Hypothermia for Cardioprotection in Acute Coronary Syndrome Patients: From Bench to Bedside
Abstract
:1. Introduction
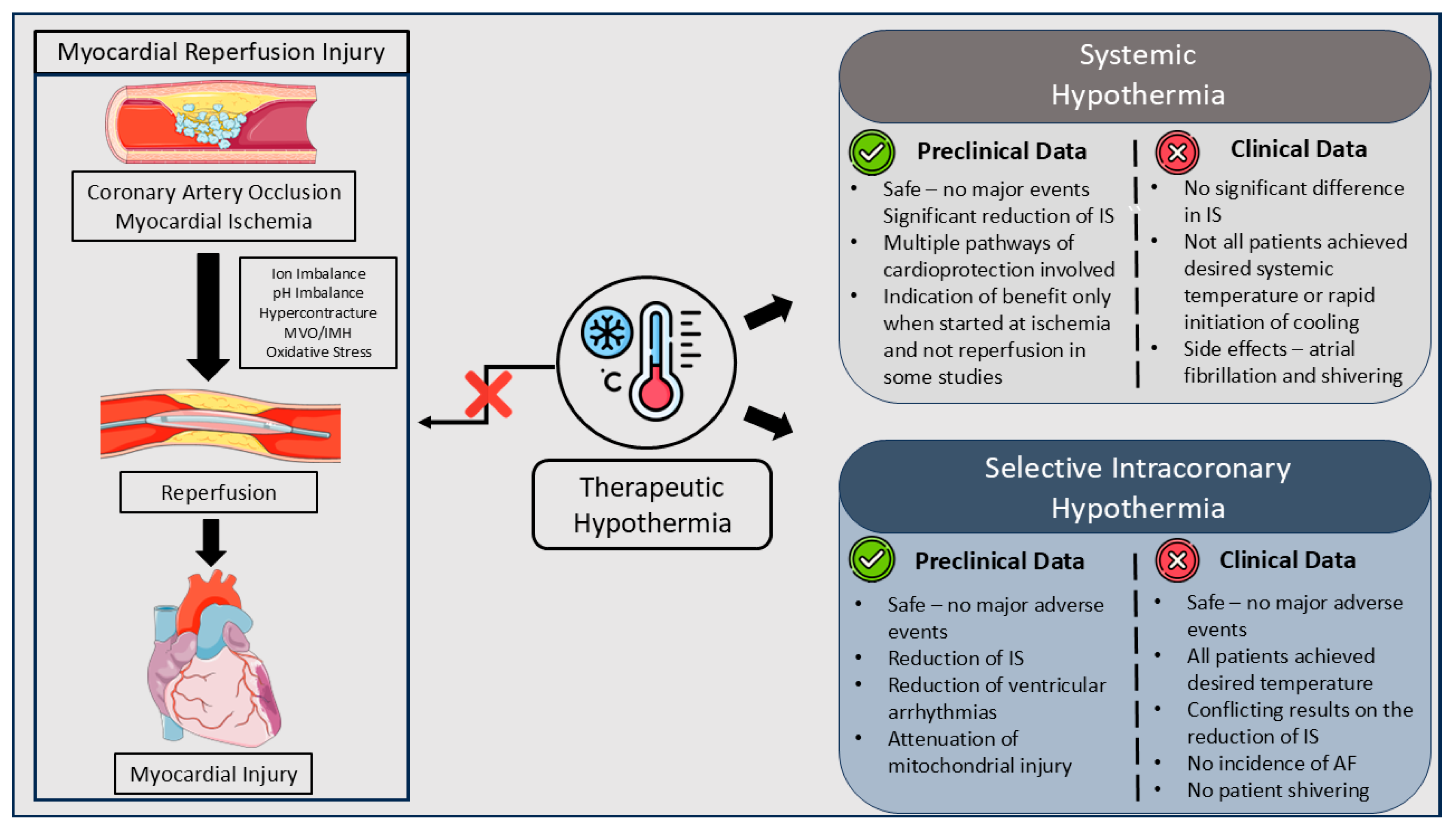
2. Pathophysiology of Myocardial Reperfusion Injury
2.1. Key Pathophysiological Concepts
2.2. Myocardial Reperfusion Injury Types
3. The Role of Systemic Hypothermia
4. Selective Intracoronary Hypothermia
5. Future Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ralapanawa, U.; Sivakanesan, R. Epidemiology and the Magnitude of Coronary Artery Disease and Acute Coronary Syndrome: A Narrative Review. J. Epidemiol. Glob. Health 2021, 11, 169. [Google Scholar] [CrossRef] [PubMed]
- Ong, K.L.; Stafford, L.K.; McLaughlin, S.A.; Boyko, E.J.; Vollset, S.E.; Smith, A.E.; Dalton, B.E.; Duprey, J.; Cruz, J.A.; Hagins, H.; et al. Global, Regional, and National Burden of Diabetes from 1990 to 2021, with Projections of Prevalence to 2050: A Systematic Analysis for the Global Burden of Disease Study 2021. Lancet 2023, 402, 203–234. [Google Scholar] [CrossRef] [PubMed]
- Timmis, A.; Kazakiewicz, D.; Townsend, N.; Huculeci, R.; Aboyans, V.; Vardas, P. Global Epidemiology of Acute Coronary Syndromes. Nat. Rev. Cardiol. 2023, 20, 778–788. [Google Scholar] [CrossRef] [PubMed]
- Khera, S.; Kolte, D.; Aronow, W.S.; Palaniswamy, C.; Subramanian, K.S.; Hashim, T.; Mujib, M.; Jain, D.; Paudel, R.; Ahmed, A.; et al. Non-ST-Elevation Myocardial Infarction in the United States: Contemporary Trends in Incidence, Utilization of the Early Invasive Strategy, and In-Hospital Outcomes. J. Am. Heart Assoc. 2014, 3, e000995. [Google Scholar] [CrossRef] [PubMed]
- André, R.; Bongard, V.; Elosua, R.; Kirchberger, I.; Farmakis, D.; Häkkinen, U.; Fusco, D.; Torre, M.; Garel, P.; Araújo, C.; et al. International Differences in Acute Coronary Syndrome Patients’ Baseline Characteristics, Clinical Management and Outcomes in Western Europe: The EURHOBOP Study. Heart 2014, 100, 1201–1207. [Google Scholar] [CrossRef]
- Kanakakis, I.; Stafylas, P.; Tsigkas, G.; Nikas, D.; Synetos, A.; Avramidis, D.; Tsiafoutis, I.; Dagre, A.; Tzikas, S.; Latsios, G.; et al. Epidemiology, Reperfusion Management, and Outcomes of Patients with Myocardial Infarction in Greece: The ILIAKTIS Study. Hell. J. Cardiol. 2022, 67, 1–8. [Google Scholar] [CrossRef]
- Dégano, I.R.; Elosua, R.; Marrugat, J. Epidemiology of Acute Coronary Syndromes in Spain: Estimation of the Number of Cases and Trends From 2005 to 2049. Rev. Española Cardiol. (Engl. Ed.) 2013, 66, 472–481. [Google Scholar] [CrossRef]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.-A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the Management of Acute Coronary Syndromes. Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef]
- Lawton, J.S.; Tamis-Holland, J.E.; Bangalore, S.; Bates, E.R.; Beckie, T.M.; Bischoff, J.M.; Bittl, J.A.; Cohen, M.G.; DiMaio, J.M.; Don, C.W.; et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e4–e17. [Google Scholar] [CrossRef]
- Stone, G.W.; Selker, H.P.; Thiele, H.; Patel, M.R.; Udelson, J.E.; Ohman, E.M.; Maehara, A.; Eitel, I.; Granger, C.B.; Jenkins, P.L.; et al. Relationship Between Infarct Size and Outcomes Following Primary PCI. J. Am. Coll. Cardiol. 2016, 67, 1674–1683. [Google Scholar] [CrossRef]
- Selker, H.P.; Udelson, J.E.; Ruthazer, R.; D’Agostino, R.B.; Nichols, M.; Ben-Yehuda, O.; Eitel, I.; Granger, C.B.; Jenkins, P.; Maehara, A.; et al. Relationship between Therapeutic Effects on Infarct Size in Acute Myocardial Infarction and Therapeutic Effects on 1-Year Outcomes: A Patient-Level Analysis of Randomized Clinical Trials. Am. Heart J. 2017, 188, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Jennings, R.B.; Sommers, H.M.; Smyth, G.A.; Flack, H.A.; Linn, H. Myocardial Necrosis Induced by Temporary Occlusion of a Coronary Artery in the Dog. Arch. Pathol. (Chic.) 1960, 70, 68–78. [Google Scholar]
- Yellon, D.M.; Hausenloy, D.J. Myocardial Reperfusion Injury. N. Engl. J. Med. 2007, 357, 1121–1135. [Google Scholar] [CrossRef] [PubMed]
