Impact of Point-of-Care Lactate Testing for Sepsis on Bundle Adherence and Clinical Outcomes in the Emergency Department: A Pre–Post Observational Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
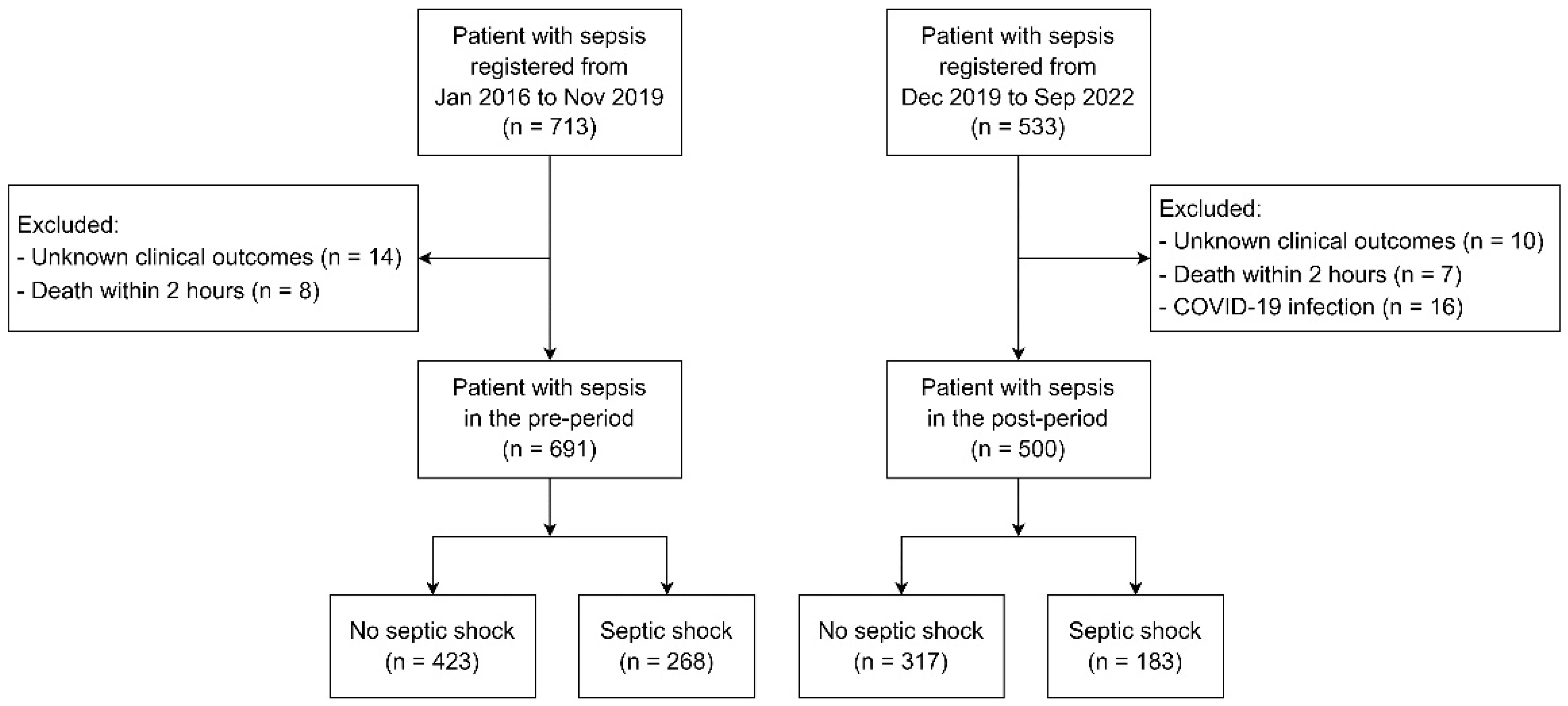
2.2. Population
2.3. Intervention
2.4. Outcomes
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shankar-Hari, M.; Phillips, G.S.; Levy, M.L.; Seymour, C.W.; Liu, V.X.; Deutschman, C.S.; Angus, D.C.; Rubenfeld, G.D.; Singer, M.; Sepsis Definitions Task Force. Developing a New Definition and Assessing New Clinical Criteria for Septic Shock: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 775–787. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, K.; Lee, H.; Ahn, S. Epidemiology of sepsis in Korea: A population-based study of incidence, mortality, cost and risk factors for death in sepsis. Clin. Exp. Emerg. Med. 2019, 6, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef] [PubMed]
- La Via, L.; Sangiorgio, G.; Stefani, S.; Marino, A.; Nunnari, G.; Cocuzza, S.; La Mantia, I.; Cacopardo, B.; Stracquadanio, S.; Spampinato, S.; et al. The Global Burden of Sepsis and Septic Shock. Epidemiologia 2024, 5, 456–478. [Google Scholar] [CrossRef]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; McIntyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021, 47, 1181–1247. [Google Scholar] [CrossRef]
- Borthwick, H.A.; Brunt, L.K.; Mitchem, K.L.; Chaloner, C. Does lactate measurement performed on admission predict clinical outcome on the intensive care unit? A concise systematic review. Ann. Clin. Biochem. 2012, 49, 391–394. [Google Scholar] [CrossRef]
- Shapiro, N.I.; Howell, M.D.; Talmor, D.; Nathanson, L.A.; Lisbon, A.; Wolfe, R.E.; Weiss, J.W. Serum lactate as a predictor of mortality in emergency department patients with infection. Ann. Emerg. Med. 2005, 45, 524–528. [Google Scholar] [CrossRef]
