Abstract
Objectives: Arterial hypertension (AH) is one of the major risk factors for cardiovascular diseases. An association between untreated AH and arrhythmia is observed. Cardiac magnetic resonance (CMR) assesses myocardial fibrosis by detecting foci of late gadolinium enhancement (LGE). Clinical significance of LGE at the right ventricular insertion point (RVIP) is not fully established. This study aimed to assess the relationship between the presence of LGE at the RVIP determined by CMR and the incidence of arrhythmia in a group suffering from arterial hypertension. Methods: The study group consisted of 81 patients with AH (37 men and 44 women, age: 56.7 ± 7.1 years). All subjects underwent CMR and 24 h Holter ECG monitoring. Two subgroups were distinguished in the study group based on the criterion of the presence of LGE at the RVIP in CMR. The RVIP+ subgroup consisted of patients with LGE at the RVIP, while the RVIP− group consisted of patients without LGE at the RVIP. Results: The RVIP+ subgroup was characterized by higher maximum and minimum heart rates in 24 h Holter ECG recordings compared to the RVIP− subgroup (p < 0.05). The RVIP+ subgroup had a statistically significantly higher number of single premature supraventricular beats, supraventricular tachycardias, and single premature ventricular beats than the RVIP− subgroup (p < 0.05). Regression analysis documented that a longer duration of AH (counted from diagnosis) as well as the occurrence of LGE at the RVIP (assessed by CMR) are independent risk factors for arrhythmia (p < 0.05). Conclusions: Due to the possibility of detecting LGE at the RVIP, CMR may be a useful diagnostic method in estimating the risk of arrhythmias in the group of patients with AH.
1. Introduction
Arterial hypertension (AH) is one of the main modifiable cardiovascular risk factors. AH affects around 1.3 billion people aged 30–70 [,]. The etiology of arterial hypertension is very complex, with the majority (90–95%) of patients suffering from primary arterial hypertension with a multifactorial gene–environment etiology []. The most common complications of arterial hypertension mainly involve the kidney [], the heart [,,], and retinal vessel lesions [,,]. Simultaneously, arterial hypertension is the most common cause of hypertensive heart disease [], including left ventricular hypertrophy [], left atrial enlargement [], functional mitral regurgitation [], and neurohormonal changes. Untreated arterial hypertension increases the risk of arrhythmia as well as sudden cardiac death [,,].
Cardiac magnetic resonance (CMR) is a non-invasive cardiac imaging technique to evaluate volume, function, and perform an analysis of myocardial tissue characteristics. CMR is the gold standard for assessing, e.g., the ventricular ejection fraction. The information obtained from CMR allows a comprehensive evaluation of the heart and provides additional information on the etiology of heart failure []. CMR allows us to determine the etiology of myocardial damage. The ischemic changes are characterized by rapid myocardial edema, which can be detected in T2-weighted sequences as early as 30 min after the onset of symptoms, as well as late gadolinium enhancement (LGE) with subendocardial to transmural distribution. Non-ischemic cardiomyopathy shows LGE with a mid-wall or subepicardial distribution pattern [,]. In patients with hypertrophic cardiomyopathy, two main types of LGE patterns have been distinguished: subepicardial or midmyocardial LGE, which corresponds to descending fibrosis, and right ventricular insertion point (RVIP) LGE, corresponding to interstitial fibrosis and/or myocyte dysfunction [,]. The presence of LGE at the RVIP on CMR in patients with other heart diseases, including structural heart diseases, is generally believed to be nonspecific. Additionally, in patients with other structural heart diseases, the RVIP is usually not associated with a worse prognosis []. However, the clinical significance of LGE in the RVIP has not been fully established. Grigoratos et al. conducted a retrospective among 2000 patients from which 420 ones with normal CMR images were selected, and in this group LGE was diagnosed in the RVIP in 36 patients. Based on follow-up, they showed that patients with LGE at the RVIP were less likely to have cardiac events than patients without LGE. In contrast, patients with LGE elsewhere (with a non-ischemic pattern) in the myocardium had a worse prognosis than patients with LGE in the RVIP [].
This study aimed to assess the relationship between the presence of late gadolinium enhancement at the right ventricular insertion point (RVIP) determined by CMR and the occurrence of cardiac arrhythmias in patients with arterial hypertension.
2. Materials and Methods
This study is a single-point observational study, conducted between 2019 and 2023. This study included patients of the hypertension department of the University Hospital in Wroclaw, hospitalized to assess the effectiveness of hypertension treatment, assess the organ consequences of arterial hypertension, and possibly optimize the current therapy. The following inclusion criteria were used: age > 18 years, hypertension diagnosed according to the European Society of Hypertension guidelines [], and willingness to participate in the study. The exclusion criteria were as follows: secondary arterial hypertension, heart failure, coronary artery disease, cardiomyopathy, respiratory insufficiency, renal insufficiency, severe mental disorders, active malignancy, and active inflammation.
This study enrolled 81 patients diagnosed with arterial hypertension. The mean age was 56.7 ± 7.1. Women comprised 54.3% and men 45.7%. A total of 64.2% of the participants were treated with combination therapy. The mean duration of arterial hypertension (counted from diagnosis) was 14.3 ± 4.8 years.
The basic characteristics of the group are shown in Table 1.

Table 1.
Clinical characteristics and characteristics of treatment of arterial hypertension in the whole study group.
