Abstract
Introduction: The spinal rehabilitation process plays a crucial role in SCI patients’ lives, and recent developments in VR have the potential to efficiently engage SCI patients in therapeutic activities and promote neuroplasticity. Objective: The primary objective of this study is to assess a complete review of the extended impacts of VR-assisted training on spine rehabilitation in SCI patients. Methods: This systematic review was conducted according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) through a single database search in PubMed/Medline between the dates 1 January 2010 and 1 February 2024. MESH terms and keywords were combined in the following search strategy: (Augmented Reality OR VR OR Virtual Reality) AND (Spine OR Spinal) AND Rehabilitation. Included articles were written in English, involved adults with SCI, included an intervention with VR, AR, or any mixed reality system, and assessed changes in outcomes after the intervention. Results: The search produced 257 articles, and 46 of them were allocated for data extraction to evaluate 652 patients. Both when VR training was analyzed and reviewed separately, and when compared to traditional training, the findings exhibited predominantly promising outcomes, reflecting a favorable trend in the study. VR technologies were used in different settings and customizations, and the medium total time of VR training among the studies was 60.46 h per patient. Conclusions: This auspicious outcome of the study further motivates the intervention of VR and AR in the rehabilitation of SCI patients along with ameliorating their overall holistic well-being.
1. Introduction
The trajectory of virtual reality (VR) technology, from its inception as a concept to its present-day implementation across various sectors such as healthcare and rehabilitation, has been nothing short of impressive [1]. Though initially created for entertainment purposes like gaming, VR has transformed into an influential therapeutic tool currently used in the medical field, with the potential to bring about life-altering changes for selected patients, especially in the field of spinal cord injury (SCI) rehabilitation [2]. Virtual reality (VR) is still in the early stages of integration into clinical practice, with several barriers slowing its adoption. Key challenges include a lack of time and expertise on how to use VR in treatment, a lack of personalization of some VR applications to patient needs and treatment goals, or a gap in knowledge on the added value of VR in a specific setting. For these technologies to achieve their intended impact, they must be seamlessly integrated into existing healthcare practices and aligned with the needs of patients and healthcare providers [1]. Throughout history, spinal cord injuries have presented considerable hurdles for both patients and healthcare providers, which have frequently led to persistent disability and decreased quality of life [3,4]. Conventional methods used in rehabilitation efforts were moderately successful but failed to address the intricate physical and cognitive challenges that accompany SCI completely [5]. Nevertheless, with the emergence of VR technology, new opportunities exist for improving rehabilitative outcomes while helping those affected by SCI attain greater functional autonomy that enhances their overall health and wellness. VR-based interventions have become increasingly popular in recent years as a means of enhancing traditional rehabilitation techniques for patients with SCI. These cutting-edge approaches utilize the interactive qualities of VR environments to engage patients in personalized exercises, activities, and simulations that specifically target motor skills, sensory abilities, and cognitive functions [6]. By creating realistic situations and delivering immediate feedback, this technology enables individuals dealing with an SCI to safely hone their movements while improving muscle strength and coordination as well as overall sensorimotor integration capabilities [7].
The utilization of virtual reality technology in spinal cord injury rehabilitation is diverse and includes a range of interventions, with assessed benefits that range from motor learning and gait balance to pain management and psychological well-being [8,9]. Patients are immersed in simulated virtual environments through head-mounted displays with motion-tracking sensors, which replicate everyday tasks like walking, reaching, or navigating obstacles [10]. For patients requiring flexibility towards their levels of sensory input or interaction, fully immersive, semi-immersive as well as non-immersive VR setups utilizing screens or projections can be useful alternative approaches. Incorporating VR technology with advanced innovations such as body–machine interface (BMI) and brain–computer interface (BCI) systems not only broadens the scope of customized rehabilitation interventions for SCI patients but also presents new avenues to enhance their recovery [11]. The ability to harness neural signals or brain activity towards controlling virtual avatars or prosthetic devices holds significant potential in improving motor functionality, neuroplasticity, and functional autonomy among individuals suffering from severe spinal cord injuries [12]. Although VR’s potential in SCI rehabilitation is increasingly recognized, more systematic evaluation and synthesis of existing evidence are necessary to determine its efficacy, feasibility, and clinical relevance.
The objective of this systematic review is to conduct a thorough examination and evaluation of the available literature to extensively investigate the effects of virtual reality as a therapeutic tool in spinal cord injury rehabilitation.
2. Methods
This systematic review was conducted according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [13]. The review was not registered.
2.1. Search Strategy
A systematic search was performed in a single database (PubMed/Medline) up to the 1st of March 2024. Articles published from 1 January 2010 to 1 February 2024 were included in the search. MESH terms and keywords were combined in the following search strategy: (Augmented Reality OR VR OR Virtual Reality) AND (Spine OR Spinal) AND Rehabilitation. A language filter was applied to include only articles written in English.
2.2. Eligibility Criteria
The inclusion criteria of the selected studies were as follows: (1) written in English, (2) comprised adults (>18 years old) with SCI, (3) included an intervention with VR, augmented reality (AR), or any mixed reality system, and (4) assessed changes in outcomes after the intervention.
All study designs were deemed eligible for inclusion, except for reviews or meta-analyses.
Three reviewers (M.S., T.S.B., and C.Z.) independently screened articles for inclusion, extracted data, and evaluated the methodological quality of the trials. Conflicts were resolved by a senior author.