- ZWEIER, J.; TALUKDER, M. The Role of Oxidants and Free Radicals in Reperfusion Injury. Cardiovasc. Res. 2006, 70, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Allen, D. Role of the Cardiac Na+/H+ Exchanger during Ischemia and Reperfusion. Cardiovasc. Res. 2003, 57, 934–941. [Google Scholar] [CrossRef]
- Lemasters, J.J.; Bond, J.M.; Chacon, E.; Harper, I.S.; Kaplan, S.H.; Ohata, H.; Trollinger, D.R.; Herman, B.; Cascio, W.E. The PH Paradox in Ischemia-Reperfusion Injury to Cardiac Myocytes. In Myocardial Ischemia: Mechanisms, Reperfusion, Protection; Birkhäuser Basel: Basel, Switzerland, 1996; pp. 99–114. [Google Scholar]
- Ruiz-Meana, M.; García-Dorado, D. Pathophysiology of Ischemia-Reperfusion Injury: New Therapeutic Options for Acute Myocardial Infarction. Rev. Española Cardiol. (Engl. Ed.) 2009, 62, 199–209. [Google Scholar] [CrossRef]
- Barrabés, J.A.; Garcia-Dorado, D.; Ruiz-Meana, M.; Piper, H.M.; Solares, J.; González, M.A.; Oliveras, J.; Herrejón, M.P.; Soler Soler, J. Myocardial Segment Shrinkage during Coronary Reperfusion in Situ. Pflugers Arch. 1996, 431, 519–526. [Google Scholar] [CrossRef]
- Ganote, C. Contraction Band Necrosis and Irreversible Myocardial Injury. J. Mol. Cell Cardiol. 1983, 15, 67–73. [Google Scholar] [CrossRef]
- Abdallah, Y.; Gkatzoflia, A.; Gligorievski, D.; Kasseckert, S.; Euler, G.; Schluter, K.; Schafer, M.; Piper, H.; Schafer, C. Insulin Protects Cardiomyocytes against Reoxygenation-Induced Hypercontracture by a Survival Pathway Targeting SR Ca2+ Storage. Cardiovasc. Res. 2006, 70, 346–353. [Google Scholar] [CrossRef]
- Siegmund, B.; Schlack, W.; Ladilov, Y.V.; Balser, C.; Piper, H.M. Halothane Protects Cardiomyocytes Against Reoxygenation-Induced Hypercontracture. Circulation 1997, 96, 4372–4379. [Google Scholar] [CrossRef]
- Griffiths, E.J.; Halestrap, A.P. Mitochondrial Non-Specific Pores Remain Closed during Cardiac Ischaemia, but Open upon Reperfusion. Biochem. J. 1995, 307, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Halestrap, A. Mitochondrial Permeability Transition Pore Opening during Myocardial Reperfusion—A Target for Cardioprotection. Cardiovasc. Res. 2004, 61, 372–385. [Google Scholar] [CrossRef] [PubMed]
- Hausenloy, D. Inhibiting Mitochondrial Permeability Transition Pore Opening: A New Paradigm for Myocardial Preconditioning? Cardiovasc. Res. 2002, 55, 534–543. [Google Scholar] [CrossRef] [PubMed]
- Behera, R.; Sharma, V.; Grewal, A.K.; Kumar, A.; Arora, B.; Najda, A.; Albadrani, G.M.; Altyar, A.E.; Abdel-Daim, M.M.; Singh, T.G. Mechanistic Correlation between Mitochondrial Permeability Transition Pores and Mitochondrial ATP Dependent Potassium Channels in Ischemia Reperfusion. Biomed. Pharmacother. 2023, 162, 114599. [Google Scholar] [CrossRef]
- Inserte, J.; Hernando, V.; Garcia-Dorado, D. Contribution of Calpains to Myocardial Ischaemia/Reperfusion Injury. Cardiovasc. Res. 2012, 96, 23–31. [Google Scholar] [CrossRef]
- Zhang, H.; Hu, H.; Zhai, C.; Jing, L.; Tian, H. Cardioprotective Strategies after Ischemia–Reperfusion Injury. Am. J. Cardiovasc. Drugs 2024, 24, 5–18. [Google Scholar] [CrossRef]
- Algoet, M.; Janssens, S.; Himmelreich, U.; Gsell, W.; Pusovnik, M.; Van den Eynde, J.; Oosterlinck, W. Myocardial Ischemia-Reperfusion Injury and the Influence of Inflammation. Trends Cardiovasc. Med. 2023, 33, 357–366. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. Inflammation in Cardiac Injury, Repair and Regeneration. Curr. Opin. Cardiol. 2015, 30, 240–245. [Google Scholar] [CrossRef]
- Veltman, D.; Wu, M.; Pokreisz, P.; Claus, P.; Gillijns, H.; Caluwé, E.; Vanhaverbeke, M.; Gsell, W.; Himmelreich, U.; Sinnaeve, P.R.; et al. Clec4e-Receptor Signaling in Myocardial Repair after Ischemia-Reperfusion Injury. JACC Basic. Transl. Sci. 2021, 6, 631–646. [Google Scholar] [CrossRef]