This study was conducted based on the principles of the Helsinki Declaration. The study project was positively reviewed by the Bioethics Committee of the Wrocław Medical University. The clinical examination methodology included a medical history, measurement of total cholesterol, fasting glucose, and cardiac magnetic resonance. Blood pressure values were measured using the Korothov method. Total blood cholesterol concentration and fasting blood glucose concentration were determined using standard tests, based on the manufacturer’s instructions.
Twenty-four-hour (from 6:00 to 6:00 the next day) Holter ECG recordings were made with a Lifecard CF 12-channel recorder serial number LIFE-045348/2015 and recording analysis with Sentinel Spacelabs Healthcare Pathfinder SL version 1.7.1.5164 with serial number 8395 (Delmar Reynolds, Hertford, UK). Two experienced cardiologists reviewed the study. ECG recordings were analyzed for quantitative and categorical changes. The variable “sum of categorical changes in 24 h Holter ECG” is a variable that comprehensively summarizes the analysis of the ECG recording in terms of categorical changes. This variable was calculated as follows. Each categorical change that was found in the patient’s ECG recording was assigned 1 point. After analyzing the ECG recording in terms of all assessed categorical variables, the summed points gave the quantitative variable “sum of categorical changes in 24 h Holter ECG”.
Cardiac magnetic resonance (CMR) was performed using one 1.5T Magneton Aera machine (Siemens Healthcare, Forchheim, Germany), according to the same protocol, with a bolus of 0.2 mmol/kg body weight of gadobutrol (Gadovist, Bayer Healthcare, Leverkusen, Germany) administered through the veins of the antecubital fossa. CINE-type SSFP (steady-state free precession sequence), short-tau inversion-recovery (STIR) sequences, late gadolinium enhancement (LGE) sequences, as well as T1 mapping and T2 mapping sequences were performed. LGE imaging was performed using a T1-weighted segmented inversion-recovery pulse sequence, 10 min after administration of the contrast agent. The following LGE sequence parameters were used: slice/gap thickness: 10/0 mm, matrix: 256 × 192, in-plane resolution: 1.4 × 1.4 mm2, TR/TE: 650/4.9 ms, flip angle: 30°, inversion time set to null normal myocardium []. T1 and T2 mapping sequences were obtained in end-diastole in short-axis orientation in three slices (basal, midventricular, and apical). For myocardial T1 mapping, a MOLLI (modified Look-Locker inversion) recovery acquisition scheme with motion correction (MOCO) was applied. The following T1 mapping sequence parameters were used: slice/gap thickness: 10/0 mm, matrix: 256 × 192, in-plane resolution: 1.4 × 1.4 mm2, TR/TE: 305.8/4.9 ms, flip angle: 35°. T2 mapping was performed using a T2-prepared balanced steady-state free precession sequence. The following T2 mapping sequence parameters were used: slice/gap thickness: 10/0 mm, matrix: 256 × 192, in-plane resolution: 1.4 × 1.4 mm2, TR/TE: 218.2/1.0 ms, flip angle: 70° [].
Post-processing evaluation of CMR images was performed using Medis Suite 4.0 software (Medis, Leiden, The Netherlands). CINE sequences were performed in the short axis of the left ventricle and in the long axis in 2-chamber, 3-chamber, and 4-chamber views. In CINE sequences, the dimensions of the cardiac chambers and the functional parameters of the left ventricle were assessed. Images in the short axis and 2-chamber projections in the long axis of the left ventricle were used to estimate the parameters of left ventricular function using the volumetric method. The end-diastolic and end-systolic volumes (EDV and ESV) were calculated as the sum of the left ventricular cavity surface in subsequent layers in images in the short axis multiplied by the thickness of the layer. The stroke volume (SV) was the difference between EDV and ESV. The ejection fraction (EF) was obtained by stroke volume divided by end-diastolic volume. Functional parameters of the left ventricle are presented as body surface area (BSA) indexed values. The left ventricular mass index (LVMI) was calculated too. Foci of left ventricular myocardial edema were assessed in the STIR sequence, and foci of late gadolinium enhancement (foci of myocardial damage) were assessed in the LGE sequence. The myocardium was assessed for the presence of 4 typical types of LGE foci: subendocardial (adjacent to the endocardium but not the epicardium), midwall (not adjacent to the endocardium and the epicardium), subepicardial (adjacent to the epicardium but not the endocardium), and transmural (adjacent to the endocardium and the epicardium). The presence of LGE foci was assessed in 16 segments of the left ventricular myocardium: 6 basal, 6 midventricular, and 4 apical. In addition, the presence of LGE foci in the RVIP was assessed []. In T1 and T2 mapping sequences performed before administration of the contrast agent, the native T1 and T2 times of the myocardium were measured. In the T1 mapping sequence performed 20 min after administration of the contrast agent, the post-contrast T1 time (T1 C+) of the myocardium was measured. T1 and T2 relaxation times were analyzed using a dedicated Syngo.via post-processing system (Siemens Healthcare, Forchheim, Germany). The interventricular septum (excluding areas with LGE at the RVIP) was outlined in all three slices (basal, midventricular, and apical) and the averages of all three measurements were obtained []. In the assessment of the mapping sequence, the standards estimated for our CMR laboratory were used: up to 1052 ms for T1 time and up to 52 ms for T2 myocardial time.