- Karon, B.S.; Tolan, N.V.; Wockenfus, A.M.; Block, D.R.; Baumann, N.A.; Bryant, S.C.; Clements, C.M. Evaluation of lactate, white blood cell count, neutrophil count, procalcitonin and immature granulocyte count as biomarkers for sepsis in emergency department patients. Clin. Biochem. 2017, 50, 956–958. [Google Scholar] [CrossRef]
- Baez, A.A.; Cochon, L. Acute Care Diagnostics Collaboration: Assessment of a Bayesian clinical decision model integrating the Prehospital Sepsis Score and point-of-care lactate. Am. J. Emerg. Med. 2016, 34, 193–196. [Google Scholar] [CrossRef]
- Martín-Rodríguez, F.; López-Izquierdo, R.; Medina-Lozano, E.; Ortega Rabbione, G.; Del Pozo Vegas, C.; Carbajosa Rodríguez, V.; Castro Villamor, M.; Sánchez-Soberon, I.; Sanz-García, A. Accuracy of prehospital point-of-care lactate in early in-hospital mortality. Eur. J. Clin. Investig. 2020, 50, e13341. [Google Scholar] [CrossRef]
- Guerra, W.F.; Mayfield, T.R.; Meyers, M.S.; Clouatre, A.E.; Riccio, J.C. Early detection and treatment of patients with severe sepsis by prehospital personnel. J. Emerg. Med. 2013, 44, 1116–1125. [Google Scholar] [CrossRef] [PubMed]
- Charoentanyarak, S.; Sawunyavisuth, B.; Deepai, S.; Sawanyawisuth, K. A Point-of-Care Serum Lactate Level and Mortality in Adult Sepsis Patients: A Community Hospital Setting. J. Prim. Care Community Health 2021, 12, 21501327211000233. [Google Scholar] [CrossRef] [PubMed]
- Mullen, M.; Cerri, G.; Murray, R.; Talbot, A.; Sanseverino, A.; McCahill, P.; Mangolds, V.; Volturo, J.; Darling, C.; Restuccia, M. Use of point-of-care lactate in the prehospital aeromedical environment. Prehosp. Disaster Med. 2014, 29, 200–203. [Google Scholar] [CrossRef] [PubMed]
- Ismail, F.; Mackay, W.G.; Kerry, A.; Staines, H.; Rooney, K.D. The accuracy and timeliness of a Point of Care lactate measurement in patients with Sepsis. Scand. J. Trauma Resusc. Emerg. Med. 2015, 23, 68. [Google Scholar] [CrossRef] [PubMed]
