Emerging Applications of Intraoperative Optical Coherence Tomography in Corneal Surgery: A Narrative Review
Abstract
1. Introduction
2. Optical Coherence Tomography—Principles and Technology
3. iOCT in Corneal Surgery
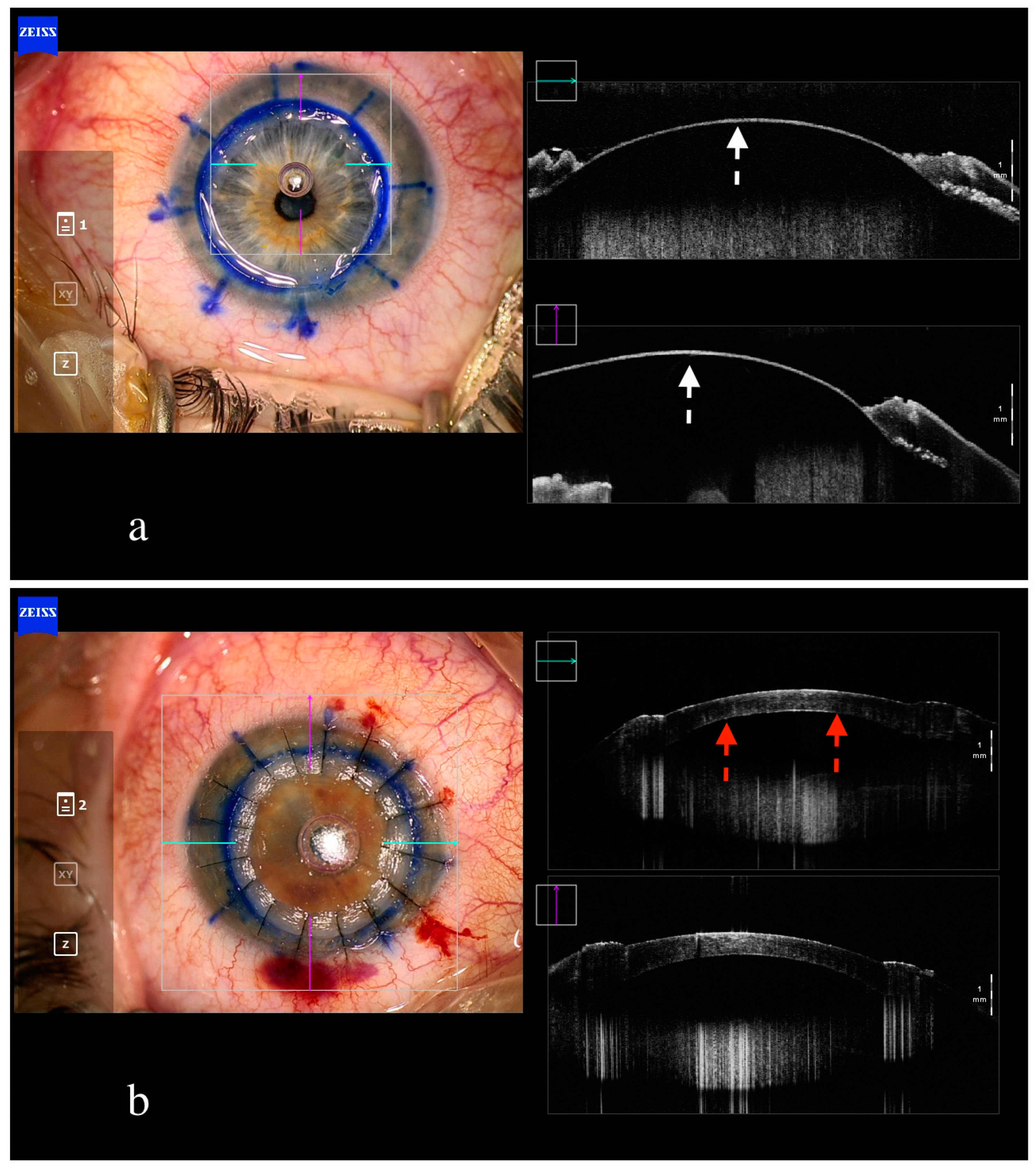
4. Penetrating Keratoplasty
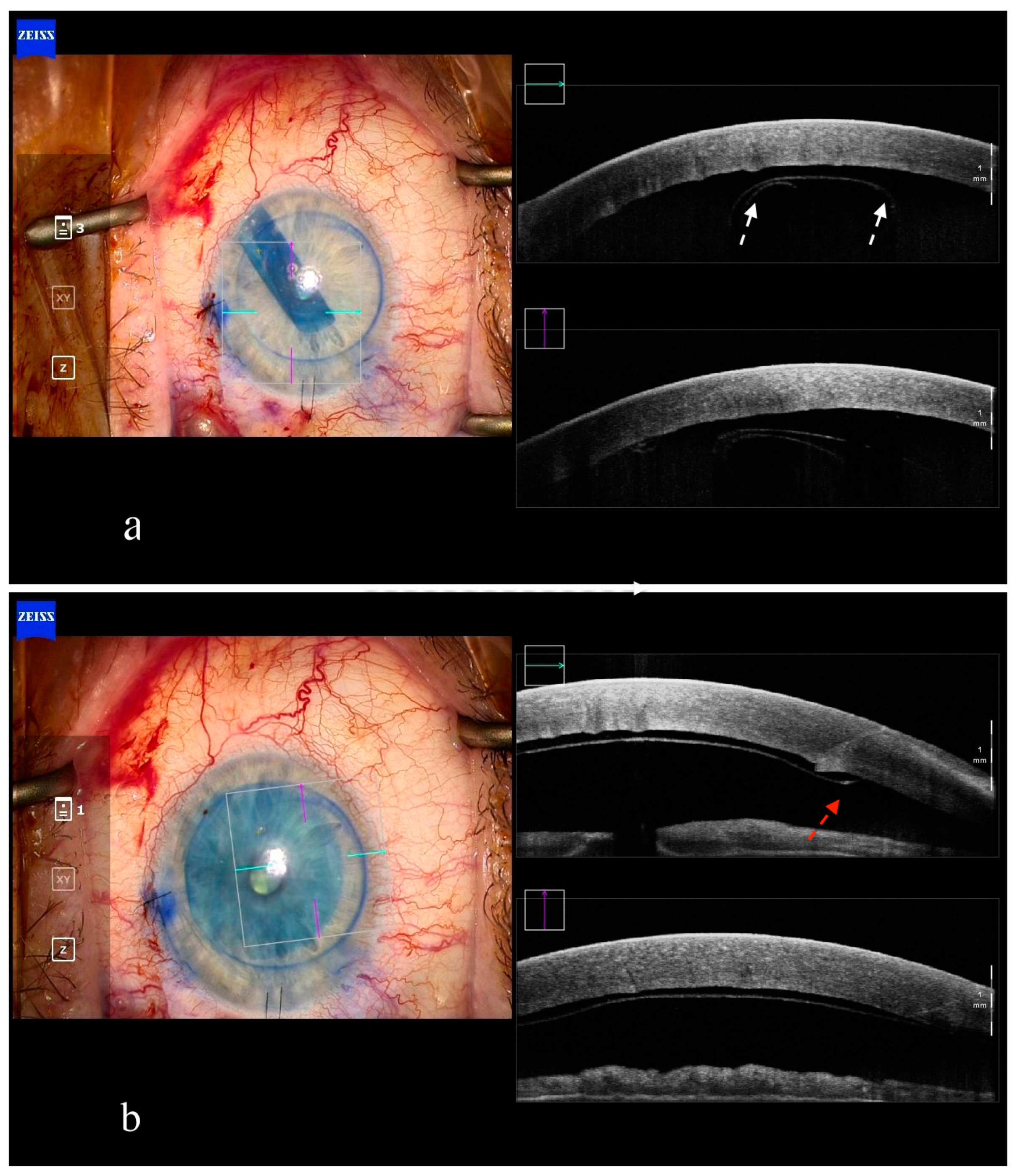
5. Deep Anterior Lamellar Keratoplasty
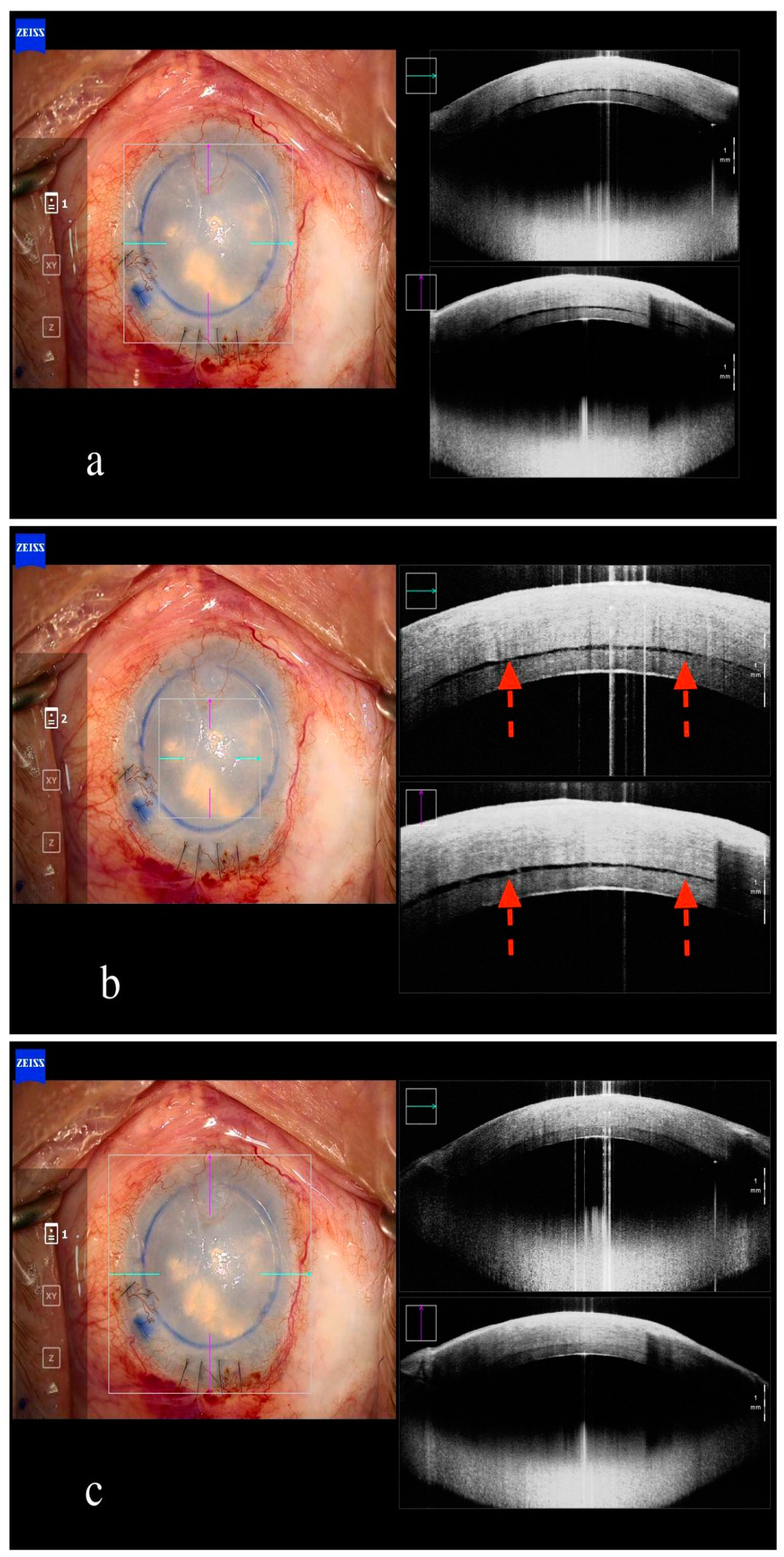
6. Endothelial Keratoplasty
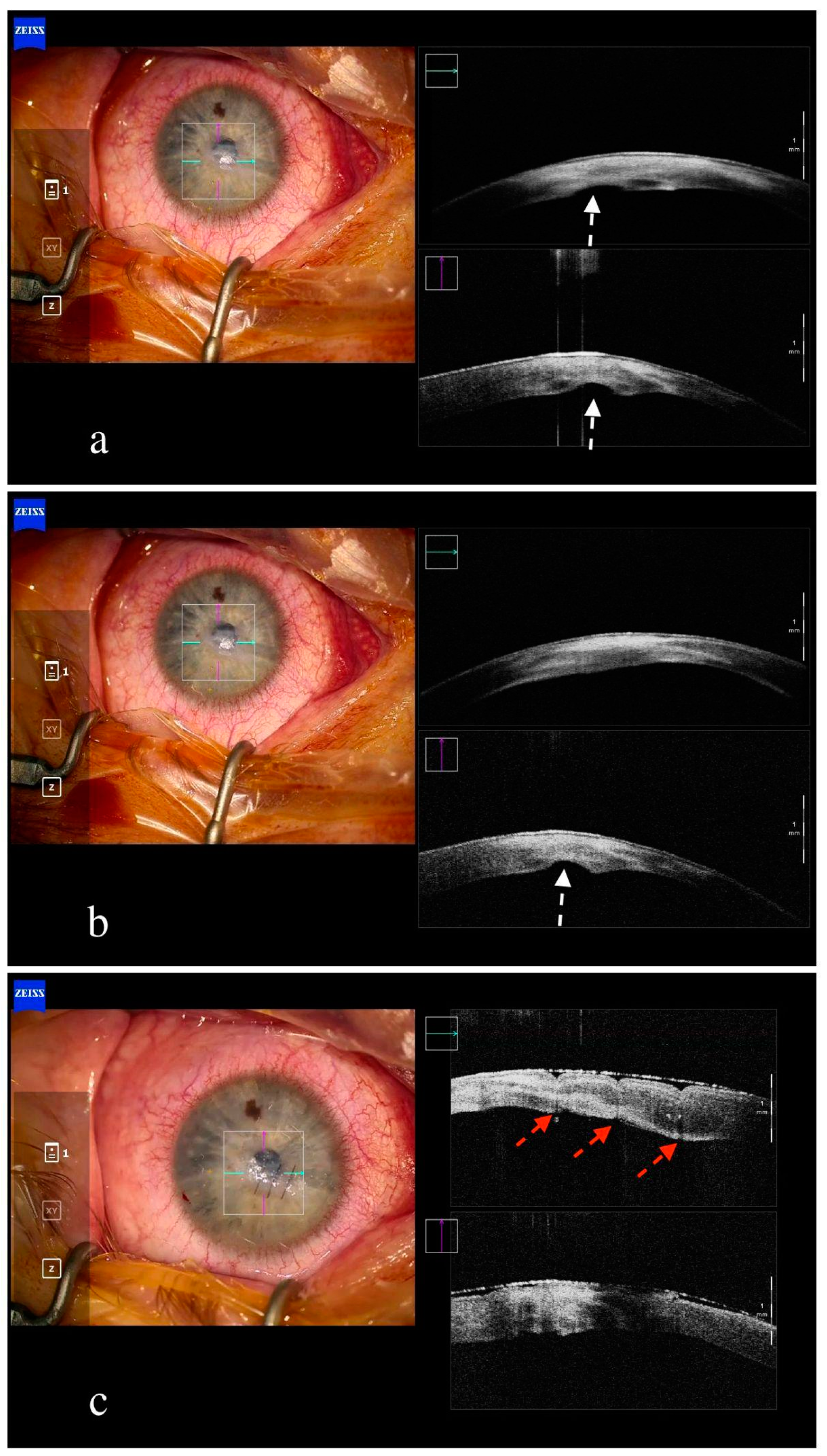
7. Other Corneal Surgeries
8. Conclusions
Funding
Conflicts of Interest
Abbreviations
| OCT | optical coherence tomography |
| DALK | deep anterior lamellar keratoplasty |
| DMEK | Descemet membrane endothelial keratoplasty |
| DSAEK | Descemet’s stripping endothelial keratoplasty |
| PK | penetrating keratoplasty |
| DM | Descemet membrane |
References
- Izatt, J.A.; Hee, M.R.; Swanson, E.A.; Lin, C.P.; Huang, D.; Schuman, J.S.; Puliafito, C.A.; Fujimoto, J.G. Micrometer-scale resolution imaging of the anterior eye in vivo with optical coherence tomography. Arch. Ophthalmol. Chic. 1994, 112, 1584–1589. [Google Scholar] [CrossRef] [PubMed]
