zurick, A.; Barnard, J.; Gillinov, A.M.; Chung, M.K.; Van Wagoner, D.R. Association of Left Atrial Endothelin-1 With Atrial Rhythm, Size, and Fibrosis in Patients with Structural Heart Disease. Circ. Arrhythmia Electrophysiol. 2010, 3, 369–379. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vaziri, S.M.; Larson, M.G.; Benjamin, E.J.; Levy, D. Echocardiographic predictors of nonrheumatic atrial fibrillation. The Framingham Heart Study. Circulation 1994, 89, 724–730. [Google Scholar] [CrossRef] [PubMed]
- Abecasis, J.; Dourado, R.; Ferreira, A.; Saraiva, C.; Cavaco, D.; Santos, K.R.; Morgado, F.B.; Adragão, P.; Silva, A. Left atrial volume calculated by multi-detector computed tomography may predict successful pulmonary vein isolation in catheter ablation of atrial fibrillation. Europace 2009, 11, 1289–1294. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, M.; Grimes, M.M.; Perez, F.J.; Kenigsberg, D.N.; Koneru, J.; Kasirajan, V.; Wood, M.A.; Ellenbogen, K.A. Histopathologic Characterization of Chronic Radiofrequency Ablation Lesions for Pulmonary Vein Isolation. J. Am. Coll. Cardiol. 2012, 59, 930–938. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Kilicaslan, F.; Pisano, E.; Marrouche, N.F.; Fanelli, R.; Brachmann, J.; Geunther, J.; Potenza, D.; Martin, D.O.; Cummings, J.; et al. Response of Atrial Fibrillation to Pulmonary Vein Antrum Isolation Is Directly Related to Resumption and Delay of Pulmonary Vein Conduction. Circulation 2005, 112, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Mulder, M.J.; Kemme, M.J.; Hagen, A.M.; Hopman, L.H.; van de Ven, P.M.; Hauer, H.A.; Tahapary, G.J.; Götte, M.J.; van Rossum, A.C.; Allaart, C.P. Impact of local left atrial wall thickness on the incidence of acute pulmonary vein reconnection after Ablation Index-guided atrial fibrillation ablation. IJC Hearth Vasc. 2020, 29, 100574. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Oh, S.; Joo, Y.H.; Lee, E.; Lee, S.-R.; Cha, M.-J.; Choi, E.-K.; Lee, J.C.; Lee, W. Left atrial wall thickness and its relationship with reconnection after pulmonary vein isolation in patients with atrial fibrillation evaluated using a three-dimensional wall thickness map. Int. J. Arrhythmia 2021, 22, 16. [Google Scholar] [CrossRef]
- Maesen, B.; Zeemering, S.; Afonso, C.; Eckstein, J.; Burton, R.A.; van Hunnik, A.; Stuckey, D.J.; Tyler, D.; Maessen, J.; Grau, V.; et al. Rearrangement of Atrial Bundle Architecture and Consequent Changes in Anisotropy of Conduction Constitute the 3-Dimensional Substrate for Atrial Fibrillation. Circ. Arrhythmia Electrophysiol. 2013, 6, 967–975. [Google Scholar] [CrossRef]
- Ho, S.Y.; Cabrera, J.A.; Sanchez-Quintana, D. Left Atrial Anatomy Revisited. Circ. Arrhythmia Electrophysiol. 2012, 5, 220–228. [Google Scholar] [CrossRef]