- Singer, A.J.; Taylor, M.; Domingo, A.; Ghazipura, S.; Khorasonchi, A.; Thode, H.C., Jr.; Shapiro, N.I. Diagnostic characteristics of a clinical screening tool in combination with measuring bedside lactate level in emergency department patients with suspected sepsis. Acad. Emerg. Med. 2014, 21, 853–857. [Google Scholar] [CrossRef]
- Contenti, J.; Corraze, H.; Lemoël, F.; Levraut, J. Effectiveness of arterial, venous, and capillary blood lactate as a sepsis triage tool in ED patients. Am. J. Emerg. Med. 2015, 33, 167–172. [Google Scholar] [CrossRef]
- Goyal, M.; Pines, J.M.; Drumheller, B.C.; Gaieski, D.F. Point-of-care testing at triage decreases time to lactate level in septic patients. J. Emerg. Med. 2010, 38, 578–581. [Google Scholar] [CrossRef]
- Shapiro, N.I.; Fisher, C.; Donnino, M.; Cataldo, L.; Tang, A.; Trzeciak, S.; Horowitz, G.; Wolfe, R.E. The feasibility and accuracy of point-of-care lactate measurement in emergency department patients with suspected infection. J. Emerg. Med. 2010, 39, 89–94. [Google Scholar] [CrossRef]
- Gaieski, D.F.; Drumheller, B.C.; Goyal, M.; Fuchs, B.D.; Shofer, F.S.; Zogby, K. Accuracy of Handheld Point-of-Care Fingertip Lactate Measurement in the Emergency Department. West. J. Emerg. Med. 2013, 14, 58–62. [Google Scholar] [CrossRef]
- Baig, M.A.; Shahzad, H.; Hussain, E.; Mian, A. Validating a point of care lactate meter in adult patients with sepsis presenting to the emergency department of a tertiary care hospital of a low- to middle-income country. World J. Emerg. Med. 2017, 8, 184–189. [Google Scholar] [CrossRef]
- Song, J.; Cho, H.; Park, D.W.; Ahn, S.; Kim, J.Y.; Seok, H.; Park, J.; Moon, S. The Effect of the Intelligent Sepsis Management System on Outcomes among Patients with Sepsis and Septic Shock Diagnosed According to the Sepsis-3 Definition in the Emergency Department. J. Clin. Med. 2019, 8, 1800. [Google Scholar] [CrossRef] [PubMed]