- Venkateswaran, N.; Galor, A.; Wang, J.; Karp, C.L. Optical coherence tomography for ocular surface and corneal diseases: A review. Eye Vis. Lond. Engl. 2018, 5, 13. [Google Scholar] [CrossRef]
- Yim, M.; Galor, A.; Nanji, A.; Joag, M.; Palioura, S.; Feuer, W.; Karp, C.L. Ability of novice clinicians to interpret high-resolution optical coherence tomography for ocular surface lesions. Can. J. Ophthalmol. J. Can. Ophtalmol. 2018, 53, 150–154. [Google Scholar] [CrossRef]
- Han, S.B.; Liu, Y.C.; Mohamed-Noriega, K.; Mehta, J.S. Application of Intraoperative Optical Coherence Tomography Technology in Anterior Segment Surgery. J. Ophthalmol. 2022, 2022, 1568406. [Google Scholar] [CrossRef]
- Geerling, G.; Müller, M.; Winter, C.; Hoerauf, H.; Oelckers, S.; Laqua, H.; Birngruber, R. Intraoperative 2-dimensional optical coherence tomography as a new tool for anterior segment surgery. Arch. Ophthalmol. 2005, 123, 253–257. [Google Scholar] [CrossRef]
- Ehlers, J.P.; Ohr, M.P.; Kaiser, P.K.; Srivastava, S.K. Novel microarchitectural dynamics in rhegmatogenous retinal detachments identified with intraoperative optical coherence tomography. Retina 2013, 33, 1428–1434. [Google Scholar] [CrossRef] [PubMed]