- Boovarahan, S.R.; Kurian, G.A. Investigating the Role of DNMT1 Gene Expression on Myocardial Ischemia Reperfusion Injury in Rat and Associated Changes in Mitochondria. Biochim. Biophys. Acta (BBA)—Bioenerg. 2022, 1863, 148566. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, T.; Yuan, H.; Sun, H.; Liu, S.; Zhang, S.; Liu, L.; Jiang, S.; Tang, Y.; Liu, Z. Bioinformatics Integration Reveals Key Genes Associated with Mitophagy in Myocardial Ischemia-Reperfusion Injury. BMC Cardiovasc. Disord. 2024, 24, 183. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Song, Q.; E, S.; Guan, X.; Zhang, Z.; Juan, Z.; Sun, X.; Liang, Y. RNA-Seq Based Transcriptomic Map Reveals Multiple Pathways of Necroptosis in Treating Myocardial Ischemia Reperfusion Injury. Gene 2024, 906, 148217. [Google Scholar] [CrossRef] [PubMed]
- Braunwald, E.; Kloner, R.A. The Stunned Myocardium: Prolonged, Postischemic Ventricular Dysfunction. Circulation 1982, 66, 1146–1149. [Google Scholar] [CrossRef] [PubMed]
- Solomon, S.D.; Glynn, R.J.; Greaves, S.; Ajani, U.; Rouleau, J.-L.; Menapace, F.; Arnold, J.M.O.; Hennekens, C.; Pfeffer, M.A. Recovery of Ventricular Function after Myocardial Infarction in the Reperfusion Era: The Healing and Early Afterload Reducing Therapy Study. Ann. Intern. Med. 2001, 134, 451. [Google Scholar] [CrossRef] [PubMed]
- Heyndrickx, G.R.; Millard, R.W.; McRitchie, R.J.; Maroko, P.R.; Vatner, S.F. Regional Myocardial Functional and Electrophysiological Alterations after Brief Coronary Artery Occlusion in Conscious Dogs. J. Clin. Investig. 1975, 56, 978–985. [Google Scholar] [CrossRef]
- Charlat, M.L.; O’Neill, P.G.; Hartley, C.J.; Roberts, R.; Bolli, R. Prolonged Abnormalities of Left Ventricular Diastolic Wall Thinning in the “Stunned” Myocardium in Conscious Dogs: Time Course and Relation to Systolic Function. J. Am. Coll. Cardiol. 1989, 13, 185–194. [Google Scholar] [CrossRef]
- DeBoer, L.W.V.; Ingwall, J.S.; Kloner, R.A.; Braunwald, E. Prolonged Derangements of Canine Myocardial Purine Metabolism after a Brief Coronary Artery Occlusion Not Associated with Anatomic Evidence of Necrosis. Proc. Natl. Acad. Sci. USA 1980, 77, 5471–5475. [Google Scholar] [CrossRef]
- McCormick, L.M.; Hoole, S.P.; White, P.A.; Read, P.A.; Axell, R.G.; Clarke, S.J.; O’Sullivan, M.; West, N.E.J.; Dutka, D.P. Pre-Treatment with Glucagon-Like Peptide-1 Protects Against Ischemic Left Ventricular Dysfunction and Stunning without a Detected Difference in Myocardial Substrate Utilization. JACC Cardiovasc. Interv. 2015, 8, 292–301. [Google Scholar] [CrossRef]
- Wdowiak-Okrojek, K.; Wejner-Mik, P.; Kasprzak, J.D.; Lipiec, P. Recovery of Regional Systolic and Diastolic Myocardial Function after Acute Myocardial Infarction Evaluated by Two-dimensional Speckle Tracking Echocardiography. Clin. Physiol. Funct. Imaging 2019, 39, 177–181. [Google Scholar] [CrossRef]
- van der Weg, K.; Prinzen, F.W.; Gorgels, A.P. Editor’s Choice-Reperfusion Cardiac Arrhythmias and Their Relation to Reperfusion-Induced Cell Death. Eur. Heart J. Acute Cardiovasc. Care 2019, 8, 142–152. [Google Scholar] [CrossRef]
- del Monte, F.; Lebeche, D.; Guerrero, J.L.; Tsuji, T.; Doye, A.A.; Gwathmey, J.K.; Hajjar, R.J. Abrogation of Ventricular Arrhythmias in a Model of Ischemia and Reperfusion by Targeting Myocardial Calcium Cycling. Proc. Natl. Acad. Sci. USA 2004, 101, 5622–5627. [Google Scholar] [CrossRef] [PubMed]
- Akar, J.G.; Akar, F.G. Regulation of Ion Channels and Arrhythmias in the Ischemic Heart. J. Electrocardiol. 2007, 40, S37–S41. [Google Scholar] [CrossRef]
- Majidi, M.; Kosinski, A.S.; Al-Khatib, S.M.; Lemmert, M.E.; Smolders, L.; van Weert, A.; Reiber, J.H.C.; Tzivoni, D.; Bar, F.W.H.M.; Wellens, H.J.J.; et al. Reperfusion Ventricular Arrhythmia “bursts” Predict Larger Infarct Size despite TIMI 3 Flow Restoration with Primary Angioplasty for Anterior ST-Elevation Myocardial Infarction. Eur. Heart J. 2008, 30, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Majidi, M.; Kosinski, A.S.; Al-Khatib, S.M.; Smolders, L.; Cristea, E.; Lansky, A.J.; Stone, G.W.; Mehran, R.; Gibbons, R.J.; Crijns, H.J.; et al. Implications of Ventricular Arrhythmia “Bursts” with Normal Epicardial Flow, Myocardial Blush, and ST-Segment Recovery in Anterior ST-Elevation Myocardial Infarction Reperfusion: A Biosignature of Direct Myocellular Injury “Downstream of Downstream”. Eur. Heart J. Acute Cardiovasc. Care 2015, 4, 51–59. [Google Scholar] [CrossRef] [PubMed]