- Singer, A.J.; Taylor, M.; LeBlanc, D.; Williams, J.; Thode, H.C., Jr. ED bedside point-of-care lactate in patients with suspected sepsis is associated with reduced time to iv fluids and mortality. Am. J. Emerg. Med. 2014, 32, 1120–1124. [Google Scholar] [CrossRef] [PubMed]
- De Backer, D.; Cecconi, M.; Chew, M.S.; Hajjar, L.; Monnet, X.; Ospina-Tascón, G.A.; Ostermann, M.; Pinsky, M.R.; Vincent, J.-L. A plea for personalization of the hemodynamic management of septic shock. Crit. Care 2022, 26, 372. [Google Scholar] [CrossRef] [PubMed]
- La Via, L.; Vasile, F.; Perna, F.; Zawadka, M. Prediction of fluid responsiveness in critical care: Current evidence and future perspective. Trends Anaesth. Crit. Care 2024, 54, 101316. [Google Scholar] [CrossRef]
- Park, H.; Shin, T.G.; Kim, W.Y.; Jo, Y.H.; Hwang, Y.J.; Choi, S.H.; Lim, T.H.; Han, K.S.; Shin, J.; Suh, G.J.; et al. A quick Sequential Organ Failure Assessment-negative result at triage is associated with low compliance with sepsis bundles: A retrospective analysis of a multicenter prospective registry. Clin. Exp. Emerg. Med. 2022, 9, 84–92. [Google Scholar] [CrossRef]
- Ward, M.J.; Self, W.H.; Singer, A.; Lazar, D.; Pines, J.M. Cost-effectiveness analysis of early point-of-care lactate testing in the emergency department. J. Crit. Care 2016, 36, 69–75. [Google Scholar] [CrossRef]
- The National Heart, Lung, and Blood Institute Prevention and Early Treatment of Acute Lung Injury Clinical Trials Network; Shapiro, N.I.; Douglas, I.S.; Brower, R.G.; Brown, S.M.; Exline, M.C.; Ginde, A.A.; Gong, M.N.; Grissom, C.K. Early Restrictive or Liberal Fluid Management for Sepsis-Induced Hypotension. N. Engl. J. Med. 2023, 388, 499–510. [Google Scholar] [CrossRef]
- Meyhoff, T.S.; Hjortrup, P.B.; Wetterslev, J.; Sivapalan, P.; Laake, J.H.; Cronhjort, M.; Jakob, S.M.; Cecconi, M.; Nalos, M.; Ostermann, M.; et al. Restriction of Intravenous Fluid in ICU Patients with Septic Shock. N. Engl. J. Med. 2022, 386, 2459–2470. [Google Scholar] [CrossRef]