- Lytvynchuk, L.M.; Glittenberg, C.G.; Ansari-Shahrezaei, S.; Binder, S. Intraoperative optical coherence tomography assisted analysis of pars Plana vitrectomy for retinal detachment in morning glory syndrome: A case report. BMC Ophthalmol. 2017, 17, 134. [Google Scholar] [CrossRef] [PubMed]
- Abraham, J.R.; Srivastava, S.K.; Reese, J.L.; Ehlers, J.P. Intraoperative OCT Features and Postoperative Ellipsoid Mapping in Primary Macula-Involving Retinal Detachments from the PIONEER Study. Ophthalmol. Retina 2019, 3, 252–257. [Google Scholar] [CrossRef]
- Abraham, J.R.; Srivastava, S.K.; Le, T.K.; Sharma, S.; Rachitskaya, A.; Reese, J.L.; Ehlers, J.P. Intraoperative OCT-Assisted Retinal Detachment Repair in the DISCOVER Study: Impact and Outcomes. Ophthalmol. Retina 2020, 4, 378–383. [Google Scholar] [CrossRef]
- Ray, R.; Barañano, D.E.; Fortun, J.A.; Chwent, B.J.; Cribbs, B.E.; Bergstrom, C.S.; Hubbard, G.B.; Srivastava, S.K. Intraoperative microscope-mounted spectral domain optical coherence tomography for evaluation of retinal anatomy during macular surgery. Ophthalmology 2011, 118, 2212–2217. [Google Scholar] [CrossRef]