CMR images analysis was performed consensually by one cardiovascular radiologist with over 10 years of experience in CMR assessment who had passed the ESC EACVI Cardiovascular Magnetic Resonance Exam and achieved national certification in cardiovascular radiology and one cardiologist with over 20 years of experience in cardiac and vascular imaging.
Statistical analysis was performed using the statistical application “Dell Statistica 13.1” (Dell Inc., Round Rock, TX, USA). Quantitative variables were presented in the format of arithmetic means ± standard deviations. Normality of the distributions of variables was verified using the Lilliefors and W–Shapiro–Wilk tests. For quantitative variables meeting the condition of normal distribution, a parametric test, i.e., the t test, was used in the comparative analysis. For quantitative variables that did not meet the condition of normal distribution, the hypotheses in the comparative analysis were verified using a nonparametric test, i.e., the Mann–Whitney U test. Categorical variables were presented in the format of numbers/percentages. For categorical variables, the Chi-squared test was used in the comparative analysis. The relationships between variables were assessed in the regression analysis using the backward stepwise multivariate method. The estimation parameters in the regression analysis were obtained using the least squares method. The results were statistically significant at two-sided p < 0.05.
3. Results
The mean values of the left and right atrial areas were 27.8 ± 7.6 and 21.4 ± 4.3 cm2, respectively. The mean left ventricular mass index was 74.3 ± 14.7 g/m2. The mean left ventricular ejection fraction was 66.1 ± 6.1%. The late gadolinium enhancement occurred in 33.3% of patients. All LGEs were localized at the right ventricular insertion point. No LGE was demonstrated elsewhere. Myocardial T1 time was 1024.9 ± 8.1 ms, and myocardial T2 time was 42.4 ± 2.7 ms. In all patients, myocardial T1 time and myocardial T2 time were normal.
Patients were divided into two subgroups based on the presence of LGE at the RVIP. The first subgroup consisted of subjects with LGE at the right ventricular insertion point (RVIP+ subgroup), while the second subgroup consisted of subjects without LGE at the right ventricular insertion point (RVIP− subgroup), Figure 1.
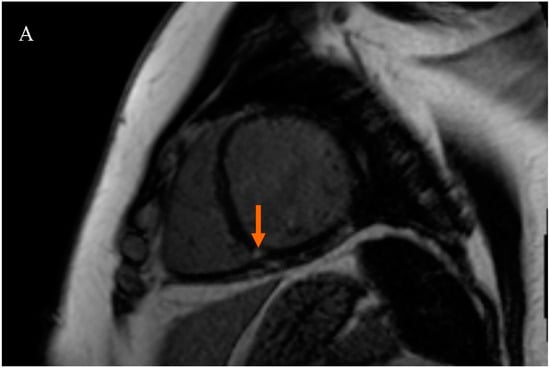
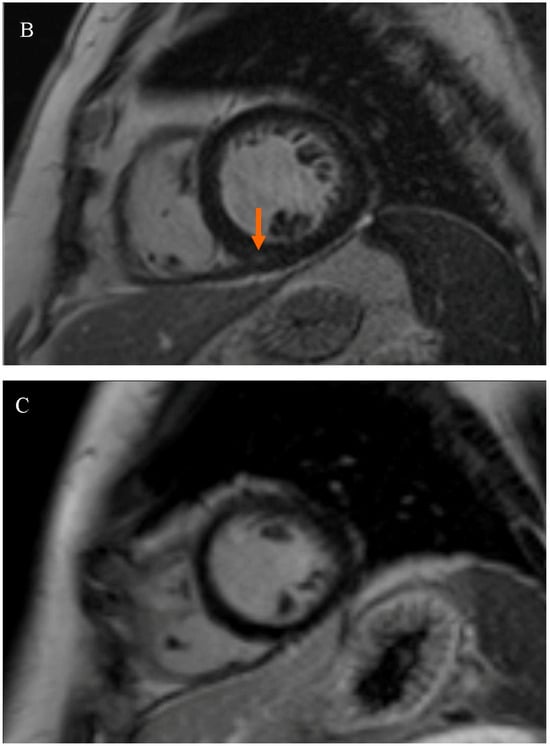
Figure 1.
Late gadolinium enhancement (LGE) sequence images in cardiac magnetic resonance (CMR): (A) in a female patient with an LGE focus on the right ventricular insertion point (indicated by an orange arrow), (B) in a male patient with a subtle LGE focus on the right ventricular insertion point (indicated by an orange arrow), and (C) in a patient without LGE foci.
Details of the parameters analyzed based on CMR are shown in Table 2.

Table 2.
Cardiac magnetic resonance parameters in the study group.
CMR showed that patients in the RVIP+ subgroup had a statistically significantly higher mean left atrial area compared to the RVIP− subgroup (33.0 ± 5.3 vs. 24.4 ± 5.6 cm2; p < 0.05). Details of CMR parameters in the studied subgroups differing in the occurrence of the RVIP are presented in Table 3.

Table 3.
Cardiac magnetic resonance parameters in the study subgroups: A—subgroup with late gadolinium enhancement at the right ventricular insertion point (RVIP+); B—subgroup without any late gadolinium enhancement at the right ventricular insertion point (RVIP−).