2.3. Data Extraction
The data were entered in a customized Excel® (Version 16.89) spreadsheet. Data included studies’ characteristics (authors, time of publication, continent/country, and journal of publication); methodological details (study design, type of VR intervention, and duration of VR training as hours/week, hours/month, and total hours); and information on participants characteristics, VR effects, and outcome measurements.
Overall, this review comprises studies in which VR system training was compared to traditional training, as well as studies in which outcomes were assessed after patients underwent “VR training” or “VR + BMI/BCI” or “VR + conventional therapy” (with no direct comparison to traditional training).
2.4. Risk of Bias Assessment
This study was thoroughly assessed for potential sources of bias that could impact the validity of its findings. Three independent reviewers (M.S., T.S.B., and C.Z.) examined the articles, with each article being reviewed by a different individual. Any discrepancies were resolved by senior reviewers (A.S. and D.C.).
No specific tools were used to assess the methodological quality of the studies.
Although each analyzed study had errors, they could not be addressed or corrected. The cumulative risks associated with individual extractions could not be resolved.
3. Results
3.1. Selection of Studies
The search produced 257 articles. In total, 205 were excluded due to unmatching titles or abstracts. A total of 52 underwent full-text review, and 6 articles were excluded for either wrong intervention (n = 2), wrong settings (n = 3), or wrong population (n = 1). In conclusion, 46 articles fulfilled the inclusion criteria and were selected for the systematic review.
Figure 1 shows the PRISMA 2020 flow diagram for systematic reviews with the selection process of studies identified and included.
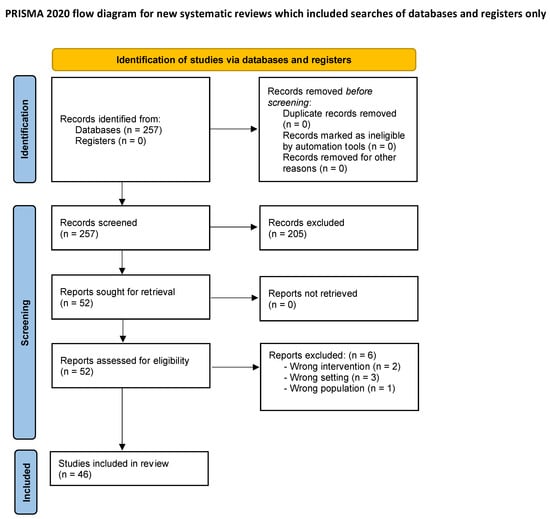
Figure 1.
PRISMA flow diagram for systematic reviews.
3.2. Participant Characteristics
Data from the 46 articles were extracted, and, considering only patients with evaluation of outcome data, the sample included 652 patients.
A majority of 478 were men, 162 were women, and the sex of about 12 patients was not specified. (478 M; 162 F; 12 not specified (n.s.))
The mean age of patients who underwent intervention was 44.47 and ranged greatly between different studies spanning from 23 to 60 years old (41.5 ± 18.5).
The American Spinal Injury Association (ASIA) impairment scale or AIS [14] was used to categorize patients based on their SCI type.
Table 1 contains all the information on the characteristics of the studies, including patients’ characteristics.

Table 1.
Characteristics of the studies, including patients’ characteristics (age, gender, and level of injury).
3.3. Design of the Studies
Among the 46 studies analyzed, 23 are clinical trials, 6 are case reports, 8 have a pretest–posttest design, 1 has an interrupted time series design, 1 has a concurrent nested design, 1 is a longitudinal pilot study, 1 is a correlational study, 1 is a case–control study, 1 is a single-arm study of neuroimaging data, 1 is a preliminary study, and 2 have experimental designs that were not specified.
Another aspect that deserves attention is the sample size, which ranged from 1 to 59 participants.
3.4. VR Characteristics
VR technologies used in different settings and studies can be categorized into immersive VR (18 articles), semi-immersive VR (10 articles), non-immersive VR (13 articles), and a combination of VR and BMI/BCI systems (5 articles).
3.5. Time of Training
Duration of the training was considered as hours/week, hours/month, and total hours per patient. The medium total time of VR training among the studies was 60.44 h per patient. This average is calculated among 40 articles of the 46 included: 6 studies did not specify the total hours of training for patients (the names being Al Nattah et al., 2024 [16], Casadio et al., 2011 [20], Putrino et al., 2021 [45], Jordan et al., 2016 [55], Trincado-Alonso et al., 2014 [59], and Tamplin et al., 2020 [60]). Among the considered studies, two present with notable outliers compared to the others: Donati et al., 2016 [54] had a total training time of 1958 h while Miguel A.L. Nicolelis et al., 2022 [36] reported a total training time of 118 h, where the median value is 12 h of training.
The intervals between VR-based interventions varied between studies, with a frequency ranging from two to five times a week, with sessions lasting from 20 to 150 min. However, some studies did not clearly report the number of sessions.
3.6. Outcome Measures
In the selected studies, a wide variety of outcome measures were assessed. To analyze the results, they have been categorized into five different subgroups: (1) upper limb mobility/strength; (2) lower limb mobility, postural stability, gait and walking, and sitting balance; (3) quality of life, daily life independence, functional/cognitive ability, and psychological outcomes; (4) pain; and (5) other outcome measures and feasibility of the VR system.
Further details about the scales used to assess VR use’s outcomes can be found in the respective tables.
Considering the whole five subgroups of the outcome measures, the analysis/review demonstrated overall positive results of VR training both compared to traditional training and considered independently.