- Yee, P.; Sevgi, D.D.; Abraham, J.; Srivastava, S.K.; Le, T.; Uchida, A.; Figueiredo, N.; Rachitskaya, A.V.; Sharma, S.; Reese, J.; et al. iOCT-assisted macular hole surgery: Outcomes and utility from the DISCOVER study. Br. J. Ophthalmol. 2021, 105, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Bruyère, E.; Philippakis, E.; Dupas, B.; Nguyen-Kim, P.; Tadayoni, R.; Couturier, A. Benefit of Intraoperative Optical Coherence Tomography for Vitreomacular Surgery in Highly Myopic Eyes. Retina 2018, 38, 2035–2044. [Google Scholar] [CrossRef] [PubMed]
- Falkner-Radler, C.I.; Glittenberg, C.; Gabriel, M.; Binder, S. Intrasurgical Microscope-Integrated Spectral Domain Optical Coherence Tomography-Assisted Membrane Peeling. Retina 2015, 35, 2100–2106. [Google Scholar] [CrossRef]
- Ehlers, J.P.; Khan, M.; Petkovsek, D.; Stiegel, L.; Kaiser, P.K.; Singh, R.P.; Reese, J.L.; Srivastava, S.K. Outcomes of Intraoperative OCT-Assisted Epiretinal Membrane Surgery from the PIONEER Study. Ophthalmol. Retina 2018, 2, 263–267. [Google Scholar] [CrossRef]