- Pashakhanloo, F.; Herzka, D.A.; Ashikaga, H.; Mori, S.; Gai, N.; Bluemke, D.A.; Trayanova, N.A.; McVeigh, E.R. Myofiber Architecture of the Human Atria as Revealed by Submillimeter Diffusion Tensor Imaging. Circ. Arrhythmia Electrophysiol. 2016, 9, e004133. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Otsuka, N.; Okumura, Y.; Kuorkawa, S.; Nagashima, K.; Wakamatsu, Y.; Hayashida, S.; Ohkubo, K.; Nakai, T.; Takahashi, R.; Taniguchi, Y. Characteristics of tissue temperature during ablation with THERMOCOOL SMARTTOUCH SF versus TactiCath versus QDOT MICRO catheters (Qmode and Qmode+): An in vivo porcine study. J. Cardiovasc. Electrophysiol. 2023, 35, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Solimene, F.; Compagnucci, P.; Tondo, C.; La Fazia, V.M.; Schillaci, V.; Mohanty, S.; Cipolletta, L.; Fassini, G.M.; Chiariello, P.; Mottola, G.; et al. Direct Epicardial Validation of Posterior Wall Electroporation in Persistent Atrial Fibrillation. JACC: Clin. Electrophysiol. 2024, 10, 1200–1202. [Google Scholar] [CrossRef] [PubMed]
- Teres, C.; Soto-Iglesias, D.; Penela, D.; Jáuregui, B.; Ordoñez, A.; Chauca, A.; Carreño, J.M.; Scherer, C.; Antonio, R.S.; Huguet, M.; et al. Personalized paroxysmal atrial fibrillation ablation by tailoring ablation index to the left atrial wall thickness: The ‘Ablate by-LAW’ single-centre study—A pilot study. EP Eur. 2021, 24, 390–399. [Google Scholar] [CrossRef] [PubMed]





| Baseline Characteristics | |
|---|---|
| Age (years) | 55.6 ± 11.2 |
| BMI (kg/m2) | 27.5 ± 4.0 |
| LAVI (mL/m2) | 58.4 ± 17.1 |
| LAV (mL) | 132.1 ± 36.4 |
| Ejection Fraction (%) | 56.0 ± 9.8 |
| AF type | |
| Paroxysmal (n, %) | 91 (71) |
| Persistent (n, %) | 37 (29) |
| Female Sex | 43 (33.6) |
| Hypertension | 79 (61.7) |
| Hyperlipidemia | 35 (30.8) |
| Diabetes mellitus | 9 (7) |
| OSAS | 16 (12.5) |
| Smoking | 21 (16.4) |
| COPD | 4 (3.1) |
| Chronic Kidney Disease | 4 (3.1) |
| Coronary Disease | 5 (3.9) |
| Vascular Disease | 8 (6.3) |
| Previous Stroke | 3 (2.3) |
| Thromboembolism | 1 (0.8) |
| Nr comorbidities: | |
| 0 | 20 (15.6) |
| 1 | 50 (39.1) |
| 2 | 35 (27.3) |
| 3 | 9 (7) |
| 4 | 6 (4.7) |
| 5 | 6 (4.7) |
| 6 | 1 (0.8) |
| CHA2DS2_VASc Score | |
| 0 | 26 (20.3) |
| 1 | 48 (37.5) |
| 2 | 31 (25) |
| 3 | 18 (14.1) |
| 4 | 3 (2.3) |
| 5 | 1 (0.8) |
| Recurrence (%) | 55 (43) |
| Follow up time | 25.8 ± 17.3 |
| Sex | Thickness (mm) | Mass (g) | ||
|---|---|---|---|---|
| Superior Wall | Segment 1 | Female | 1.289 ± 0.254 | 0.573 ± 0.214 |
| Male | 1.377 ± 0.678 | 0.679 ± 0.281 | ||
| Segment 2 | Female | 1.560 ± 0.426 | 0.649 ± 0.215 | |
| Male | 1.708 ± 0.851 | 0.789 ± 0.292 | ||
| Segment 3 | Female | 1.320 ± 0.280 | 0.603 ± 0.248 | |