The Holter ECG showed that patients in the RVIP+ subgroup had a statistically significantly higher mean heart rate compared to the RVIP− subgroup (71.3 ± 6.9 vs. 65.9 ± 6.8 bpm; p < 0.05). It was observed that patients with LGE at the RVIP had a significantly higher maximum heart rate of 104.8 ± 13.4 bpm compared to patients without LGE at the RVIP at 98.6 ± 14.1 bpm; p < 0.05. Patients in the group with LGE also had significantly more supraventricular premature complex and supraventricular premature complex pairs than the other study participants, 217.2 ± 401.6 vs. 8.1 ± 10.8; p < 0.05 and 18.6 ± 45.3 vs. 1.2 ± 1.4; p < 0.05, respectively. Details of the other parameters analyzed are presented in Table 4.

Table 4.
Quantitative parameters of 24 h Holter ECG monitoring in the study subgroups: A—subgroup with late gadolinium enhancement at the right ventricular insertion point (RVIP+); B—subgroup without any late gadolinium enhancement at the right ventricular insertion point (RVIP−).
Interestingly, patients with LGE at the RVIP were significantly more likely to be diagnosed with atrial fibrillation than patients without LGE at the RVIP, 14.8 vs. 1.8%; p < 0.05. Patients in the RVIP+ subgroup were significantly more likely to have tachycardia than participants in the RVIP− subgroup, 18.5 vs. 3.7%, respectively; p < 0.05. LGE at the RVIP was not observed to affect the presence of bradycardia, pauses, or atrioventricular blocks. However, patients in the RVIP+ subgroup had significantly more frequent ST-T segment changes than the others, 22.2 vs. 13.0%; p < 0.05. Considering the sum of all changes observed on the Holter ECG, patients with LGE in the RVIP had significantly more changes than patients without LGE in the RVIP, 4.2 ± 0.6 vs. 0.9 ± 0.5%; p < 0.05. Detailed results are presented in Table 5.

Table 5.
Categorical parameters of 24 h Holter ECG monitoring in the study subgroups: A—subgroup with late gadolinium enhancement at the right ventricular insertion point (RVIP+); B—subgroup without any late gadolinium enhancement at the right ventricular insertion point (RVIP−).
To verify the relationship between the occurrence of LGE in the RVIP and arrhythmias, regression analysis was performed using the “sum of categorical changes in 24 h Holter ECG” variable as the dependent variable. The potential independent variables associated with the sum of categorical changes in the 24 h Holter ECG were the clinical parameters presented in Table 1 (i.e., anthropometric variables, variables characterizing arterial hypertension and treatment of arterial hypertension, variables of lipid and carbohydrate metabolism and smoking) and CMR parameters (variables related to the size and mass of the left ventricle and RVIP). Multivariable, stepwise, backward analysis was used. The assessed model is presented in Table 6.

Table 6.
Results of regression analysis in the study group: risk factors for higher ∑ categorical changes in 24 h Holter ECG monitoring.
Regression analysis showed that LGE at the RVIP and a longer duration of arterial hypertension (counted from diagnosis) are independent risk factors for a larger sum of categorical changes in the 24 h Holter ECG.
4. Discussion
This study provides additional information on the importance of LGE in the RVIP and its relations with arrhythmias determined by Holter ECG in patients with arterial hypertension. According to the latest guidelines [], CMR is a technique aiming to recognize cardiomyopathies and various causes of heart failure. Our study showed that patients with arterial hypertension and LGE at the RVIP had a significantly higher average heart rate and maximum heart rate compared to patients without LGE at the RVIP. Furthermore, these patients significantly more often experienced supraventricular premature complexes than patients without LGE. The presence of LGE at the RVIP led to a significantly higher frequency of diagnosed atrial fibrillation in patients compared to those without LGE at the RVIP. Regression analysis demonstrated that LGE at the RVIP and a longer duration of arterial hypertension (counted from diagnosis) were independent risk factors for observed changes in the 24 h Holter ECG.
Arterial hypertension causes endothelial dysfunction, exacerbates the atherosclerotic process, and contributes to the instability of atherosclerotic plaques []. Moreover, it leads to left ventricular hypertrophy. All of the abovementioned processes contribute to decreased coronary reserve and increased myocardial oxygen demand, causing myocardial ischemia []. Furthermore, arterial hypertension causes harmful effects on the structure of the heart muscle, resulting in left ventricular hypertrophy and excessive growth of fibroblasts and extracellular matrix (fibrosis), death of cardiomyocytes, and heart failure []. The intercellular matrix is involved in influencing cardiac cell physiology. Cardiovascular diseases have been proven to affect the remodeling of the matrix and can consequently give rise to impaired cardiac function []. Additionally, arterial hypertension is a significant origin in the development of non-rheumatic atrial fibrillation and other supraventricular arrhythmias [,]. Hennersdorf et al., based on analyzed studies, showed that the presence of arterial hypertension even without left ventricular hypertrophy significantly increases the risk of ventricular arrhythmias compared to patients with controlled blood pressure. In contrast, left ventricular hypertrophy increases the risk of ventricular tachycardia and mortality fourfold [].
One of the classic consequences of arterial hypertension is left atrial enlargement. In addition to eliciting left ventricular hypertrophy, dysfunction, and heart failure, hypertension also causes left atrial remodeling that may culminate in atrial contractile dysfunction and atrial fibrillation. The data imply that left atrial remodeling is multifactorial, starts early in hypertension, and is an important contributor to the progression of hypertensive heart disease, including the development of atrial fibrillation and heart failure []. Volume and pressure overload is one of the mechanisms of the development of left atrial enlargement in arterial hypertension. Similarly, some researchers believe that the nonspecific symptom of the RVIP is the result of changes in pressure–volume relationships during the cardiac cycle []. In our study of patients with hypertension, the subgroup of patients with the RVIP+ symptom was characterized by higher LAA and a more frequent occurrence of supraventricular arrhythmias (including atrial fibrillation) than the RVIP− subgroup. The mechanism explaining this fact may be atrial volume–pressure overload—a common mechanism, as indicated by literature data, for the occurrence of RVIP and left atrial enlargement in arterial hypertension.