- Lang, S.J.; Heinzelmann, S.; Böhringer, D.; Reinhard, T.; Maier, P. Indications for intraoperative anterior segment optical coherence tomography in corneal surgery. Int. Ophthalmol. 2020, 40, 2617–2625. [Google Scholar] [CrossRef]
- Ehlers, J.P.; Dupps, W.J.; Kaiser, P.K.; Goshe, J.; Singh, R.P.; Petkovsek, D.; Srivastava, S.K. The Prospective Intraoperative and Perioperative Ophthalmic ImagiNg with Optical CoherEncE TomogRaphy (PIONEER) Study: 2-year results. Am. J. Ophthalmol. 2014, 158, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Ehlers, J.P.; Modi, Y.S.; Pecen, P.E.; Goshe, J.; Dupps, W.J.; Rachitskaya, A.; Sharma, S.; Yuan, A.; Singh, R.; Kaiser, P.K.; et al. The DISCOVER Study 3-Year Results: Feasibility and Usefulness of Microscope-Integrated Intraoperative OCT during Ophthalmic Surgery. Ophthalmology 2018, 125, 1014–1027. [Google Scholar] [CrossRef]
- Podoleanu, A.G. Optical coherence tomography. J. Microsc. 2012, 247, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Drexler, W.; Fujimoto, J.G. State-of-the-art retinal optical coherence tomography. Prog. Retin. Eye Res. 2008, 27, 45–88. [Google Scholar] [CrossRef]