| Male | 1.308 ± 0.666 | 0.736 ± 0.274 | ||
| Segment 4 | Female | 1.354 ± 0.301 | 0.537 ± 0.188 | |
| Male | 1.361 ± 0.692 | 0.670 ± 0.290 | ||
| Posterior Wall | Segment 5 | Female | 1.349 ± 0.283 | 1.293 ± 0.537 |
| Male | 1.357 ± 0.682 | 1.428 ± 0.541 | ||
| Segment 6 | Female | 1.382 ±0.284 | 1.804 ± 0.568 | |
| Male | 1.414 ± 0.703 | 2.088 ± 0.719 | ||
| Septal Wall | Segment 7 | Female | 1.245 ± 0.268 | 1.733 ± 0.567 |
| Male | 1.364 ± 0.678 | 2.045 ± 0.824 | ||
| Anterior Wall | Segment 8 | Female | 1.739 ± 0.562 | 2.452 ± 0.825 |
| Male | 1.983 ± 0.993 | 2.806 ± 0.956 | ||
| Segment 9 | Female | 1.256 ± 0.265 | 0.482 ± 0.152 | |
| Male | 1.286 ± 0.651 | 0.546 ± 0.197 | ||
| Segment 10 | Female | 1.978 ± 0.707 | 1.510 ± 0.518 | |
| Male | 2.226 ± 1.131 | 1.788 ± 0.547 | ||
| Segment 11 | Female | 1.337 ± 0.291 | 0.378 ± 0.159 | |
| Male | 1.338 ± 0.680 | 0.438 ± 0.201 | ||
| Left Lateral Wall | Segment 12 | Female | 1.304 ± 0.328 | 0.815 ± 0.391 |
| Male | 1.351 ± 0.701 | 0.818 ± 0.397 | ||
| Between Superior Veins | Segment 13 | Female | 1.805 ± 0.647 | 0.189 ± 0.117 |
| Male | 1.804 ± 1.004 | 0.298 ± 0.172 | ||
| Between Inferior Veins | Segment 14 | Female | 1.469 ± 0.355 | 0.423 ± 0.187 |
| Male | 1.590 ± 0.786 | 0.548 ± 0.322 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva Cunha, P.; Laranjo, S.; Monteiro, S.; Almeida, I.G.; Mendonça, T.; Fontes, I.; Ferreira, R.C.; Almeida, A.G.; Didenko, M.; Oliveira, M.M. Left Atrial Wall Thickness Estimated by Cardiac CT: Implications for Catheter Ablation of Atrial Fibrillation. J. Clin. Med. 2024, 13, 5379. https://doi.org/10.3390/jcm13185379
Silva Cunha P, Laranjo S, Monteiro S, Almeida IG, Mendonça T, Fontes I, Ferreira RC, Almeida AG, Didenko M, Oliveira MM. Left Atrial Wall Thickness Estimated by Cardiac CT: Implications for Catheter Ablation of Atrial Fibrillation. Journal of Clinical Medicine. 2024; 13(18):5379. https://doi.org/10.3390/jcm13185379
Chicago/Turabian StyleSilva Cunha, Pedro, Sérgio Laranjo, Sofia Monteiro, Inês Grácio Almeida, Tiago Mendonça, Iládia Fontes, Rui Cruz Ferreira, Ana G. Almeida, Maxim Didenko, and Mário Martins Oliveira. 2024. "Left Atrial Wall Thickness Estimated by Cardiac CT: Implications for Catheter Ablation of Atrial Fibrillation" Journal of Clinical Medicine 13, no. 18: 5379. https://doi.org/10.3390/jcm13185379
APA StyleSilva Cunha, P., Laranjo, S., Monteiro, S., Almeida, I. G., Mendonça, T., Fontes, I., Ferreira, R. C., Almeida, A. G., Didenko, M., & Oliveira, M. M. (2024). Left Atrial Wall Thickness Estimated by Cardiac CT: Implications for Catheter Ablation of Atrial Fibrillation. Journal of Clinical Medicine, 13(18), 5379. https://doi.org/10.3390/jcm13185379