Few of the studies found no statistically significant difference in outcomes between VR and traditional systems, but no article assessed inferior results for VR compared to traditional training. Even when there was no statistically significant difference in outcomes between VR and conventional therapy, there were still better results in the experimental group (EG) compared to the control group (CG), only not enough to be considered statistically significant, which could be related to the population of patients selected by the studies.
To ensure a clearer understanding of these results, studies comparing VR training with traditional therapy will be labeled as “comparative studies”, in both the written content and tables.
Upper limb mobility and strength were evaluated in seven studies through 12 different outcome scales, with overall positive results of VR training both compared to traditional training and considered individually. Few of the studies found no significant difference in upper limb (UL) outcomes between VR and traditional systems, but no article assessed inferior results for VR compared to traditional training. Taking comparative studies into consideration, 27% of the tests defined a better outcome compared to traditional training, especially when “muscle strength” was tested.
In studies without a comparison group, 100% of tests saw a significant improvement related to VR training when compared to baseline.
All specific data are outlined in Table 2.

Table 2.
Upper limb mobility and strength.
Lower limb mobility, gait and walking, postural stability, and balance were evaluated in 18 studies through 20 different outcomes. Even for lower limbs (LLs), the overall results were positive, both comparing VR to traditional training and considering VR individually. None of the studies, however, demonstrated an inferior performance of VR systems for the explored purposes.
Focusing on comparative studies, 71% of the tests defined a better outcome compared to traditional training.
In the other studies, 80% of scales saw a significant improvement with VR training when compared to baseline.
All specific data are outlined in Table 3.

Table 3.
Lower limb mobility, gait and walking, postural stability, and balance.
Quality of life, daily life independence, functional/cognitive ability, and psychological outcomes were evaluated in 17 studies through 21 different outcome scales. In total, 10 studies were comparative ones, and 56% of the tests analyzed found a statistically significant improvement in the assessed measures compared to the control group. The remaining 7 articles reported outstanding results, with 100% of the tests improving after VR training compared to baseline.
All specific data are outlined in Table 4.

Table 4.
Quality of life, daily life independence, functional/cognitive ability, and psychological outcomes.
Pain was evaluated in 12 studies through seven different outcome scales. Among these studies, 3 articles compared the efficacy of VR systems in pain management with control groups and found a significant improvement in 100% of tests. The remaining 9 articles with no control groups demonstrated positive results in pain control after the use of VR in their experimental group across 66.6% of tests.
All specific data are outlined in Table 5.

Table 5.
Pain.
Other outcome measures and feasibility of the VR system: The last subgroup comprises scales assessing various outcomes such as the feasibility of the VR system, changes in body ownership, perceived exertion using the system, changes in sensory parameters, absence of side effects, improvement of driving abilities, improved effectiveness of BCI, use of VR in diagnostics and assessment, perceived interaction and immersion, and motor imagery. These have been grouped into 14 different groups of parameters, ranging from the feasibility of the VR system to side effects of VR use and quality of the perceived VR environment, changes in body ownership and changes in sensory parameters, brain/brainstem plasticity, and improvement in driving abilities after using a dedicated simulator.
Two of these studies assessed the respective outcomes with the comparison with a control group, and better results in VR-assisted training were found across 100% of criteria, encouraging the use of VR systems. Twelve studies assessed the experimental group, without a control group, across various parameters: in 93.3% of cases, the use of VR systems had a positive impact on the outcome measured. Notably, the only negative result, reported by Pozeg et al., 2017 [50], is merely an absence of significant improvement in one of the outcomes measured (full body illusion) after the intervention.
All specific data are outlined in Table 6.

Table 6.
Other outcome measures and feasibility of the VR system.
4. Discussion
The current literature comprises different reviews on the examined topic; however, this is the first of its kind to refine a holistic analysis comprising all outcomes associated with VR rehabilitation. We indeed take into consideration a whole range of different benefits and changes related to the rehabilitation: upper limb mobility/strength; lower limb mobility, postural stability, gait and walking, and sitting balance; quality of life, daily life independence, functional/cognitive ability, and psychological outcome; pain; and other outcome measures and feasibility of the VR system. Upper and lower limb mobility and strength are surely the most analyzed outcomes in previous reviews [7,34,61,62,63,64], together with pain changes [41,65]. In this review we try to implement different and new points of view, analyzing outcomes in quality of life, psychological well-being, and variables that measure the feasibility of different VR systems.
The introduction of VR technology in spinal rehabilitation is a big step from traditional methods. Conventional treatments are moderately successful but do not adequately cater to the complex cognitive and physical challenges associated with SCI like VR-based interventions do. Unlike them, these types of therapy are more holistic and interesting, thus improving the outcomes of rehabilitation as well as functional independence among patients. Each of the outcomes was categorized into one of the five different subgroups and fully analyzed.
4.1. Upper Limb Mobility and Strength
For upper limb rehabilitation, setting VR and traditional training side by side, this review shows confident results mostly when “Muscle Strength” was studied, with 100% of the related scales showing better outcomes for VR. On the contrary, “Hand dexterity” measures showed no valuable difference between the two training types.
When taking the duration of training into account, we noticed that patients with a higher total number of training hours (especially when higher than 15 h) underwent better outcomes overall compared to patients with shorter periods of rehabilitation. This emphasizes the need, particularly for mobility and strength effects, to set a higher baseline as standard hours of VR training.
4.2. Lower Limb Mobility, Postural Stability, Gait and Walking, and Sitting Balance
The second group was the most represented one of all, with 18 studies evaluating lower limbs’ strength together with physical mobility and balance. A highly positive trend was observed when analyzing data from these studies, and this probably depicts one of the best results shown, especially considering that body mobility and balance are among the outcomes patients prioritize the most.