- Marschall, S.; Sander, B.; Mogensen, M.; Jørgensen, T.M.; Andersen, P.E. Optical coherence tomography-current technology and applications in clinical and biomedical research. Anal. Bioanal. Chem. 2011, 400, 2699–2720. [Google Scholar] [CrossRef]
- Elliott, A.D. Confocal Microscopy: Principles and Modern Practices. Curr. Protoc. Cytom. 2020, 92, e68. [Google Scholar] [CrossRef] [PubMed]
- Ang, M.; Baskaran, M.; Werkmeister, R.M.; Chua, J.; Schmidl, D.; dos Santos, V.A.; Garhöfer, G.; Mehta, J.S.; Schmetterer, L. Anterior segment optical coherence tomography. Prog. Retin. Eye Res. 2018, 66, 132–156. [Google Scholar] [CrossRef] [PubMed]
- Auksorius, E. Fourier-domain full-field optical coherence tomography with real-time axial imaging. Opt. Lett. 2021, 46, 4478–4481. [Google Scholar] [CrossRef] [PubMed]
- Rollins, A.M.; Kulkarni, M.D.; Yazdanfar, S.; Ung-arunyawee, R.; Izatt, J.A. In vivo video rate optical coherence tomography. Opt. Express. 1998, 3, 219–229. [Google Scholar] [CrossRef]
- Live Volumetric (4D) Visualization and Guidance of in vivo Human Ophthalmic Surgery with Intraoperative Optical Coherence Tomography—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/27538478/ (accessed on 25 November 2023).