- Hernández, G.; Ospina-Tascón, G.A.; Damiani, L.P.; Estenssoro, E.; Dubin, A.; Hurtado, J.; Friedman, G.; Castro, R.; Alegría, L.; Teboul, J.-L.; et al. Effect of a Resuscitation Strategy Targeting Peripheral Perfusion Status vs Serum Lactate Levels on 28-Day Mortality Among Patients with Septic Shock: The ANDROMEDA-SHOCK Randomized Clinical Trial. JAMA 2019, 321, 654–664. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, S.G.; Ahn, S.; Kim, J.Y.; Song, J.; Moon, S.; Cho, H. Strategies to prevent COVID-19 transmission in the emergency department of a regional base hospital in Korea: From index patient until pandemic declaration. Am. J. Emerg. Med. 2021, 46, 247–253. [Google Scholar] [CrossRef]
- Chun, S.Y.; Kim, H.J.; Kim, H.B. The effect of COVID-19 pandemic on the length of stay and outcomes in the emergency department. Clin. Exp. Emerg. Med. 2022, 9, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Lee, J.; Cho, Y.; Oh, J.; Kang, H.; Lim, T.H.; Ko, B.S. The impact of the COVID-19 pandemic on in-hospital mortality in patients admitted through the emergency department. Clin. Exp. Emerg. Med. 2023, 10, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Morris, E.; McCartney, D.; Lasserson, D.; Van den Bruel, A.; Fisher, R.; Hayward, G. Point-of-care lactate testing for sepsis at presentation to health care: A systematic review of patient outcomes. Br. J. Gen. Pract. 2017, 67, e859–e870. [Google Scholar] [CrossRef] [PubMed]

| Variables | Pre-Period Group (n = 691) | Post-Period Group (n = 500) | p Value |
|---|---|---|---|
| Sex (male), n (%) | 400 (57.9%) | 287 (57.4%) | 0.905 |
| Age, median (IQR) | 75 (64, 81) | 78 (69, 84) | <0.001 |
| Underlying disease, n (%) | |||
| Diabetes mellitus | 269 (38.9%) | 212 (42.4%) | 0.308 |