- van der Weg, K.; Majidi, M.; Haeck, J.D.E.; Tijssen, J.G.P.; Green, C.L.; Koch, K.T.; Kuijt, W.J.; Krucoff, M.W.; Gorgels, A.P.M.; de Winter, R.J. Ventricular Arrhythmia Burst Is an Independent Indicator of Larger Infarct Size Even in Optimal Reperfusion in STEMI. J. Electrocardiol. 2016, 49, 345–352. [Google Scholar] [CrossRef] [PubMed]
- van der Weg, K.; Kuijt, W.J.; Bekkers, S.C.; Tijssen, J.G.; Green, C.L.; Lemmert, M.E.; Krucoff, M.W.; Gorgels, A.P. Reperfusion Ventricular Arrhythmia Bursts Identify Larger Infarct Size in Spite of Optimal Epicardial and Microvascular Reperfusion Using Cardiac Magnetic Resonance Imaging. Eur. Heart J. Acute Cardiovasc. Care 2018, 7, 246–256. [Google Scholar] [CrossRef]
- Galli, M.; Niccoli, G.; De Maria, G.; Brugaletta, S.; Montone, R.A.; Vergallo, R.; Benenati, S.; Magnani, G.; D’Amario, D.; Porto, I.; et al. Coronary Microvascular Obstruction and Dysfunction in Patients with Acute Myocardial Infarction. Nat. Rev. Cardiol. 2024, 21, 283–298. [Google Scholar] [CrossRef]
- Durante, A.; Laricchia, A.; Benedetti, G.; Esposito, A.; Margonato, A.; Rimoldi, O.; De Cobelli, F.; Colombo, A.; Camici, P.G. Identification of High-Risk Patients after ST-Segment–Elevation Myocardial Infarction. Circ. Cardiovasc. Imaging 2017, 10, e005841. [Google Scholar] [CrossRef]
- van Kranenburg, M.; Magro, M.; Thiele, H.; de Waha, S.; Eitel, I.; Cochet, A.; Cottin, Y.; Atar, D.; Buser, P.; Wu, E.; et al. Prognostic Value of Microvascular Obstruction and Infarct Size, as Measured by CMR in STEMI Patients. JACC Cardiovasc. Imaging 2014, 7, 930–939. [Google Scholar] [CrossRef]
- Betgem, R.P.; de Waard, G.A.; Nijveldt, R.; Beek, A.M.; Escaned, J.; van Royen, N. Intramyocardial Haemorrhage after Acute Myocardial Infarction. Nat. Rev. Cardiol. 2015, 12, 156–167. [Google Scholar] [CrossRef]
- Konijnenberg, L.S.F.; Damman, P.; Duncker, D.J.; Kloner, R.A.; Nijveldt, R.; van Geuns, R.-J.M.; Berry, C.; Riksen, N.P.; Escaned, J.; van Royen, N. Pathophysiology and Diagnosis of Coronary Microvascular Dysfunction in ST-Elevation Myocardial Infarction. Cardiovasc. Res. 2020, 116, 787–805. [Google Scholar] [CrossRef] [PubMed]
- Rochitte, C.E.; Lima, J.A.C.; Bluemke, D.A.; Reeder, S.B.; McVeigh, E.R.; Furuta, T.; Becker, L.C.; Melin, J.A. Magnitude and Time Course of Microvascular Obstruction and Tissue Injury after Acute Myocardial Infarction. Circulation 1998, 98, 1006–1014. [Google Scholar] [CrossRef] [PubMed]
- Mathey, D.G.; Schofer, J.; Kuck, K.H.; Beil, U.; Kloppel, G. Transmural, Haemorrhagic Myocardial Infarction after Intracoronary Streptokinase. Clinical, Angiographic, and Necropsy Findings. Heart 1982, 48, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Dorado, D.; Ruiz-Meana, M.; Piper, H.M. Lethal Reperfusion Injury in Acute Myocardial Infarction: Facts and Unresolved Issues. Cardiovasc. Res. 2009, 83, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Chien, G.L.; Wolff, R.A.; Davis, R.F.; Winkle, D.M. V. “Normothermic Range” Temperature Affects Myocardial Infarct Size. Cardiovasc. Res. 1994, 28, 1014–1017. [Google Scholar] [CrossRef]
- Miki, T.; Liu, G.S.; Cohen, M.V.; Downey, J.M. Mild Hypothermia Reduces Infarct Size in the Beating Rabbit Heart: A Practical Intervention for Acute Myocardial Infarction? Basic. Res. Cardiol. 1998, 93, 372–383. [Google Scholar] [CrossRef]
- Dae, M.W.; Gao, D.W.; Sessler, D.I.; Chair, K.; Stillson, C.A. Effect of Endovascular Cooling on Myocardial Temperature, Infarct Size, and Cardiac Output in Human-Sized Pigs. Am. J. Physiol.-Heart Circ. Physiol. 2002, 282, H1584–H1591. [Google Scholar] [CrossRef]