- Eguchi, H.; Kusaka, S.; Arimura-Koike, E.; Tachibana, K.; Tsujioka, D.; Fukuda, M.; Shimomura, Y. Intraoperative optical coherence tomography (RESCAN® 700) for detecting iris incarceration and iridocorneal adhesion during keratoplasty. Int. Ophthalmol. 2017, 37, 761–765. [Google Scholar] [CrossRef]
- Steven, P.; Le Blanc, C.; Velten, K.; Lankenau, E.; Krug, M.; Oelckers, S.; Heindl, L.M.; Gehlsen, U.; Hüttmann, G.; Cursiefen, C. Optimizing descemet membrane endothelial keratoplasty using intraoperative optical coherence tomography. JAMA Ophthalmol. 2013, 131, 1135–1142. [Google Scholar] [CrossRef]
- Ehlers, J.P. Intraoperative optical coherence tomography: Past, present, and future. Eye Lond. Engl. 2016, 30, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Price, F.W. Intraoperative Optical Coherence Tomography: Game-Changing Technology. Cornea 2021, 40, 675–678. [Google Scholar] [CrossRef]
- Boucenna, W.; Bourges, J.L. [Penetrating keratoplasty]. J. Fr. Ophtalmol. 2022, 45, 543–558. [Google Scholar] [CrossRef]
- Eguchi, H.; Hotta, F.; Kusaka, S.; Shimomura, Y. Intraoperative Optical Coherence Tomography Imaging in Corneal Surgery: A Literature Review and Proposal of Novel Applications. J. Ophthalmol. 2020, 2020, 1497089. [Google Scholar] [CrossRef]
- Deshmukh, R.; Nair, S.; Vaddavalli, P.K.; Agrawal, T.; Rapuano, C.J.; Beltz, J.; Vajpayee, R.B. Post-penetrating keratoplasty astigmatism. Surv. Ophthalmol. 2022, 67, 1200–1228. [Google Scholar] [CrossRef] [PubMed]
- Titiyal, J.S.; Kaur, M.; Falera, R. Intraoperative optical coherence tomography in anterior segment surgeries. Indian. J. Ophthalmol. 2017, 65, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Priyadarshini, K.; Agarwal, R.; Bafna, R.K.; Nagpal, R.; Sinha, R.; Agarwal, T.; Maharana, P.K.; Titiyal, J.S. Role of Microscope-Intraoperative Optical Coherence Tomography in Pediatric Keratoplasty: AComparative Study. Am. J. Ophthalmol. 2021, 221, 190–198. [Google Scholar] [CrossRef]
- Mohamed-Noriega, K.; Angunawela, R.I.; Tan, D.; Mehta, J.S. Corneal transplantation: Changing techniques. Transplantation 2011, 92, e31–e32, author reply e32–e33. [Google Scholar] [CrossRef]
- Luengo-Gimeno, F.; Tan, D.T.; Mehta, J.S. Evolution of deep anterior lamellar keratoplasty (DALK). Ocul. Surf. 2011, 9, 98–110. [Google Scholar] [CrossRef]
- Au, J.; Goshe, J.; Dupps, W.J.; Srivastava, S.K.; Ehlers, J.P. Intraoperative Optical Coherence Tomography for Enhanced Depth Visualization in Deep Anterior Lamellar Keratoplasty from the PIONEER Study. Cornea 2015, 34, 1039–1043. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Queratoplastia Lamelar Anterior Profunda (DALK Pachy Bubble) Guiada por OCT Transoperatorio. Caso Clínico. Available online: https://www.medigraphic.com/cgi-bin/new/resumen.cgi?IDARTICULO=85712 (accessed on 25 November 2023).
- Patil, M.; Ang, M.; Mehta, J. Anterior Segment OCT: Real-Time Intraoperative OCT in Corneal Surgery. In Atlas of Anterior Segment Optical Coherence Tomography; Springer: Cham, Switzerland, 2021; pp. 181–189. [Google Scholar] [CrossRef]
- Saad, A.; Guilbert, E.; Grise-Dulac, A.; Sabatier, P.; Gatinel, D. Intraoperative OCT-Assisted DMEK: 14 Consecutive Cases. Cornea 2015, 34, 802–807. [Google Scholar] [CrossRef]
- Pujari, A.; Agarwal, D.; Chawla, R.; Kumar, A.; Sharma, N. Intraoperative Optical Coherence Tomography Guided Ocular Surgeries: Critical Analysis of Clinical Role and Future Perspectives. Clin. Ophthalmol. 2020, 14, 2427–2440. [Google Scholar] [CrossRef]