| Hypertension | 367 (53.1%) | 275 (55.0%) | 0.679 |
| Chronic liver diseases | 38 (5.5%) | 27 (5.4%) | 0.905 |
| Chronic kidney disease | 91 (13.2%) | 61 (12.2%) | 0.571 |
| Chronic respiratory disease | 159 (23.0%) | 78 (15.6%) | 0.001 |
| Cardiovascular disease | 125 (18.3%) | 92 (18.4%) | 0.962 |
| Malignancy | 122 (17.7%) | 127 (25.5%) | 0.002 |
| Source of infection, n (%) | |||
| Respiratory | 456 (66.0%) | 316 (63.2%) | 0.389 |
| Genitourinary | 268 (38.8%) | 141 (28.2%) | <0.001 |
| Gastrointestinal | 73 (10.6%) | 34 (6.8%) | 0.026 |
| Other | 118 (17.1%) | 70 (14.0%) | 0.151 |
| Septic shock, n (%) | 268 (38.8%) | 183 (36.6%) | 0.468 |
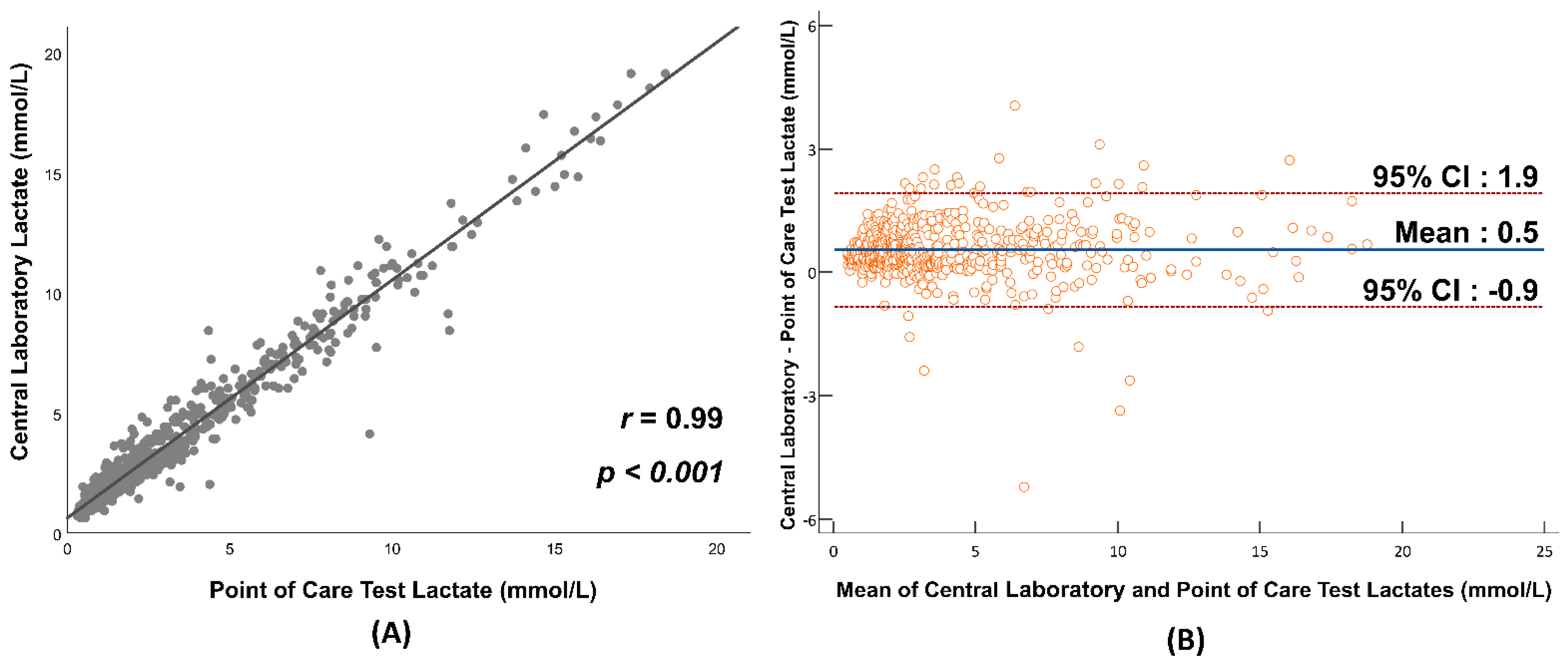
| Initial serum lactate measured with central lab (mmol/L), median (IQR) | 2.9 (1.7, 5.4) | 3.1 (2.0, 6.0) | 0.073 |
| Time to lactate result (min), median (IQR) | |||
| Time from arrival to first lactate result | 53 (42, 72) | 33 (21, 50) | <0.001 |
| Time from arrival to lactate result with POCT | 33 (21, 50) | ||
| Time from arrival to lactate result with central lab | 53 (42, 72) | 67 (49, 89) | <0.001 |
| Severity score, median (IQR) | |||
| APACHE II score | 18 (14, 22) | 19 (14, 24) | 0.128 |
| SOFA score | 8 (6, 11) | 9 (6, 11) | 0.499 |
| Surviving sepsis campaign guidelines adherence | |||
| Overall bundle adherence, n (%) | 328 (47.5%) | 225 (45.0%) | 0.410 |
| Appropriate fluid resuscitation in 3 h, n (%) | 496 (71.8%) | 350 (70.0%) | 0.568 |
| Blood culture before antibiotics administration, n (%) | 678 (98.1%) | 491 (98.2%) | 0.735 |
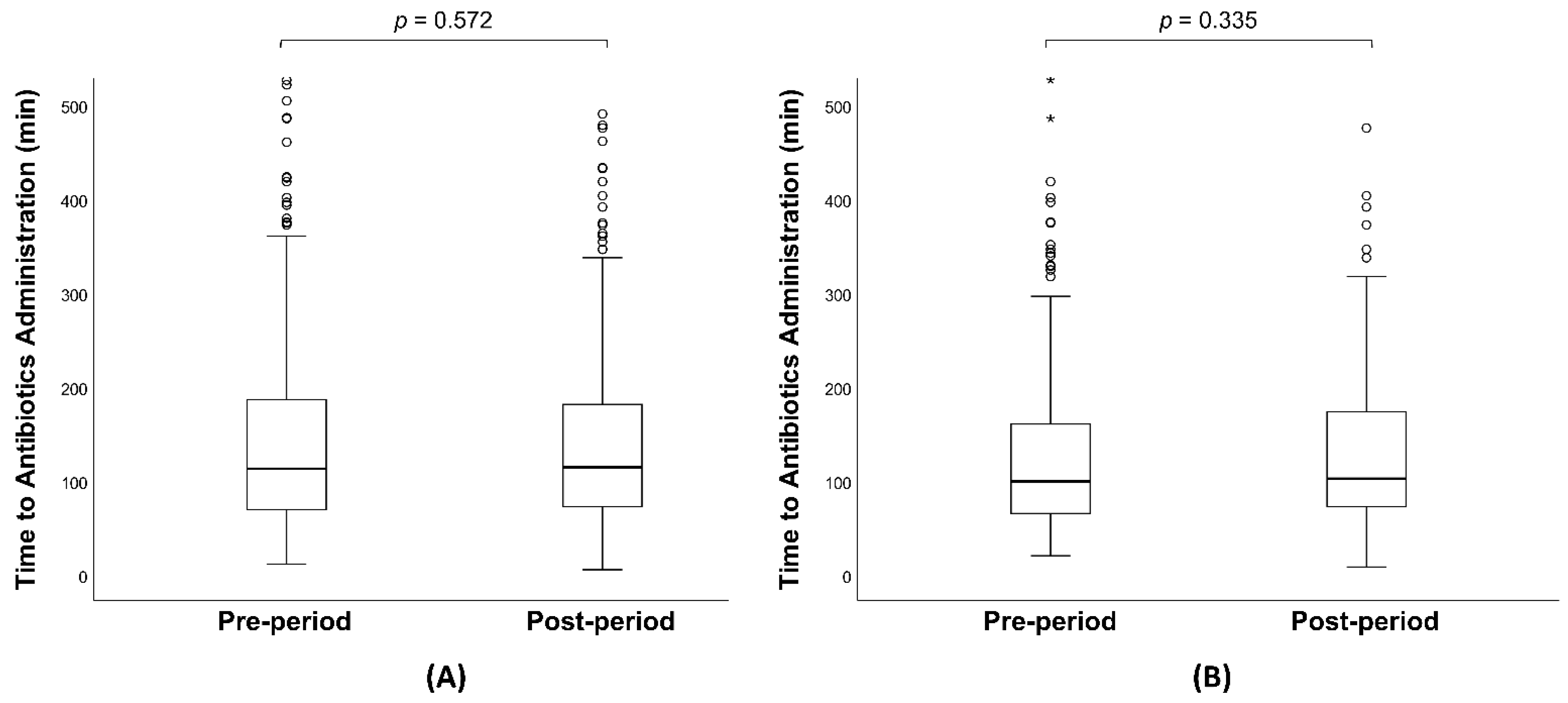
| Antibiotics administration in 3 h, n (%) | 543 (78.6%) | 386 (77.2%) | 0.572 |
| Subsequent lactate measurement (if indicated), n (%) | 567 (82.1%) | 441 (88.2%) | 0.004 |
| Sepsis management | |||
| Time from arrival to antibiotics (min), median (IQR) | 111 (69, 183) | 114 (72, 178) | 0.752 |