- Jung, K.T.; Bapat, A.; Kim, Y.-K.; Hucker, W.J.; Lee, K. Therapeutic Hypothermia for Acute Myocardial Infarction: A Narrative Review of Evidence from Animal and Clinical Studies. Korean J. Anesthesiol. 2022, 75, 216–230. [Google Scholar] [CrossRef]
- Maeng, M.; Mortensen, U.M.; Kristensen, J.; Kristiansen, S.B.; Andersen, H.R. Hypothermia during Reperfusion Does Not Reduce Myocardial Infarct Size in Pigs. Basic. Res. Cardiol. 2006, 101, 61–68. [Google Scholar] [CrossRef]
- JONES, R. Effect of Hypothermia on Changes in High-Energy Phosphate Production and Utilization in Total Ischemia*1. J. Mol. Cell Cardiol. 1982, 14, 123–130. [Google Scholar] [CrossRef]
- Götberg, M.; Olivecrona, G.K.; Engblom, H.; Ugander, M.; van der Pals, J.; Heiberg, E.; Arheden, H.; Erlinge, D. Rapid Short-Duration Hypothermia with Cold Saline and Endovascular Cooling before Reperfusion Reduces Microvascular Obstruction and Myocardial Infarct Size. BMC Cardiovasc. Disord. 2008, 8, 7. [Google Scholar] [CrossRef] [PubMed]
- Hale, S.L.; Herring, M.J.; Kloner, R.A. Delayed Treatment with Hypothermia Protects Against the No-Reflow Phenomenon Despite Failure to Reduce Infarct Size. J. Am. Heart Assoc. 2013, 2, e004234. [Google Scholar] [CrossRef] [PubMed]
- Berg, J.; Jablonowski, R.; Nordlund, D.; Ryd, D.; Heiberg, E.; Carlsson, M.; Arheden, H. Mild Hypothermia Attenuates Ischaemia/Reperfusion Injury: Insights from Serial Non-Invasive Pressure–Volume Loops. Cardiovasc. Res. 2023, 119, 2230–2243. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q.; Zhang, Q.; Guo, Z.; Wu, L.; Chen, Y.; Chen, Z.; Yang, K.; Cao, J. Therapeutic Hypothermia Inhibits Hypoxia-Induced Cardiomyocyte Apoptosis Via the MiR-483-3p/Cdk9 Axis. J. Am. Heart Assoc. 2023, 12, e026160. [Google Scholar] [CrossRef]
- Inoue, K.; Ando, S.; Gyuan, F.; Takaba, T. A Study of the Myocardial Protective Effect of Rapid Cooling Based on Intracellular Ca, Intracellular PH, and HSP70. Ann. Thorac. Cardiovasc. Surg. 2003, 9, 301–306. [Google Scholar]
- Shao, Z.-H.; Sharp, W.W.; Wojcik, K.R.; Li, C.-Q.; Han, M.; Chang, W.-T.; Ramachandran, S.; Li, J.; Hamann, K.J.; Vanden Hoek, T.L. Therapeutic Hypothermia Cardioprotection via Akt- and Nitric Oxide-Mediated Attenuation of Mitochondrial Oxidants. Am. J. Physiol.-Heart Circ. Physiol. 2010, 298, H2164–H2173. [Google Scholar] [CrossRef]
- El Farissi, M.; Buscone, S.; Bax, N.A.M.; van Rijswijk, J.W.; Veenendaal, T.; Keulards, D.C.J.; Zelis, J.M.; van Tuijl, S.; Eerdekens, R.; Demandt, J.; et al. Ultrastructural Characteristics of Myocardial Reperfusion Injury and Effect of Selective Intracoronary Hypothermia: An Observational Study in Isolated Beating Porcine Hearts. Ther. Hypothermia Temp. Manag. 2022, 12, 129–137. [Google Scholar] [CrossRef]
- Dixon, S.R.; Nguyen, T.T.; O’Neill, W.W.; Whitbourn, R.J.; Dae, M.W.; Grube, E.; Sherman, W.; Schaer, G.L.; Jenkins, J.S.; Baim, D.S.; et al. Induction of Mild Systemic Hypothermia with Endovascular Cooling during Primary Percutaneous Coronary Intervention for Acute Myocardial Infarction. J. Am. Coll. Cardiol. 2002, 40, 1928–1934. [Google Scholar] [CrossRef]
- Ly, H.Q.; Denault, A.; Dupuis, J.; Vadeboncoeur, A.; Harel, F.; Arsenault, A.; Gibson, C.M.; Bonan, R. A Pilot Study: The Noninvasive Surface Cooling Thermoregulatory System for Mild Hypothermia Induction in Acute Myocardial Infarction (The NICAMI Study). Am. Heart J. 2005, 150, 933.e9–933.e13. [Google Scholar] [CrossRef]
- Götberg, M.; Olivecrona, G.K.; Koul, S.; Carlsson, M.; Engblom, H.; Ugander, M.; van der Pals, J.; Algotsson, L.; Arheden, H.; Erlinge, D. A Pilot Study of Rapid Cooling by Cold Saline and Endovascular Cooling Before Reperfusion in Patients with ST-Elevation Myocardial Infarction. Circ. Cardiovasc. Interv. 2010, 3, 400–407. [Google Scholar] [CrossRef]