- Hinojosa, J.L.D.; Domene-Hickman, J.L.; Hurtado, N.J.A.; Hinojosa, J.L.D.; Domene-Hickman, J.L.; Hurtado, N.J.A. Intraoperative OCT in Lamellar Corneal Transplants (DALK, DSAEK, DMEK). In OCT—Applications in Ophthalmology; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Patel, A.S.; Goshe, J.M.; Srivastava, S.K.; Ehlers, J.P. Intraoperative Optical Coherence Tomography-Assisted Descemet Membrane Endothelial Keratoplasty in the DISCOVER Study: First 100 Cases. Am. J. Ophthalmol. 2020, 210, 167–173. [Google Scholar] [CrossRef]
- Yahia Chérif, H.; Gueudry, J.; Afriat, M.; Delcampe, A.; Attal, P.; Gross, H.; Muraine, M. Efficacy and safety of pre-Descemet’s membrane sutures for the management of acute corneal hydrops in keratoconus. Br. J. Ophthalmol. 2015, 99, 773–777. [Google Scholar] [CrossRef]
- Reinhold, A.; Hasler, P.W.; Scholl, H.P.N.; Gatzioufas, Z. iOCT-Assisted DSAEK Tamponade for Spontaneous Corneal Perforation with Secondary Bleb Formation in Pellucid Marginal Degeneration. Klin. Monbl. Augenheilkd. 2023, 240, 398–399. [Google Scholar] [CrossRef] [PubMed]
- Chatzimichail, E.; Martinho, A.C.; Pfau, K.; Gugleta, K.; Gatzioufas, Z. Intraoperative Anterior Segment OCT-Assisted Removal of Misdirected Intracorneal Viscoelastic Substance in Iatrogenic Descemetolysis. Klin. Monbl. Augenheilkd. 2024, 241, 381–384. [Google Scholar] [CrossRef] [PubMed]
- Ruland, K.; Bouldin, T.W.; Davis, R.M.; Fleischman, D. Intraoperative optical coherence tomography-assisted retrocorneal fibrous membrane biopsy and excision. Am. J. Ophthalmol. Case Rep. 2018, 11, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, E.M.; Stiefel, H.C.; Houghton, D.C.; Chamberlain, W.D. Intraoperative Optical Coherence Tomography to Guide Corneal Biopsy: A Case Report. Cornea 2019, 38, 639–641. [Google Scholar] [CrossRef] [PubMed]
- Siebelmann, S.; Horstmann, J.; Scholz, P.; Bachmann, B.; Matthaei, M.; Hermann, M.; Cursiefen, C. Intraoperative changes in corneal structure during excimer laser phototherapeutic keratectomy (PTK) assessed by intraoperative optical coherence tomography. Graefes Arch. Clin. Exp. Ophthalmol. Albrecht Von. Graefes Arch. Klin. Exp. Ophthalmol. 2018, 256, 575–581. [Google Scholar] [CrossRef]
- Tong, C.M.; Parker, J.S.; Dockery, P.W.; Birbal, R.S.; Melles, G.R.J. Use of intraoperative anterior segment optical coherence tomography for Bowman layer transplantation. Acta Ophthalmol. 2019, 97, E1031–E1032. [Google Scholar] [CrossRef]
- El-Haddad, M.T.; Tao, Y.K. Automated stereo vision instrument tracking for intraoperative OCT guided anterior segment ophthalmic surgical maneuvers. Biomed. Opt. Express 2015, 6, 3014–3031. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chatzimichail, E.; Chondrozoumakis, G.; Doroodgar, F.; Vounotrypidis, E.; Panos, G.D.; Gatzioufas, Z. Emerging Applications of Intraoperative Optical Coherence Tomography in Corneal Surgery: A Narrative Review. J. Clin. Med. 2024, 13, 5426. https://doi.org/10.3390/jcm13185426
Chatzimichail E, Chondrozoumakis G, Doroodgar F, Vounotrypidis E, Panos GD, Gatzioufas Z. Emerging Applications of Intraoperative Optical Coherence Tomography in Corneal Surgery: A Narrative Review. Journal of Clinical Medicine. 2024; 13(18):5426. https://doi.org/10.3390/jcm13185426
Chicago/Turabian StyleChatzimichail, Eleftherios, Georgios Chondrozoumakis, Farideh Doroodgar, Efstathios Vounotrypidis, Georgios D. Panos, and Zisis Gatzioufas. 2024. "Emerging Applications of Intraoperative Optical Coherence Tomography in Corneal Surgery: A Narrative Review" Journal of Clinical Medicine 13, no. 18: 5426. https://doi.org/10.3390/jcm13185426
APA StyleChatzimichail, E., Chondrozoumakis, G., Doroodgar, F., Vounotrypidis, E., Panos, G. D., & Gatzioufas, Z. (2024). Emerging Applications of Intraoperative Optical Coherence Tomography in Corneal Surgery: A Narrative Review. Journal of Clinical Medicine, 13(18), 5426. https://doi.org/10.3390/jcm13185426