| Time from arrival to vasopressor administration (min), median (IQR) | 114 (59, 221) | 129 (68, 257) | 0.051 |
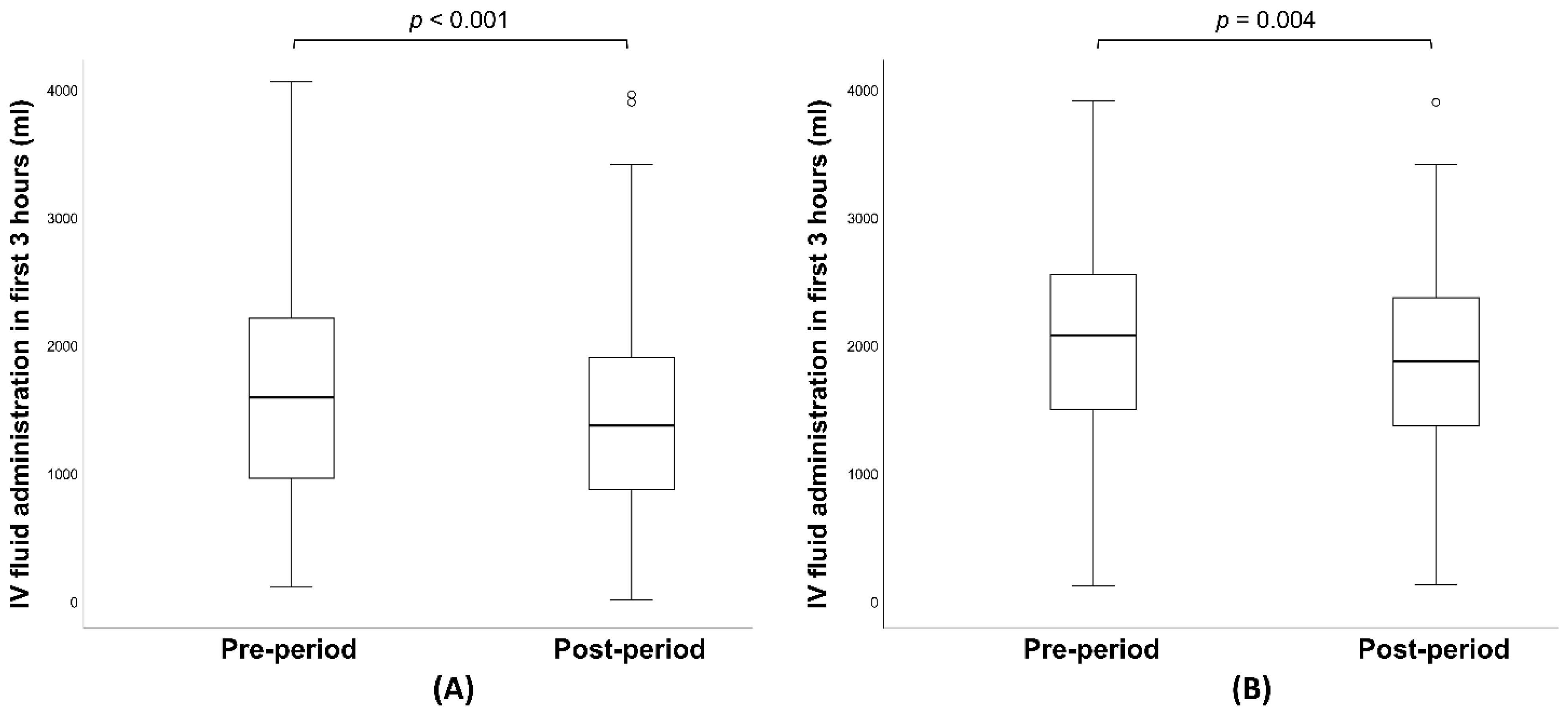
| IV fluid in first 3 h (ml), median (IQR) | 1560 (900, 2162) | 1360 (860, 1899) | <0.001 |
| All-cause mortality, n (%) | |||
| 7-day mortality | 136 (19.7%) | 97 (19.4%) | 0.493 |
| 14-day mortality | 173 (25.0%) | 133 (26.6%) | 0.425 |
| 30-day mortality | 215 (31.1%) | 157 (31.4%) | 0.950 |
| Variables | Pre-Period Group (n = 268) | Post-Period Group (n = 183) | p Value |
|---|---|---|---|
| Sex (male), n (%) | 97 (36.2%) | 100 (54.6%) | 0.051 |
| Age, median (IQR) | 75 (61, 82) | 79 (69, 84) | 0.002 |
| Underlying disease, n (%) | |||
| Diabetes mellitus | 117 (44.2%) | 75 (41.0%) | 0.505 |
| Hypertension | 137 (51.7%) | 99 (54.1%) | 0.617 |
| Chronic liver diseases | 23 (8.7%) | 10 (5.5%) | 0.269 |
| Chronic kidney disease | 33 (12.5%) | 25 (13.7%) | 0.775 |
| Chronic respiratory disease | 47 (17.7%) | 17 (9.3%) | 0.013 |
| Cardiovascular disease | 46 (17.4%) | 38 (20.8%) | 0.390 |
| Malignancy | 49 (18.5%) | 47 (25.7%) | 0.079 |
| Source of infection, n (%) | |||
| Respiratory | 164 (61.2%) | 106 (57.9%) | 0.139 |
| Genitourinary | 108 (40.3%) | 61 (33.3%) | 0.495 |
| Gastrointestinal | 42 (15.7%) | 19 (10.4%) | 0.123 |
| Other | 45 (16.8%) | 27 (14.8%) | 0.602 |
| Initial serum lactate measured with central lab (mmol/L), median (IQR) | 5.3 (3.2, 8.4) | 6.0 (3.1, 9.4) | 0.472 |
| Time to lactate result (min), median (IQR) | |||
| Time from arrival to first lactate result | 50 (39, 65) | 31 (21, 44) | <0.001 |
| Time from arrival to lactate result with POCT | 31 (21, 44) | ||
| Time from arrival to lactate result with central lab | 50 (39, 65) | 64 (48, 80) | <0.001 |
| Severity score, median (IQR) | |||
| APACHE II score | 21 (17, 24) | 21 (17, 26) | 0.660 |
| SOFA score | 11 (9, 13) | 12 (10, 13) | 0.092 |
| Survival sepsis campaign guidelines adherence | |||