- Erlinge, D.; Götberg, M.; Lang, I.; Holzer, M.; Noc, M.; Clemmensen, P.; Jensen, U.; Metzler, B.; James, S.; Bötker, H.E.; et al. Rapid Endovascular Catheter Core Cooling Combined with Cold Saline as an Adjunct to Percutaneous Coronary Intervention for the Treatment of Acute Myocardial Infarction. J. Am. Coll. Cardiol. 2014, 63, 1857–1865. [Google Scholar] [CrossRef] [PubMed]
- Nichol, G.; Strickland, W.; Shavelle, D.; Maehara, A.; Ben-Yehuda, O.; Genereux, P.; Dressler, O.; Parvataneni, R.; Nichols, M.; McPherson, J.; et al. Prospective, Multicenter, Randomized, Controlled Pilot Trial of Peritoneal Hypothermia in Patients with ST-Segment—Elevation Myocardial Infarction. Circ. Cardiovasc. Interv. 2015, 8, e001965. [Google Scholar] [CrossRef] [PubMed]
- Noc, M.; Erlinge, D.; Neskovic, A.; Kafedzic, S.; Merkely, B.; Zima, E.; Fister, M.; Petrović, M.; Čanković, M.; Veress, G.; et al. COOL AMI EU Pilot Trial: A Multicentre, Prospective, Randomised Controlled Trial to Assess Cooling as an Adjunctive Therapy to Percutaneous Intervention in Patients with Acute Myocardial Infarction. EuroIntervention 2017, 13, e531–e539. [Google Scholar] [CrossRef] [PubMed]
- Dallan, L.A.P.; Giannetti, N.S.; Rochitte, C.E.; Polastri, T.F.; San Martin, C.Y.B.; Hajjar, L.A.; Lima, F.G.; Nicolau, J.C.; de Oliveira, M.T.; Dae, M.; et al. Cooling as an Adjunctive Therapy to Percutaneous Intervention in Acute Myocardial Infarction: COOL-MI InCor Trial. Ther. Hypothermia Temp. Manag. 2021, 11, 135–144. [Google Scholar] [CrossRef]
- Noc, M.; Laanmets, P.; Neskovic, A.N.; Petrović, M.; Stanetic, B.; Aradi, D.; Kiss, R.G.; Ungi, I.; Merkely, B.; Hudec, M.; et al. A Multicentre, Prospective, Randomised Controlled Trial to Assess the Safety and Effectiveness of Cooling as an Adjunctive Therapy to Percutaneous Intervention in Patients with Acute Myocardial Infarction: The COOL AMI EU Pivotal Trial. EuroIntervention 2021, 17, 466–473. [Google Scholar] [CrossRef]
- Testori, C.; Beitzke, D.; Mangold, A.; Sterz, F.; Loewe, C.; Weiser, C.; Scherz, T.; Herkner, H.; Lang, I. Out-of-Hospital Initiation of Hypothermia in ST-Segment Elevation Myocardial Infarction: A Randomised Trial. Heart 2019, 105, 531–537. [Google Scholar] [CrossRef]
- El Farissi, M.; Keulards, D.C.J.; Zelis, J.M.; van’t Veer, M.; Zimmermann, F.M.; Pijls, N.H.J.; Otterspoor, L.C. Hypothermia for Reduction of Myocardial Reperfusion Injury in Acute Myocardial Infarction: Closing the Translational Gap. Circ. Cardiovasc. Interv. 2021, 14, e010326. [Google Scholar] [CrossRef]
- Otterspoor, L.; Van’t Veer, M.; Van Nunen, L.; Brueren, G.; Tonino, P.; Wijnbergen, I.; Helmes, H.; Zimmermann, F.; Van Hagen, E.; Johnson, N.; et al. Safety and Feasibility of Selective Intracoronary Hypothermia in Acute Myocardial Infarction. EuroIntervention 2017, 13, e1475–e1482. [Google Scholar] [CrossRef]
- Kim, H.; Lee, J.; Song, W.; Shin, J.; Oh, D.; Harrison, K.; Jakkula, M.; Chiu Wong, S.; Hong, M.K. Feasibility and Safety of Regional Myocardial Hypothermia during Myocardial Ischemia and Infarction in Pigs. Coron. Artery Dis. 2005, 16, 125–129. [Google Scholar] [CrossRef]
- Otake, H.; Shite, J.; Paredes, O.L.; Shinke, T.; Yoshikawa, R.; Tanino, Y.; Watanabe, S.; Ozawa, T.; Matsumoto, D.; Ogasawara, D.; et al. Catheter-Based Transcoronary Myocardial Hypothermia Attenuates Arrhythmia and Myocardial Necrosis in Pigs with Acute Myocardial Infarction. J. Am. Coll. Cardiol. 2007, 49, 250–260. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Li, Y.; Chen, B.; Khurwolah, M.R.; Tian, Y.; Shi, H.; Yang, Z.; Wang, L. A Pilot Clinical Study of Adjunctive Therapy with Selective Intracoronary Hypothermia in Patients with ST-segment Elevation Myocardial Infarction. Catheter. Cardiovasc. Interv. 2018, 92, E433–E440. [Google Scholar] [CrossRef] [PubMed]
- El Farissi, M.; Pijls, N.H.J.; Good, R.; Engström, T.; Keeble, T.R.; Beleslin, B.; De Bruyne, B.; Fröbert, O.; Erlinge, D.; Teeuwen, K.; et al. A Randomised Trial of Selective Intracoronary Hypothermia during Primary PCI. EuroIntervention 2024, 20, e740–e749. [Google Scholar] [CrossRef] [PubMed]