Therefore, the common pathophysiology linking the occurrence of the RVIP in CMR, left atrial enlargement, supraventricular arrhythmias, and higher heart rate values may be the volume–pressure overload of the left heart chambers in hypertension. Therefore, it is not surprising that in our study, patients with AH from the RVIP+ subgroup showed higher HR values than patients with AH from the RVIP− subgroup. The above observation could be important in the context of recognizing tachycardia as a potential cardiovascular risk factor. It should be noted, however, that heart rates in the RVIP+ group remained within the normal range. Furthermore, maximum HR obviously depends on what the subject did during the Holter’s 24 h.
CMR is the gold standard for cardiac imaging and has advantages over echocardiography in evaluating and differentiating left ventricular hypertrophy. The finding of left ventricular hypertrophy alone does not establish a clear etiology, which may be related to myocardial fibrosis and abnormal protein deposition. A detailed assessment of the structure of the muscle can be analyzed by CMR []. There are various techniques used in CMR. One of them is late gadolinium enhancement, which is a standard evaluation in cardiac imaging. It allows differentiation of ischemic and non-ischemic cardiac damage through characteristic enhancement patterns and is ideal for detecting areas of focal scarring and or fibrosis [,]. Other recognized methods include native T1 mapping, which is sensitive to increased tissue water content and is prolonged in cases of inflammation, myocardial edema, and fibrosis and decreased by high iron or lipid content [,]. T2 mapping, on the other hand, is used to detect acute inflammation and myocardial edema [].
Our study has examined whether LGE at the RVIP is significantly associated with arrhythmias. Wang et al. conducted a study showing that the native T1 value of the intraventricular RVIP can play an essential role in the risk stratification of patients with pulmonary hypertension, which may be necessary for deciding the appropriate treatment []. Similar observations were indicated in connective tissue disease patients with pulmonary hypertension []. Cabanis et al., who based on their studies on sheep and human hearts, showed that the RVIP is a well-organized aggregated cardiomyocyte arrangement. Altering the structure of this site, the presence of LGE may play a role in the formation of cardiac arrhythmias [].
In contrast to our study, Grigoratos et al. showed that patients with LGE at the RVIP had significantly fewer cardiac events than patients with LGE at another site. In contrast, the Kaplan–Meier curve analysis showed no significant differences between patients with RVIP-LGE and those without LGE at the RVIP. Patients with another LGE had a worse prognosis than patients with RVIP-LGE or no LGE []. Similar observations were demonstrated by Yi et al., who showed, based on a study, that LGE found at the RVIP among patients with non-ischemia-related dilated cardiomyopathy did not significantly increase the risk of adverse cardiac events and provided a better prognosis than LV-localized LGE in the same range []. On the other hand, Nitsche et al. showed that interstitial proliferation in the anterior RVIP was associated with hemodynamic changes recorded during cardiac catheterization and was independently associated with the prognosis of patients with heart failure [].
The presence of LGE correlates with the risk of cardiac events in patients with hypertrophic cardiomyopathy. The absence of LGE was associated with a lower risk of events, while the degree of LGE also predicted the development of end-stage heart failure with reduced ejection fraction [].
Not many studies are available in the literature on the relationship between LGE at the RVIP and cardiac arrhythmias. Available studies show that LGE at the RVIP is not significantly associated with cardiac arrhythmias. It has been shown that the presence of fibrosis at the RVIP can be used in the prognosis of patients with pulmonary hypertension. We, in turn, have shown that patients with LGE at the RVIP and with arterial hypertension had significantly more frequent cardiac events than patients without LGE.
Our results may also be important in the ongoing discussion on heart remodeling in athletes. As Allwood et al. point out, the development of myocardial fibrosis in athletes appears to be a multifactorial process, with genetics, hormones, the exercise dose, and an adverse cardiovascular risk profile playing key roles. Myocardial fibrosis is not a benign finding and warrants a comprehensive evaluation and follow-up regarding potential cardiac disease []. Małek et al. documented that in their study group, 73.3% of athletes fulfilled the volumetric criteria for dilated cardiomyopathy or arrhythmogenic right ventricular cardiomyopathy. Non-ischemic, small-volume LGE was found in 27% of athletes and in 10% of controls. It was localized at the RVIP or in the septum or inferolateral wall. Athletes with LGE at the RVIP had a higher right ventricular end-diastolic volume index in comparison to athletes without LGE (p < 0.05), which suggests its relation to volume overload []. In this context, the relationship we have shown between the presence of LGE at the RVIP and arrhythmias may be a potential link in the pathogenesis of cardiac arrhythmias in athletes.
The limitations of our study were the relatively small number of study participants and the lack of patients in the study group with other types of LGE. Undoubtedly, the issue requires further study to demonstrate the clinical implications of LGE at the RVIP.