Among the comparative studies, only one (Sengupta et al., 2020 [25]) assessed no significant difference in lower limb outcomes between VR and conventional therapy; however, the p value for this assessment being p = 0.001 is surely a limitation to consider.
4.3. Quality of Life, Daily Life Independence, Functional/Cognitive Ability, and Psychological Outcomes
The general quality of life improvements constitute the starting point when assessing a rehabilitation program; in this review, we tried to implement and consider all the different aspects that influence a person’s well-being other than mobility and strength. “Daily life Independence” is surely one of the main factors to consider; on this topic, our evaluation displays quite optimistic results, enhancing the potential role of VR training in restoring dignity and liberty for patients with SCI.
A quite new point of view analyzed here is represented by the “Psychological changes and outcomes” related to rehabilitation. Patients with SCI often suffer from mild to severe types of mood disorders, with depression or depressive-like behaviors being the most common; it becomes fundamental at this point to try and observe the differences between disparate kinds of training in influencing patients’ mood. The potential benefits of VR training in enhancing the psychological well-being of SCI patients are mainly related to the higher level of enjoyment shown when compared to traditional training, especially when immersive or semi-immersive settings are used. On top of that, game-like systems are frequently incorporated in VR exercises, and this firmly contributes to the positive impact of training on patients’ spirits.
4.4. Pain
Taking comparative studies into consideration, the “Pain” measure had the most astonishing results, with 100% of scales assessing a better outcome for VR settings compared to traditional ones. The three most used scales to assess neuropathic pain were the Neuropathic pain intensity Numeric Rating Scale (NRS), the Neuropathic Pain Scale (NPS), and the Visual Analog Scale (VAS), and all three of them revealed significant improvements in most of the studies, and also when comparing VR training alone to the baseline. When looking at the articles that considered pain variables as outcomes, the duration of training varies significantly, from 0.05 to 13.5 total hours, though it does not result in a consistent related difference in results. Pain reduction remains another cardinal goal of rehabilitation, with these results thus strengthening the position of VR settings in current recovery programs.
4.5. Other Outcome Measures and Feasibility of the VR System
One more novelty of this review stands in reassessing a whole variety of different outcomes, seemingly unrelated, but quite important when assessing the feasibility of the usage of VR systems in rehabilitation programs. Through gathering these various scales, the main purpose was to report the level of practicality and viability of these technologies, with current results that show an overwhelmingly positive trend.
4.6. Limitations of the Study
The application of VR in SCI rehabilitation still has some drawbacks despite its positive results. One major point is that different studies used various VR systems and rehabilitation protocols producing issues with treatment standardization and outcome comparison. Another limitation lies in the time taken for each session, which ranges from 20 to 150 min, and training frequency, fluctuating from 2 to 5 times/week. This makes it challenging to define what is the best amount of VR therapy that one needs to achieve maximum benefits. Besides this, cost implications together with the need for specialized training among healthcare providers act as barriers to achieving widespread implementation.
VR-based rehabilitation is estimated to be more effective and accessible in future developments. Artificial intelligence (AI) systems with machine learning (ML) algorithms can provide custom adaptive programs working together with virtual reality. Real-time patient performance tracking abilities of this technology allow for adjusting task complexity levels to always keep the patients at the highest possible challenge point to maximize therapeutic gains. This can be analogized with neuromonitoring that has been commonly used during neurosurgery, and these advanced VR methodologies in question also have the potential to benefit patients who had tumor-resection surgeries and need rehabilitation post-operatively [66]. Portable wearable devices combined with wireless sensors might also enhance convenience when using VR systems for home-based recovery purposes. This transition would be very beneficial, especially for those individuals who cannot move around freely due to limited mobility or living far away from hospitals. Also, it could be even more effective if augmented reality (AR) was used alongside virtual reality in creating mixed-reality environments, where patients can seamlessly shift between tasks completed within these two worlds. If such integration happened, then skills acquired through simulated environments would easily transfer into real-life situations thereby further improving functional outcomes.
5. Conclusions
The use of VR has the potential to be a game-changer for SCI rehabilitation. It increases motor and cognitive functions better than traditional methods, particularly when used in conjunction with other non-invasive treatments. In saying that, there are still some problems that need to be solved before it can be widely adopted such as the inconsistency between VR systems and protocols, the duration of sessions, the frequency of sessions, and high costs that make standardization difficult. In the future, we may see more personalized adaptive programs that integrate AI, ML, and wearable technology thereby enhancing therapy outcomes through real-time adjustment of task difficulty based on patient performance.
Author Contributions
Conceptualization and methodology: M.S., T.S.B., C.Z., A.S., R.S., and D.C.; data extraction: M.S., T.S.B., and C.Z.; writing–original draft preparation: M.S., T.S.B., and C.Z.; writing—review and editing: M.S., C.Z., A.S., and D.C. All authors have read and agreed to the published version of the manuscript.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Data Availability Statement
All data are extractable from the references listed in Table 1.