- Bøtker, H.E.; Hausenloy, D.; Andreadou, I.; Antonucci, S.; Boengler, K.; Davidson, S.M.; Deshwal, S.; Devaux, Y.; Di Lisa, F.; Di Sante, M.; et al. Practical Guidelines for Rigor and Reproducibility in Preclinical and Clinical Studies on Cardioprotection. Basic. Res. Cardiol. 2018, 113, 39. [Google Scholar] [CrossRef] [PubMed]
- Merrill, T.L.; Mitchell, J.E.; Merrill, D.R.; Gorman, J.H.; Gorman, R.C.; Gillespie, M.J. Myocardial Tissue Salvage Is Correlated with Ischemic Border Region Temperature at Reperfusion. Catheter. Cardiovasc. Interv. 2020, 96, E593–E601. [Google Scholar] [CrossRef]
- Pei, Z.; Qiu, J.; Zhao, Y.; Song, S.; Wang, R.; Luo, W.; Cai, X.; Liu, B.; Chen, H.; Yin, J.; et al. A Novel Intracoronary Hypothermia Device Reduces Myocardial Reperfusion Injury in Pigs. Chin. Med. J. (Engl.) 2024. [Google Scholar] [CrossRef]
- Richard Spears, J.; Prcevski, P.; Xu, R.; Li, L.; Brereton, G.; DiCarli, M.; Spanta, A.; Crilly, R.; Lavine, S.; vander Heide, R. Aqueous Oxygen Attenuation of Reperfusion Microvascular Ischemia in a Canine Model of Myocardial Infarction. ASAIO J. 2003, 49, 716–720. [Google Scholar] [CrossRef]
- O’Neill, W.W.; Martin, J.L.; Dixon, S.R.; Bartorelli, A.L.; Trabattoni, D.; Oemrawsingh, P.V.; Atsma, D.E.; Chang, M.; Marquardt, W.; Oh, J.K.; et al. Acute Myocardial Infarction with Hyperoxemic Therapy (AMIHOT). J. Am. Coll. Cardiol. 2007, 50, 397–405. [Google Scholar] [CrossRef]
- Stone, G.W.; Martin, J.L.; de Boer, M.-J.; Margheri, M.; Bramucci, E.; Blankenship, J.C.; Metzger, D.C.; Gibbons, R.J.; Lindsay, B.S.; Weiner, B.H.; et al. Effect of Supersaturated Oxygen Delivery on Infarct Size after Percutaneous Coronary Intervention in Acute Myocardial Infarction. Circ. Cardiovasc. Interv. 2009, 2, 366–375. [Google Scholar] [CrossRef]
- Chen, S.; David, S.W.; Khan, Z.A.; Metzger, D.C.; Wasserman, H.S.; Lotfi, A.S.; Hanson, I.D.; Dixon, S.R.; LaLonde, T.A.; Généreux, P.; et al. One-year Outcomes of Supersaturated Oxygen Therapy in Acute Anterior Myocardial Infarction: The IC-HOT Study. Catheter. Cardiovasc. Interv. 2021, 97, 1120–1126. [Google Scholar] [CrossRef]
- Kloner, R.A.; Creech, J.L.; Stone, G.W.; O’Neill, W.W.; Burkhoff, D.; Spears, J.R. Update on Cardioprotective Strategies for STEMI. JACC Basic. Transl. Sci. 2021, 6, 1021–1033. [Google Scholar] [CrossRef]
- Jackson, T.C.; Kochanek, P.M. A New Vision for Therapeutic Hypothermia in the Era of Targeted Temperature Management: A Speculative Synthesis. Ther. Hypothermia Temp. Manag. 2019, 9, 13–47. [Google Scholar] [CrossRef] [PubMed]

| Trial | Design | n | Anterior MI | Hypothermia Technique | Target Temperature (°C) | Achieved Temperature (°C) | Door-to-Ballon Time (min) (Hypothermia vs. Control) | Patients Achieved Target Temperature | Imaging | IS (% of LVM) (Hypothermia vs. Control) | Safety (Hypothermia vs. Control) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| COOL-MI I pilot [69] | Multi-center, prospective RCT | 42 | 45% | Endovascular cooling | 33 | 33.2 ± 0.9 | 87 ± 30 vs. 104 ± 44 | 20/21 | SPECT | 2% vs. 8% (p = 0.8) | MACEs (30 days) 0% vs. 10% (p = NS) |
| NICAMI [70] | Single-arm, open-label, feasibility | 9 | 33% | Noninvasive surface cooling | 34.5 | N/R | 38 | 9/9 | SPECT | 23% | No hemodynamic or arrhythmic instability |
| RAPID-MI-ICE [71] | Single-center, prospective RCT | 20 | 72% | Endovascular cooling + cold saline infusion | 35 | 34.7 ± 0.3 | 43 ± 7 vs. 40 ± 6 | 9/10 | CMR | 13.7% vs. 20.5% (p = 0.08) | MACEs (30 days) 0% vs. 0% (p = NS) |
| CHILL-MI [72] | Multi-center, prospective RCT | 120 | 42% | Endovascular cooling + cold saline infusion | 33 | 34.7 | 42 ± 16 vs. 33 ± 21 | 46/60 | CMR | IS/MaR: 40.5% vs. 46.6% (p = 0.15) | No significant difference in thrombotic or bleeding events |