5. Conclusions
Cardiac magnetic resonance by identifying late gadolinium enhancement at the right ventricular insertion point may be a proper diagnostic method in assessing the risk of cardiac arrhythmias in patients with arterial hypertension.
Author Contributions
Conceptualization, R.P. and P.G.; funding acquisition, P.G.; investigation, A.W., P.G. and R.P.; project administration, P.G.; resources, A.W., P.M. and B.D.-M.; software, P.G. and R.P.; supervision, P.G. and R.P.; writing—original draft, A.W., P.M. and B.D.-M.; writing—review and editing, M.P., R.P. and P.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Wroclaw Medical University. Approval Code—KB 58/2022; Approval Date—26 January 2022.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Mancia, G.; Kreutz, R.; Brunström, M.; Burnier, M.; Grassi, G.; Januszewicz, A.; Muiesan, M.L.; Tsioufis, K.; Agabiti-Rosei, E.; Algharably, E.A.E.; et al. 2023 ESH Guidelines for the management of arterial hypertension The Task Force for the management of arterial hypertension of the European Society of Hypertension: Endorsed by the International Society of Hypertension (ISH) and the European Renal Association (ERA). J. Hypertens. 2023, 41, 1874–2071. [Google Scholar] [CrossRef] [PubMed]
- Oparil, S.; Acelajado, M.C.; Bakris, G.L.; Berlowitz, D.R.; Cífková, R.; Dominiczak, A.F.; Grassi, G.; Jordan, J.; Poulter, N.R.; Rodgers, A.; et al. Hypertension. Nat. Rev. Dis. Prim. 2018, 4, 18014. [Google Scholar] [CrossRef] [PubMed]
- Rimoldi, S.F.; Scherrer, U.; Messerli, F.H. Secondary arterial hypertension: When, who, and how to screen? Eur. Heart J. 2014, 35, 1245–1254. [Google Scholar] [CrossRef]
- Nagaraju, S.P.; Shenoy, S.V.; Rao, I.R.; Bhojaraja, M.V.; Rangaswamy, D.; Prabhu, R.A. Measurement of Blood Pressure in Chronic Kidney Disease: Time to Change Our Clinical Practice—A Comprehensive Review. Int. J. Nephrol. Renovasc. Dis. 2022, 15, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Di Palo, K.E.; Barone, N.J. Hypertension and Heart Failure: Prevention, Targets, and Treatment. Cardiol. Clin. 2022, 40, 237–244. [Google Scholar] [CrossRef]
- Di Palo, K.E.; Barone, N.J. Hypertension and Heart Failure: Prevention, Targets, and Treatment. Heart Fail. Clin. 2020, 16, 99–106. [Google Scholar] [CrossRef]
- Rifkin, D.E.; Kiernan, M.; Sarnak, M.J. Hitting the Mark: Blood Pressure Targets and Agents in Those with Prevalent Cardiovascular Disease and Heart Failure. Adv. Chronic Kidney Dis. 2015, 22, 140–144. [Google Scholar] [CrossRef]
- Cerasola, G.; Cottone, S.; Mulé, G.; Nardi, E.; Mangano, M.T.; Andronico, G.; Contorno, A.; Li Vecchi, M.; Galione, P.; Renda, F.; et al. Microalbuminuria, renal dysfunction and cardiovascular complication in essential hypertension. J. Hypertens. 1996, 14, 915–920. [Google Scholar] [CrossRef]
- Tien, Y.W.; McIntosh, R. Hypertensive retinopathy signs as risk indicators of cardiovascular morbidity and mortality. Br. Med. Bull. 2005, 73–74, 57–70. [Google Scholar] [CrossRef]
- Chatterjee, S.; Chattopadhya, S.; Hope-Ross, M.; Lip, P.L. Hypertension and the eye: Changing perspectives. J. Hum. Hypertens. 2002, 16, 667–675. [Google Scholar] [CrossRef]
- Xu, Y.; Bouliotis, G.; Beckett, N.S.; Antikainen, R.L.; Anderson, C.S.; Bulpitt, C.J.; Peters, R. Left ventricular hypertrophy and incident cognitive decline in older adults with hypertension. J. Hum. Hypertens. 2023, 37, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Kockskämper, J.; Pluteanu, F. Left Atrial Myocardium in Arterial hypertension. Cells 2022, 11, 3157. [Google Scholar] [CrossRef] [PubMed]
- Imbalzano, E.; Vatrano, M.; Ghiadoni, L.; Mandraffino, G.; Dalbeni, A.; Khandheria, B.K.; Costantino, R.; Trapani, G.; Manganaro, R.; Cusmà Piccione, M.; et al. Arterial stiffness and mitral regurgitation in arterial hypertension: An intriguing pathophysiological link. Vascul. Pharmacol. 2018, 111, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Shenasa, M.; Shenasa, H. Hypertension, left ventricular hypertrophy, and sudden cardiac death. Int. J. Cardiol. 2017, 237, 60–63. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, M.; Oktay, A.A.; Stewart, M.H.; Milani, R.V.; Ventura, H.O.; Lavie, C.J. Left ventricular hypertrophy and hypertension. Prog. Cardiovasc. Dis. 2020, 63, 10–21. [Google Scholar] [CrossRef]