Conflicts of Interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Abbreviations
| AI | artificial intelligence |
| AIS | ASIA impairment scale |
| AR | augmented reality |
| ASIA | American Spinal Injury Association |
| BCI | brain–computer interface |
| BMI | brain–machine interface |
| CG | control group |
| EG | experimental group |
| FBI | full body illusion |
| LL | lower limbs |
| ML | machine learning |
| n.s. | not specified |
| NPS | Neuropathic Pain Scale |
| NRS | Numeric Rating Scale |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| SCI | spinal cord injury |
| UL | upper limbs |
| VAS | Visual Analog Scale |
| VR | virtual reality |
References
- Kouijzer, M.M.T.E.; Kip, H.; Bouman, Y.H.A.; Kelders, S.M. Implementation of virtual reality in healthcare: A scoping review on the implementation process of virtual reality in various healthcare settings. Implement. Sci. Commun. 2023, 4, 67. [Google Scholar] [CrossRef]
- Bateni, H.; Carruthers, J.; Mohan, R.; Pishva, S. Use of virtual reality in physical therapy as an intervention and diagnostic tool. Rehabil. Res. Pract. 2024, 2024, 1122286. [Google Scholar] [CrossRef]
- Bennett, J.; Das, J.M.; Emmady, P.D. Spinal cord injuries. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2024. [Google Scholar]
- Eckert, M.J.; Martin, M.J. Trauma: Spinal cord injury. Surg. Clin. N. Am. 2017, 97, 1031–1045. [Google Scholar] [CrossRef]
- Maggio, M.G.; Bonanno, M.; Manuli, A.; Calabrò, R.S. Improving outcomes in people with spinal cord injury: Encouraging results from a multidisciplinary advanced rehabilitation pathway. Brain Sci. 2024, 14, 140. [Google Scholar] [CrossRef]
- Šlosar, L.; Peskar, M.; Pišot, R.; Marusic, U. Environmental enrichment through virtual reality as multisensory stimulation to mitigate the negative effects of prolonged bed rest. Front. Aging Neurosci. 2023, 15, 1169683. [Google Scholar] [CrossRef]
- Miguel-Rubio, A.D.; Rubio, M.D.; Salazar, A.; Moral-Munoz, J.A.; Requena, F.; Camacho, R.; Lucena-Anton, D. Is virtual reality effective for balance recovery in patients with spinal cord injury? A systematic review and meta-analysis. J. Clin. Med. 2020, 9, 2861. [Google Scholar] [CrossRef]
- Leemhuis, E.; Esposito, R.M.; De Gennaro, L.; Pazzaglia, M. Go virtual to get real: Virtual reality as a resource for spinal cord treatment. Int. J. Environ. Res. Public Health 2021, 18, 1819. [Google Scholar] [CrossRef]
- Riva, G.; Wiederhold, B.K.; Mantovani, F. Neuroscience of virtual reality: From virtual exposure to embodied medicine. Cyberpsychol. Behav. Soc. Netw. 2019, 22, 82–96. [Google Scholar] [CrossRef]
- Kizony, R.; Raz, L.; Katz, N.; Weingarden, H.; Weiss, P.L.T. Video-capture virtual reality system for patients with paraplegic spinal cord injury. J. Rehabil. Res. Dev. 2005, 42, 595–608. [Google Scholar] [CrossRef]
- De Miguel-Rubio, A.; Gallego-Aguayo, I.; De Miguel-Rubio, M.D.; Arias-Avila, M.; Lucena-Anton, D.; Alba-Rueda, A. Effectiveness of the combined use of a brain-machine interface system and virtual reality as a therapeutic approach in patients with spinal cord injury: A systematic review. Healthcare 2023, 11, 3189. [Google Scholar] [CrossRef]
- Awuah, W.A.; Ahluwalia, A.; Darko, K.; Sanker, V.; Tan, J.K.; Tenkorang, P.O.; Ben-Jaafar, A.; Ranganathan, S.; Aderinto, N.; Mehta, A.; et al. Bridging minds and machines: The recent advances of brain-computer interfaces in neurological and neurosurgical applications. World Neurosurg. 2024, 189, 138–153. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Roberts, T.T.; Leonard, G.R.; Cepela, D.J. Classifications in brief: American spinal injury association (ASIA) impairment scale. Clin. Orthop. Relat. Res. 2017, 475, 1499–1504. [Google Scholar] [CrossRef]
- Lim, D.Y.; Hwang, D.M.; Cho, K.H.; Moon, C.W.; Ahn, S.Y. A fully immersive virtual reality method for upper limb rehabilitation in spinal cord injury. Ann. Rehabil. Med. 2020, 44, 311–319. [Google Scholar] [CrossRef]
- Al Nattah, M.M.A.; Tiberti, S.; Segaletti, L. Semi-immersive virtual reality exercise therapy for upper limb rehabilitation in patients with spinal cord injury using the leap motion controller. Cureus 2024, 16, e52261. [Google Scholar] [CrossRef]
- Prasad, S.; Aikat, R.; Labani, S.; Khanna, N. Efficacy of virtual reality in upper limb rehabilitation in patients with spinal cord injury: A pilot randomized controlled trial. Asian Spine J. 2018, 12, 927–934. [Google Scholar] [CrossRef]
- Dimbwadyo-Terrer, I.; Gil-Agudo, A.; Segura-Fragoso, A.; De Los Reyes-Guzman, A.; Trincado-Alonso, F.; Piazza, S.; Polonio-López, B. Effectiveness of the virtual reality system toyra on upper limb function in people with tetraplegia: A pilot randomized clinical trial. BioMed Res. Int. 2016, 2016, 6397828. [Google Scholar] [CrossRef]