| VELOCITY [73] | Multi-center, prospective RCT | 54 | 46% | Peritoneal lavage | 32.5 | 34.0 ± 0.8 | 62 ± 15 vs. 47 ± 9 (p = 0.007) | 24/28 | CMR | 17.2% ± 2.3 vs. 16.0% ± 6.1 (p = 0.54) IS/MaR: 67.3% vs. 55.8% (p = 0.36) | MACEs (30 days) 6 (21.4%) vs. 0 (0%) patients (p = 0.01) |
| COOL AMI EU pilot [74] | Multi-center, prospective RCT | 50 | 100% | Endovascular cooling + cold saline infusion | 32 | 33.6 ± 1.0 | 59 ± 19 vs. 42 ± 23 | 23/25 | CMR | 16.7% vs. 23.8% (p = 0.31) | No difference in adverse events PAF (32% versus 8%; p = 0.074) |
| COOL-MI InCor [75] | Single-center, prospective RCT | 50 | 38% | Endovascular cooling + cold saline infusion | 32 | 33.1 ± 1.0 | 92.1 ± 20.5 vs. 87 ± 24.4 | 35/35 | CMR | 13.9% vs. 13.8% (p = 0.801) | No difference: -All-cause mortality (2.9% vs. 6.7%, p = 0.237) -MACEs (21.7% vs. 20%, p = 0.237) |
| COOL AMI EU Pivotal [76] | Multi-center, prospective RCT | 111 | 100% | Endovascular cooling + cold saline infusion | 32 | 33.0 ± 0.9 | 61 ± 21 vs. 32 ± 18 | 55/58 | CMR | 21.3% vs. 20.0% (p = 0.540) | Non-significant increase of MACEs (8.6% vs. 1.9%; p = 0.117) Significant difference in: -cardiogenic shock (10.3% vs. 0%, p = 0.028) -PAF (43.1% vs. 3.8%, p < 0.001) |
| STATIM [77] | Single-center, prospective RCT | 101 | 51% | Endovascular cooling + cold saline infusion | 34 | 34.4 ± 0.6 | 103 ± 21 vs. 89 ± 24 | 38/47 | CMR | MSI: 0.43 ± 0.27 vs. 0.37 ± 0.26 (p = 0.27). | No difference in MACEs Numerical trend towards increased bleeding events in the hypothermia arm |
| Study | Design | Population | Number of Participants | TIMI 0/1 | Infarct Size Imaging Method | Door to Balloon Time (minutes) | Ischemic Time Prolongation (minutes) | Time to Achieve-Target Temperature (seconds) | Target Temperature Achieved (%) | Mortality | Infarct Size | Arrhythmia Outcomes | Stent Thrombosis |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Otterspoor et al. [79] | Observational, Feasibility | STEMI (60% Anterior, 40% Inferior) | 10 | 8 | N/R | 55 (46–57) | 19.2 (18.4–19.6) | 27 (21–46) | 100% | 0% | N/R | 20% AV block 20% ventricular tachyarrhythmia | 10% |
| Wang et al. [82] | Observational | STEMI (54% Anterior, 46% Inferior) | 60 (30 intervention, 30 control) | 60 | CMR, after 7 days | 96 ± 24 vs. 83 ± 32 (p = 0.07) | 13 | 31 ± 8 | 100% | 7% vs. 10% (30 days) | Mean IS/MaR: 44.85 ± 5.89% vs. 50.69 ± 10.75%; =0.022 Mean IS/LVM: 18.76 ± 7.61 vs. 23.64 ± 10.08; p = 0.059 | Non-sustained VT: 0% | 3% vs. 3% |
| El Farissi et al. [83] | Randomized Controlled Trial | Anterior STEMI | 200 (100 intervention, 100 control) | 200 | CMR, after 3 months | 37 (33–44) vs. 22 (18–26); p < 0.001 | 15 | 43 (18–113) | 100% | 1% vs. 0% (3 months) | IS (%LVM): 23.1 ± 12.5 vs. 21.6 ± 12.2; p = 0.43 IS (g): 26.1 ± 17.8 vs. 24.5 ± 15.7; p = 0.52 Myocardial salvage index: 0.54 ± 0.24 vs. 0.55 ± 0.25; p = 0.82 | VT: 9 vs. 7 patients (p = 0.60) AF: 0 vs. 3 patients (p = 0.08) | 2 vs. 1 patients (p = 0.56) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pyrpyris, N.; Dimitriadis, K.; Iliakis, P.; Theofilis, P.; Beneki, E.; Terentes-Printzios, D.; Sakalidis, A.; Antonopoulos, A.; Aznaouridis, K.; Tsioufis, K. Hypothermia for Cardioprotection in Acute Coronary Syndrome Patients: From Bench to Bedside. J. Clin. Med. 2024, 13, 5390. https://doi.org/10.3390/jcm13185390
Pyrpyris N, Dimitriadis K, Iliakis P, Theofilis P, Beneki E, Terentes-Printzios D, Sakalidis A, Antonopoulos A, Aznaouridis K, Tsioufis K. Hypothermia for Cardioprotection in Acute Coronary Syndrome Patients: From Bench to Bedside. Journal of Clinical Medicine. 2024; 13(18):5390. https://doi.org/10.3390/jcm13185390
Chicago/Turabian StylePyrpyris, Nikolaos, Kyriakos Dimitriadis, Panagiotis Iliakis, Panagiotis Theofilis, Eirini Beneki, Dimitrios Terentes-Printzios, Athanasios Sakalidis, Alexios Antonopoulos, Konstantinos Aznaouridis, and Konstantinos Tsioufis. 2024. "Hypothermia for Cardioprotection in Acute Coronary Syndrome Patients: From Bench to Bedside" Journal of Clinical Medicine 13, no. 18: 5390. https://doi.org/10.3390/jcm13185390