- Chatterjee, S.; Bavishi, C.; Sardar, P.; Agarwal, V.; Krishnamoorthy, P.; Grodzicki, T.; Messerli, F.H. Meta-analysis of left ventricular hypertrophy and sustained arrhythmias. Am. J. Cardiol. 2014, 114, 1049–1052. [Google Scholar] [CrossRef]
- Liu, C.; Ferrari, V.A.; Han, Y. Cardiovascular Magnetic Resonance Imaging and Heart Failure. Curr. Cardiol. Rep. 2021, 23, 35. [Google Scholar] [CrossRef]
- Baritussio, A.; Scatteia, A.; Bucciarelli-Ducci, C. Role of cardiovascular magnetic resonance in acute and chronic ischemic heart disease. Int. J. Cardiovasc. Imaging 2018, 34, 67. [Google Scholar] [CrossRef]
- Patel, A.R.; Kramer, C.M. Role of Cardiac Magnetic Resonance in the Diagnosis and Prognosis of Nonischemic Cardiomyopathy. JACC Cardiovasc. Imaging 2017, 10, 1180–1193. [Google Scholar] [CrossRef]
- Bravo, P.E.; Luo, H.C.; Pozios, I.; Zimmerman, S.L.; Corona-Villalobos, C.P.; Sorensen, L.; Kamel, I.R.; Bluemke, D.A.; Wahl, R.L.; Abraham, M.R.; et al. Late gadolinium enhancement confined to the right ventricular insertion points in hypertrophic cardiomyopathy: An intermediate stage phenotype? Eur. Heart J. Cardiovasc. Imaging 2016, 17, 293–300. [Google Scholar] [CrossRef]
- Cardim, N.; Galderisi, M.; Edvardsen, T.; Plein, S.; Popescu, B.A.; D’Andrea, A.; Bruder, O.; Cosyns, B.; Davin, L.; Donal, E.; et al. Role of multimodality cardiac imaging in the management of patients with hypertrophic cardiomyopathy: An expert consensus of the European Association of Cardiovascular Imaging Endorsed by the Saudi Heart Association. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 280. [Google Scholar] [CrossRef] [PubMed]
- Grigoratos, C.; Pantano, A.; Meschisi, M.; Gaeta, R.; Ait-Ali, L.; Barison, A.; Todiere, G.; Festa, P.; Sinagra, G.; Aquaro, G.D. Clinical importance of late gadolinium enhancement at right ventricular insertion points in otherwise normal hearts. Int. J. Cardiovasc. Imaging 2020, 36, 913–920. [Google Scholar] [CrossRef]
- Miszalski-Jamka, T.; Szczeklik, W.; Karwat, K.; Sokołowska, B.; Gąsior, J.; Rucińska, M.; Mazur, W.; Skotnicki, A.; Kereiakes, D.J.; Urbańczyk, M.; et al. MRI-based evidence for myocardial involvement in women with hypereosinophilic syndrome. Magn. Reson. Med. Sci. 2015, 14, 107–114. [Google Scholar] [CrossRef]
- Muser, D.; Chahal, A.A.; Selvanayagam, J.B.; Nucifora, G. Clinical Applications of Cardiac Magnetic Resonance Parametric Mapping. Diagnostics 2024, 14, 1816. [Google Scholar] [CrossRef] [PubMed]
- Vaitiekiene, A.; Kulboke, M.; Bieseviciene, M.; Jankauskas, A.; Bartnykaite, A.; Rinkuniene, D.; Strazdiene, I.; Lidziute, E.; Jankauskaite, D.; Gaidamavicius, I.; et al. T1 Mapping in Cardiovascular Magnetic Resonance—A Marker of Diffuse Myocardial Fibrosis in Patients Undergoing Hematopoietic Stem Cell Transplantation. J. Pers. Med. 2024, 14, 412. [Google Scholar] [CrossRef] [PubMed]
- Arbelo, E.; Protonotarios, A.; Gimeno, J.R.; Arbustini, E.; Arbelo, E.; Barriales-Villa, R.; Basso, C.; Biagini, E.; Blom, N.A.; De Boer, R.A.; et al. 2023 ESC Guidelines for the management of cardiomyopathies: Developed by the task force on the management of cardiomyopathies of the European Society of Cardiology (ESC). Eur. Heart J. 2023, 44, 3503–3626. [Google Scholar] [CrossRef]
- Escobar, E. Hypertension and coronary heart disease. J. Hum. Hypertens. 2002, 16 (Suppl. S1), S61–S63. [Google Scholar] [CrossRef]
- Duncker, D.J.; Bache, R.J. Regulation of coronary blood flow during exercise. Physiol. Rev. 2008, 88, 1009–1086. [Google Scholar] [CrossRef]
- Wakatsuki, T.; Schlessinger, J.; Elson, E.L. The biochemical response of the heart to hypertension and exercise. Trends Biochem. Sci. 2004, 29, 609–617. [Google Scholar] [CrossRef]
- Sullivan, K.E.; Black, L.D. The role of cardiac fibroblasts in extracellular matrix-mediated signaling during normal and pathological cardiac development. J. Biomech. Eng. 2013, 135, 071001. [Google Scholar] [CrossRef]
- Afzal, M.R.; Savona, S.; Mohamed, O.; Mohamed-Osman, A.; Kalbfleisch, S.J. Hypertension and Arrhythmias. Heart Fail. Clin. 2019, 15, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Yiu, K.H.; Tse, H.F. Hypertension and cardiac arrhythmias: A review of the epidemiology, pathophysiology and clinical implications. J. Hum. Hypertens. 2008, 22, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Hennersdorf, M.G.; Strauer, B.E. Arterial hypertension and cardiac arrhythmias. J. Hypertens. 2001, 19, 167–177. [Google Scholar] [CrossRef]