- Dimbwadyo-Terrer, I.; Trincado-Alonso, F.; de Los Reyes-Guzmán, A.; Aznar, M.A.; Alcubilla, C.; Pérez-Nombela, S.; del Ama-Espinosa, A.; Polonio-López, B.; Gil-Agudo, Á. Upper limb rehabilitation after spinal cord injury: A treatment based on a data glove and an immersive virtual reality environment. Disabil. Rehabil. Assist. Technol. 2016, 11, 462–467. [Google Scholar] [CrossRef]
- Casadio, M.; Pressman, A.; Acosta, S.; Danzinger, Z.; Fishbach, A.; Mussa-Ivaldi, F.A.; Muir, K.; Tseng, H.; Chen, D. Body machine interface: Remapping motor skills after spinal cord injury. IEEE Int. Conf. Rehabil. Robot. 2011, 2011, 5975384. [Google Scholar] [CrossRef]
- Bressi, F.; Cricenti, L.; Bravi, M.; Pannunzio, F.; Cordella, F.; Lapresa, M.; Miccinilli, S.; Santacaterina, F.; Zollo, L.; Sterzi, S.; et al. Treatment of the paretic hand with a robotic glove combined with physiotherapy in a patient suffering from traumatic tetraparesis: A case report. Sensors 2023, 23, 3484. [Google Scholar] [CrossRef]
- Nair, M.S.; Kulkarni, V.N.; Shyam, A.K. Combined effect of virtual reality training (VRT) and conventional therapy on sitting balance in patients with spinal cord injury (SCI): Randomized control trial. Neurol. India 2022, 70, S245–S250. [Google Scholar] [CrossRef] [PubMed]
- Goel, T.; Sharma, N.; Gehlot, A.; Srivastav, A.K. Effectiveness of immersive virtual reality training to improve sitting balance control among individuals with acute and sub-acute paraplegia: A randomized clinical trial. J. Spinal Cord Med. 2023, 46, 964–974. [Google Scholar] [CrossRef] [PubMed]
- Manzanares, A.; Camblor, Á.; Romero-Arenas, S.; Segado, F.; Gil-Arias, A. Effect of a semi-immersive virtual reality navigation therapy on quality of life in persons with spinal cord injury. Disabil. Rehabil. Assist. Technol. 2021, 18, 730–735. [Google Scholar] [CrossRef]
- Sengupta, M.; Gupta, A.; Khanna, M.; Rashmi Krishnan, U.K.; Chakrabarti, D. Role of virtual reality in balance training in patients with spinal cord injury: A prospective comparative pre-post study. Asian Spine J. 2020, 14, 51–58. [Google Scholar] [CrossRef]
- Khurana, M.; Walia, S.; Noohu, M.M. Study on the effectiveness of virtual reality game-based training on balance and functional performance in individuals with paraplegia. Top. Spinal Cord Inj. Rehabil. 2017, 23, 263–270. [Google Scholar] [CrossRef]
- An, Y.; Park, C. The effects of virtual soccer game on balance, gait function, and kick speed in chronic incomplete spinal cord injury: A randomized controlled trial. Spinal Cord 2022, 60, 504–509. [Google Scholar] [CrossRef]
- Villiger, M.; Liviero, J.; Awai, L.; Stoop, R.; Pyk, P.; Clijsen, R.; Curt, A.; Eng, K.; Bolliger, M. Home-based virtual reality-augmented training improves lower limb muscle strength, balance, and functional mobility following chronic incomplete spinal cord injury. Front. Neurol. 2017, 8, 635. [Google Scholar] [CrossRef] [PubMed]
- An, C.; Park, Y. The effects of semi-immersive virtual reality therapy on standing balance and upright mobility function in individuals with chronic incomplete spinal cord injury: A preliminary study. J. Spinal Cord Med. 2018, 41, 223–229. [Google Scholar] [CrossRef]
- Wall, T.; Feinn, R.; Chui, K.; Cheng, M.S. The effects of the nintendo™ wii fit on gait, balance, and quality of life in individuals with incomplete spinal cord injury. J. Spinal Cord Med. 2015, 38, 777–783. [Google Scholar] [CrossRef][Green Version]
- Duffell, L.D.; Paddison, S.; Alahmary, A.F.; Donaldson, N.; Burridge, J. The effects of FES cycling combined with virtual reality racing biofeedback on voluntary function after incomplete SCI: A pilot study. J. Neuroeng. Rehabil. 2019, 16, 149. [Google Scholar] [CrossRef]
- Villiger, M.; Bohli, D.; Kiper, D.; Pyk, P.; Spillmann, J.; Meilick, B.; Curt, A.; Hepp-Reymond, M.-C.; Hotz-Boendermaker, S.; Eng, K. Virtual reality-augmented neurorehabilitation improves motor function and reduces neuropathic pain in patients with incomplete spinal cord injury. Neurorehabil. Neural Repair 2013, 27, 675–683. [Google Scholar] [CrossRef]
- Villiger, M.; Grabher, P.; Hepp-Reymond, M.; Kiper, D.; Curt, A.; Bolliger, M.; Hotz-Boendermaker, S.; Kollias, S.; Eng, K.; Freund, P. Relationship between structural brainstem and brain plasticity and lower-limb training in spinal cord injury: A longitudinal pilot study. Front. Hum. Neurosci. 2015, 9, 254. [Google Scholar] [CrossRef]
- Lee, M.; Lee, S. The effect of virtual reality exercise program on sitting balance ability of spinal cord injury patients. Healthcare 2021, 9, 183. [Google Scholar] [CrossRef]