| Overall bundle adherence, n (%) | 120 (44.8%) | 78 (42.6%) | 0.651 |
| Appropriate fluid resuscitation in 3 h, n (%) | 181 (67.5%) | 118 (64.5%) | 0.500 |
| Blood culture before antibiotics administration, n (%) | 263 (98.1%) | 179 (97.8%) | 0.692 |
| Antibiotics administration in 3 h, n (%) | 222 (82.8%) | 145 (79.2%) | 0.335 |
| Subsequent lactate measurement (if indicated), n (%) | 199 (76.5%) | 150 (82.0%) | 0.225 |
| Sepsis management | |||
| Time from arrival to antibiotics (min), median (IQR) | 99 (65, 159) | 106 (72, 173) | 0.619 |
| Time from arrival to vasopressor administration (min), median (IQR) | 113 (59, 220) | 112 (57, 223) | 0.686 |
| IV fluid in first 3 h (ml), median (IQR) | 2045 (1492, 2507) | 1860 (1360, 2360) | 0.004 |
| All-cause mortality, n (%) | |||
| 7-day mortality | 90 (33.6%) | 59 (32.2%) | 0.839 |
| 14-day mortality | 108 (40.3%) | 74 (40.4%) | 1.000 |
| 30-day mortality | 132 (49.3%) | 85 (46.4%) | 0.566 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Song, J.; Lee, S.; Kim, S.J.; Han, K.S.; Lee, S. Impact of Point-of-Care Lactate Testing for Sepsis on Bundle Adherence and Clinical Outcomes in the Emergency Department: A Pre–Post Observational Study. J. Clin. Med. 2024, 13, 5389. https://doi.org/10.3390/jcm13185389
Lee S, Song J, Lee S, Kim SJ, Han KS, Lee S. Impact of Point-of-Care Lactate Testing for Sepsis on Bundle Adherence and Clinical Outcomes in the Emergency Department: A Pre–Post Observational Study. Journal of Clinical Medicine. 2024; 13(18):5389. https://doi.org/10.3390/jcm13185389
Chicago/Turabian StyleLee, Sukyo, Juhyun Song, Sungwoo Lee, Su Jin Kim, Kap Su Han, and Sijin Lee. 2024. "Impact of Point-of-Care Lactate Testing for Sepsis on Bundle Adherence and Clinical Outcomes in the Emergency Department: A Pre–Post Observational Study" Journal of Clinical Medicine 13, no. 18: 5389. https://doi.org/10.3390/jcm13185389