- Nitsche, C.; Kammerlander, A.A.; Binder, C.; Duca, F.; Aschauer, S.; Koschutnik, M.; Snidat, A.; Beitzke, D.; Loewe, C.; Bonderman, D.; et al. Native T1 time of right ventricular insertion points by cardiac magnetic resonance: Relation with invasive haemodynamics and outcome in heart failure with preserved ejection fraction. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 683–691. [Google Scholar] [CrossRef]
- Burrage, M.K.; Ferreira, V.M. Cardiovascular Magnetic Resonance for the Differentiation of Left Ventricular Hypertrophy. Curr. Heart Fail. Rep. 2020, 17, 192. [Google Scholar] [CrossRef]
- Mahrholdt, H.; Wagner, A.; Judd, R.M.; Sechtem, U.; Kim, R.J. Delayed enhancement cardiovascular magnetic resonance assessment of non-ischaemic cardiomyopathies. Eur. Heart J. 2005, 26, 1461–1474. [Google Scholar] [CrossRef]
- Jackson, E.; Bellenger, N.; Seddon, M.; Harden, S.; Peebles, C. Ischaemic and non-ischaemic cardiomyopathies—Cardiac MRI appearances with delayed enhancement. Clin. Radiol. 2007, 62, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Nakou, E.; Patel, R.K.; Fontana, M.; Bucciarelli-Ducci, C. Cardiovascular Magnetic Resonance Parametric Mapping Techniques: Clinical Applications and Limitations. Curr. Cardiol. Rep. 2021, 23, 185. [Google Scholar] [CrossRef]
- Xu, Y.; Sun, J.; Wan, K.; Yu, L.; Wang, J.; Li, W.; Yang, F.; Sun, J.; Cheng, W.; Mui, D.; et al. Multiparametric cardiovascular magnetic resonance characteristics and dynamic changes in myocardial and skeletal muscles in idiopathic inflammatory cardiomyopathy. J. Cardiovasc. Magn. Reson. 2020, 22, 22. [Google Scholar] [CrossRef]
- Kim, P.K.; Hong, Y.J.; Im, D.J.; Suh, Y.J.; Park, C.H.; Kim, J.Y.; Chang, S.; Lee, H.J.; Hur, J.; Kim, Y.J.; et al. Myocardial T1 and T2 Mapping: Techniques and Clinical Applications. Korean J. Radiol. 2017, 18, 113–131. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, L.; Cao, J.; Li, X.; Wang, J.; Jing, Z.; Jin, Z.; Wang, Y. The application value of cardiac magnetic resonance quantitative T1 mapping technique for risk stratification in patients with pulmonary arterial hypertension. Zhonghua Yi Xue Za Zhi 2022, 102, 2963–2968. [Google Scholar] [CrossRef] [PubMed]
- Hesselstrand, R.; Scheja, A.; Wuttge, D.M.; Arheden, H.; Ugander, M. Enlarged right-sided dimensions and fibrosis of the right ventricular insertion point on cardiovascular magnetic resonance imaging is seen early in patients with pulmonary arterial hypertension associated with connective tissue disease. Scand. J. Rheumatol. 2011, 40, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Cabanis, P.; Magat, J.; Rodriguez-Padilla, J.; Ramlugun, G.; Yon, M.; Bihan-Poudec, Y.; Pallares-Lupon, N.; Vaillant, F.; Pasdois, P.; Jais, P.; et al. Cardiac structure discontinuities revealed by ex-vivo microstructural characterization. A focus on the basal inferoseptal left ventricle region. J. Cardiovasc. Magn. Reson. 2023, 25, 78. [Google Scholar] [CrossRef]
- Yi, J.E.; Park, J.; Lee, H.J.; Shin, D.G.; Kim, Y.; Kim, M.; Kwon, K.; Pyun, W.B.; Kim, Y.J.; Joung, B. Prognostic implications of late gadolinium enhancement at the right ventricular insertion point in patients with non-ischemic dilated cardiomyopathy: A multicenter retrospective cohort study. PLoS ONE 2018, 13, e0208100. [Google Scholar] [CrossRef] [PubMed]
- Chan, R.H.; Maron, B.J.; Olivotto, I.; Pencina, M.J.; Assenza, G.E.; Haas, T.; Lesser, J.R.; Gruner, C.; Crean, A.M.; Rakowski, H.; et al. Prognostic value of quantitative contrast-enhanced cardiovascular magnetic resonance for the evaluation of sudden death risk in patients with hypertrophic cardiomyopathy. Circulation 2014, 130, 484–495. [Google Scholar] [CrossRef]
- Allwood, R.P.; Papadakis, M.; Androulakis, E. Myocardial Fibrosis in Young and Veteran Athletes: Evidence from a Systematic Review of the Current Literature. J. Clin. Med. 2024, 13, 4536. [Google Scholar] [CrossRef]
- Małek, Ł.A.; Barczuk-Falęcka, M.; Werys, K.; Czajkowska, A.; Mróz, A.; Witek, K.; Burrage, M.; Bakalarski, W.; Nowicki, D.; Roik, D.; et al. Cardiovascular magnetic resonance with parametric mapping in long-term ultra-marathon runners. Eur. J. Radiol. 2019, 117, 89–94. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).