- Maresca, G.; Maggio, M.G.; Buda, A.; La Rosa, G.; Manuli, A.; Bramanti, P.; De Luca, R.; Calabrò, R.S. A novel use of virtual reality in the treatment of cognitive and motor deficit in spinal cord injury: A case report. Medicine 2018, 97, e13559. [Google Scholar] [CrossRef]
- Nicolelis, M.A.L.; Alho, E.J.L.; Donati, A.R.C.; Yonamine, S.; Aratanha, M.A.; Bao, G.; Campos, D.S.F.; Almeida, S.; Fischer, D.; Shokur, S. Training with noninvasive brain–machine interface, tactile feedback, and locomotion to enhance neurological recovery in individuals with complete paraplegia: A randomized pilot study. Sci. Rep. 2022, 12, 20545. [Google Scholar] [CrossRef]
- van Dijsseldonk, R.B.; de Jong, L.A.F.; Groen, B.E.; Vos-van der Hulst, M.; Geurts, A.C.H.; Keijsers, N.L.W. Gait stability training in a virtual environment improves gait and dynamic balance capacity in incomplete spinal cord injury patients. Front. Neurol. 2018, 9, 963. [Google Scholar] [CrossRef]
- Zimmerli, L.; Jacky, M.; Lünenburger, L.; Riener, R.; Bolliger, M. Increasing patient engagement during virtual reality-based motor rehabilitation. Arch. Phys. Med. Rehabil. 2013, 94, 1737–1746. [Google Scholar] [CrossRef]
- Michibata, A.; Haraguchi, M.; Murakawa, Y.; Ishikawa, H. Electrical stimulation and virtual reality-guided balance training for managing paraplegia and trunk dysfunction due to spinal cord infarction. BMJ Case Rep. 2022, 15, e244091. [Google Scholar] [CrossRef]
- Maggio, M.G.; Bonanno, M.; Manuli, A.; Onesta, M.P.; De Luca, R.; Quartarone, A.; Calabrò, R.S. Do individuals with spinal cord injury benefit from semi-immersive virtual reality cognitive training? preliminary results from an exploratory study on an underestimated problem. Brain Sci. 2023, 13, 945. [Google Scholar] [CrossRef]
- Azurdia, D.; Acuña, S.M.; Narasaki-Jara, M.; Furtado, O.J.; Jung, T. Effects of virtual reality-based aerobic exercise on perceptions of pain and fatigue in individuals with spinal cord injury. Games Health J. 2022, 11, 236–241. [Google Scholar] [CrossRef]
- Trost, Z.; Anam, M.; Seward, J.; Shum, C.; Rumble, D.; Sturgeon, J.; Mark, V.; Chen, Y.; Mitchell, L.D.; Cowan, R.; et al. Immersive interactive virtual walking reduces neuropathic pain in spinal cord injury: Findings from a preliminary investigation of feasibility and clinical efficacy. Pain 2022, 163, 350–361. [Google Scholar] [CrossRef]
- Austin, P.D.; Craig, A.; Middleton, J.W.; Tran, Y.; Costa, D.S.J.; Wrigley, P.J.; Siddall, P.J. The short-term effects of head-mounted virtual-reality on neuropathic pain intensity in people with spinal cord injury pain: A randomised cross-over pilot study. Spinal Cord 2021, 59, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Lakhani, A.; Martin, K.; Gray, L.; Mallison, J.; Grimbeek, P.; Hollins, I.; Mackareth, C. What is the impact of engaging with natural environments delivered via virtual reality on the psycho-emotional health of people with spinal cord injury receiving rehabilitation in hospital? findings from a pilot randomized controlled trial. Arch. Phys. Med. Rehabil. 2020, 101, 1532–1540. [Google Scholar] [CrossRef] [PubMed]
- Putrino, D.; Tabacof, L.; Breyman, E.; Revis, J.; Soomro, Z.; Chopra, D.; Delaney, K.; Smeragliuolo, A.; Cortes, M. Pain reduction after short exposure to virtual reality environments in people with spinal cord injury. Int. J. Environ. Res. Public Health 2021, 18, 8923. [Google Scholar] [CrossRef] [PubMed]
- Richardson, E.J.; McKinley, E.C.; Rahman, A.K.M.F.; Klebine, P.; Redden, D.T.; Richards, J.S. Effects of virtual walking on spinal cord injury-related neuropathic pain: A randomized, controlled trial. Rehabil. Psychol. 2019, 64, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Solcà, M.; Krishna, V.; Young, N.; Deogaonkar, M.; Herbelin, B.; Orepic, P.; Mange, R.; Rognini, G.; Serino, A.; Rezai, A.; et al. Enhancing analgesic spinal cord stimulation for chronic pain with personalized immersive virtual reality. Pain 2021, 162, 1641–1649. [Google Scholar] [CrossRef]
- Trujillo, M.S.; Alvarez, A.F.; Nguyen, L.; Petros, J. Embodiment in virtual reality for the treatment of chronic low back pain: A case series. J. Pain Res. 2020, 13, 3131–3137. [Google Scholar] [CrossRef]
- Pais-Vieira, C.; Gaspar, P.; Matos, D.; Alves, L.P.; da Cruz, B.M.; Azevedo, M.J.; Gago, M.; Poleri, T.; Perrotta, A.; Pais-Vieira, M. Embodiment comfort levels during motor imagery training combined with immersive virtual reality in a spinal cord injury patient. Front. Hum. Neurosci. 2022, 16, 909112. [Google Scholar] [CrossRef]
- Pozeg, P.; Palluel, E.; Ronchi, R.; Solcà, M.; Al-Khodairy, A.-W.; Jordan, X.; Kassouha, A.; Blanke, O. Virtual reality improves embodiment and neuropathic pain caused by spinal cord injury. Neurology 2017, 89, 1894–1903. [Google Scholar] [CrossRef]
- Gustin, S.M.; Bolding, M.; Willoughby, W.; Anam, M.; Shum, C.; Rumble, D.; Mark, V.W.; Mitchell, L.; Cowan, R.E.; Richardson, E.; et al. Cortical mechanisms underlying immersive interactive virtual walking treatment for amelioration of neuropathic pain after spinal cord injury: Findings from a preliminary investigation of thalamic inhibitory function. J. Clin. Med. 2023, 12, 5743. [Google Scholar] [CrossRef]
- Roosink, M.; Robitaille, N.; Jackson, P.L.; Bouyer, L.J.; Mercier, C. Interactive virtual feedback improves gait motor imagery after spinal cord injury: An exploratory study. Restor. Neurol. Neurosci. 2016, 34, 227–235. [Google Scholar] [CrossRef]
- Shokur, S.; Gallo, S.; Moioli, R.C.; Donati, A.R.C.; Morya, E.; Bleuler, H.; Nicolelis, M.A. Assimilation of virtual legs and perception of floor texture by complete paraplegic patients receiving artificial tactile feedback. Sci. Rep. 2016, 6, 32293. [Google Scholar] [CrossRef]
- Donati, A.R.C.; Shokur, S.; Morya, E.; Campos, D.S.F.; Moioli, R.C.; Gitti, C.M.; Augusto, P.B.; Tripodi, S.; Pires, C.G.; Pereira, G.A.; et al. Long-term training with a brain-machine interface-based gait protocol induces partial neurological recovery in paraplegic patients. Sci. Rep. 2016, 6, 30383. [Google Scholar] [CrossRef]
- Jordan, M.; Richardson, E.J. Effects of virtual walking treatment on spinal cord injury-related neuropathic pain: Pilot results and trends related to location of pain and at-level neuronal hypersensitivity. Am. J. Phys. Med. Rehabil. 2016, 95, 390–396. [Google Scholar] [CrossRef]
- Sung, W.; Chiu, T.; Tsai, W.; Cheng, H.; Chen, J. The effect of virtual reality-enhanced driving protocol in patients following spinal cord injury. J. Chin. Med. Assoc. 2012, 75, 600–605. [Google Scholar] [CrossRef][Green Version]
- King, C.E.; Wang, P.T.; Chui, L.A.; Do, A.H.; Nenadic, Z. Operation of a brain-computer interface walking simulator for individuals with spinal cord injury. J. Neuroeng. Rehabil. 2013, 10, 77. [Google Scholar] [CrossRef]
- Ferrero, L.; Quiles, V.; Ortiz, M.; Iáñez, E.; Gil-Agudo, Á.; Azorín, J.M. Brain-computer interface enhanced by virtual reality training for controlling a lower limb exoskeleton. iScience 2023, 26, 106675. [Google Scholar] [CrossRef]
- Trincado-Alonso, F.; Dimbwadyo-Terrer, I.; de los Reyes-Guzmán, A.; López-Monteagudo, P.; Bernal-Sahún, A.; Gil-Agudo, Á. Kinematic metrics based on the virtual reality system toyra as an assessment of the upper limb rehabilitation in people with spinal cord injury. BioMed Res. Int. 2014, 2014, 904985. [Google Scholar] [CrossRef]
- Tamplin, J.; Loveridge, B.; Clarke, K.; Li, Y.; JBerlowitz, D. Development and feasibility testing of an online virtual reality platform for delivering therapeutic group singing interventions for people living with spinal cord injury. J. Telemed. Telecare 2020, 26, 365–375. [Google Scholar] [CrossRef]
- De Miguel-Rubio, A.; Rubio, M.D.; Alba-Rueda, A.; Salazar, A.; Moral-Munoz, J.; Lucena-Anton, D. Virtual reality systems for upper limb motor function recovery in patients with spinal cord injury: Systematic review and meta-analysis. JMIR Mhealth Uhealth 2020, 8, e22537. [Google Scholar] [CrossRef]
- De Miguel-Rubio, A.; Rubio, M.D.; Salazar, A.; Camacho, R.; Lucena-Anton, D. Effectiveness of virtual reality on functional performance after spinal cord injury: A systematic review and meta-analysis of randomized controlled trials. J. Clin. Med. 2020, 9, 2065. [Google Scholar] [CrossRef]
- Yeo, E.; Chau, B.; Chi, B.; Ruckle, D.E.; Ta, P. Virtual reality neurorehabilitation for mobility in spinal cord injury: A structured review. Innov. Clin. Neurosci. 2019, 16, 13–20. [Google Scholar]
- Abou, L.; Vonjiniaina, D.M.; Yarnot, R.; Alluri, A.; Rice, L.A. Effects of virtual reality therapy on gait and balance among individuals with spinal cord injury: A systematic review and meta-analysis. Neurorehabil. Neural Repair. 2020, 34, 375–388. [Google Scholar] [CrossRef]
- Chi, B.; Chau, B.; Yeo, E.; Ta, P. Virtual reality for spinal cord injury-associated neuropathic pain: Systematic review. Ann. Phys. Rehabil. Med. 2019, 62, 49–57. [Google Scholar] [CrossRef]
- Cannizzaro, D.; Mancarella, C.; Nasi, D.; Tropeano, M.P.; Anania, C.D.; Cataletti, G.; Milani, D.; Fava, E.M.; Ghadirpour, R.; Costa, F.; et al. Intramedullary spinal cord tumors: The value of intraoperative neurophysiological monitoring in a series of 57 cases from two italian centers. J. Neurosurg. Sci. 2022, 66, 447–455. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).