Heart Rate Variability and Global Longitudinal Strain for Prognostic Evaluation and Recovery Assessment in Conservatively Managed Post-Myocardial Infarction Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Heart Rate Variability Analysis and Cardiac Performance Evaluation
2.3. Statistical Analysis
3. Results
3.1. Study Group Description
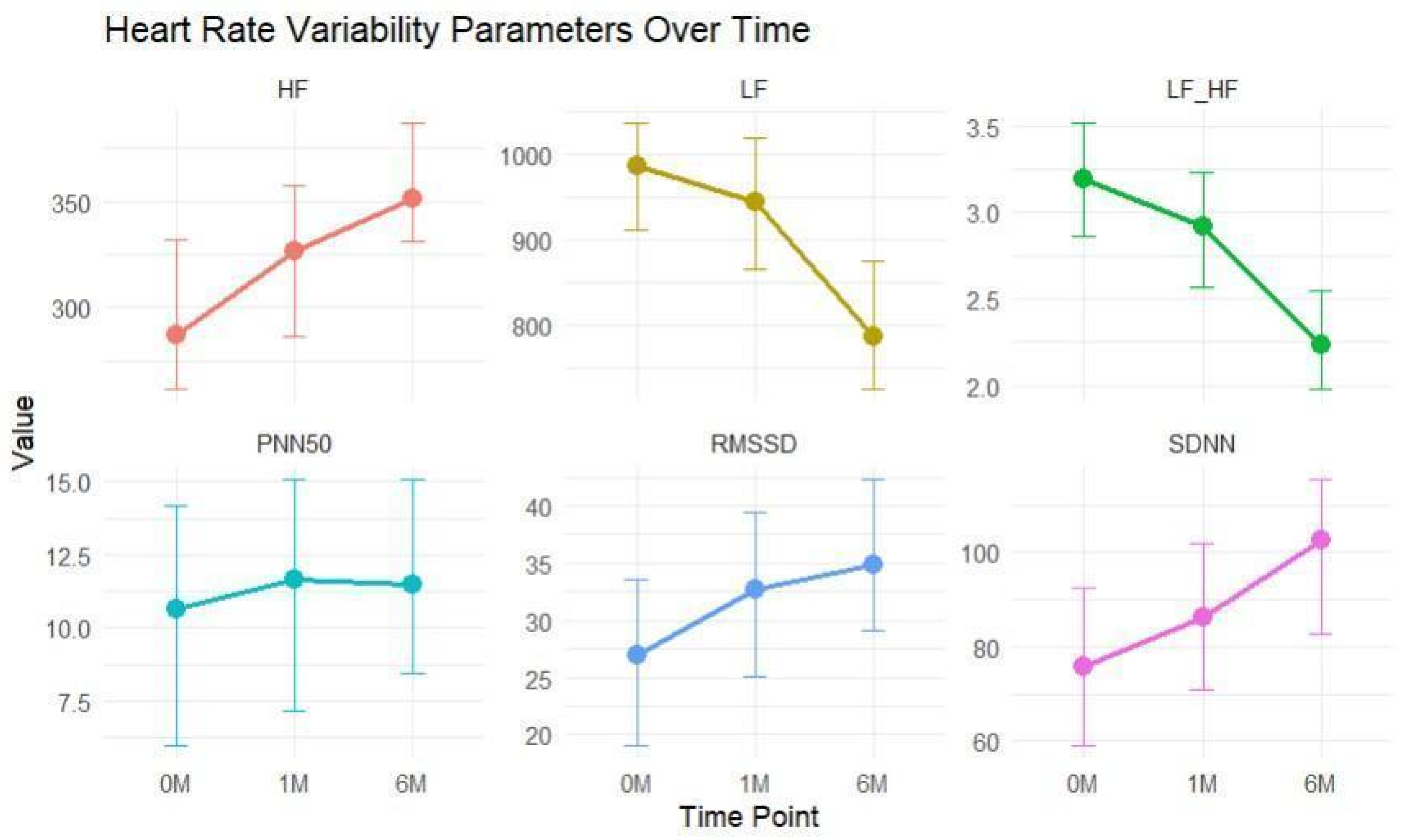
3.2. HRV Analysis
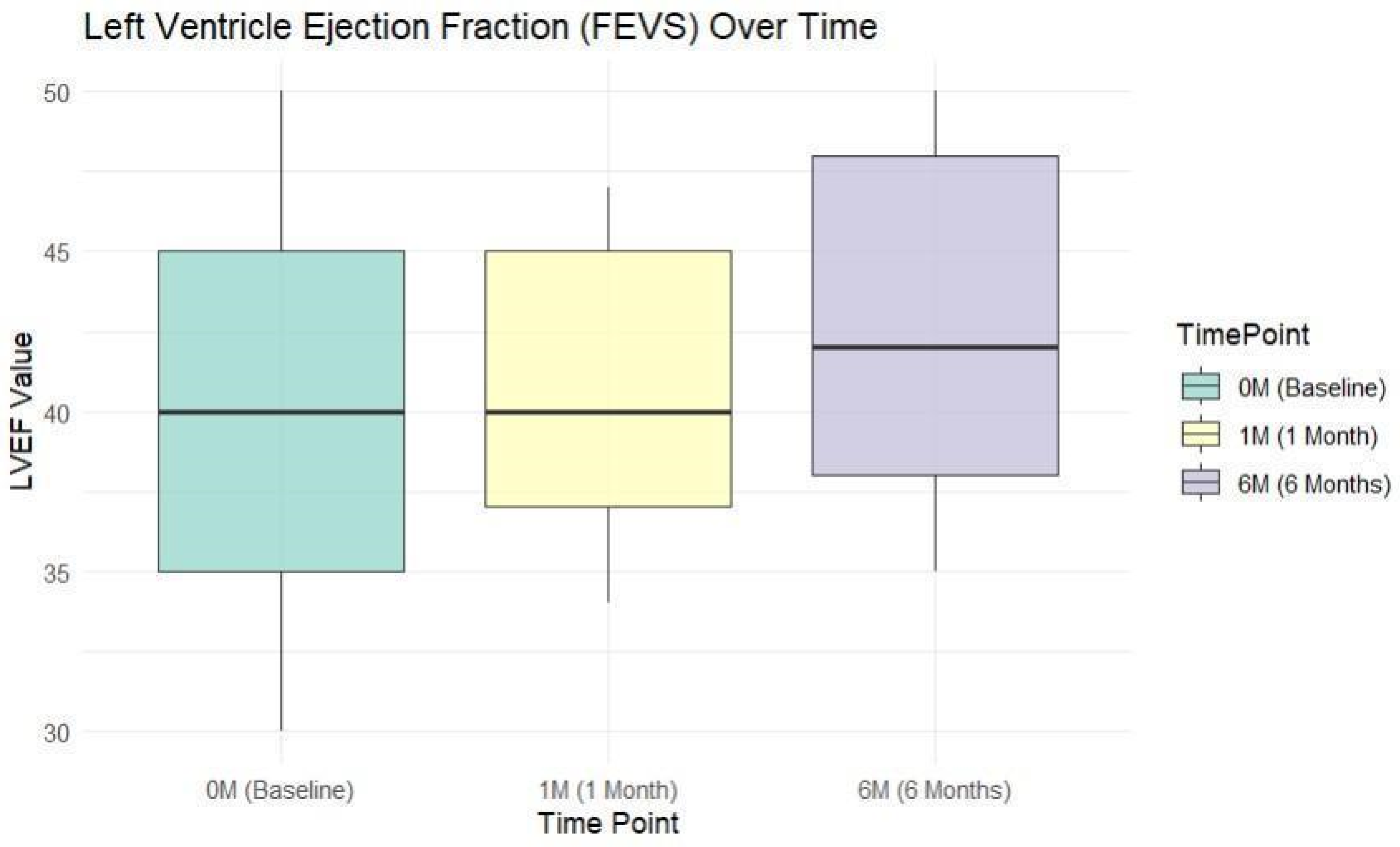
3.3. Cardiac Performance Analysis
3.4. Associations between HRV, Cardiac Performance Analysis, and Different Risk Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wehrwein, E.A.; Orer, H.S.; Barman, S.M. Overview of the Anatomy, Physiology, and Pharmacology of the Autonomic Nervous System. Compr. Physiol. 2016, 6, 1239–1278. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, N.L.; Ribeiro, F.; Alves, A.J.; Teixeira, M.; Miranda, F.; Oliveira, J. Heart Rate Variability in Myocardial Infarction Patients: Effects of Exercise Training. Rev. Port. Cardiol. 2013, 32, 687–700. [Google Scholar] [CrossRef] [PubMed]
- Hayano, J.; Ueda, N.; Kisohara, M.; Yuda, E.; Carney, R.M.; Blumenthal, J.A. Survival Predictors of Heart Rate Variability after Myocardial Infarction with and without Low Left Ventricular Ejection Fraction. Front. Neurosci. 2021, 15, 610955. [Google Scholar] [CrossRef]
- Timmis, A.; Vardas, P.; Townsend, N.; Torbica, A.; Katus, H.; De Smedt, D.; Gale, C.P.; Maggioni, A.P.; Petersen, S.E.; Huculeci, R.; et al. European Society of Cardiology: Cardiovascular Disease Statistics 2021. Eur. Heart J. 2022, 43, 716–799. [Google Scholar] [CrossRef] [PubMed]
- Thayer, J.F.; Lane, R.D. The Role of Vagal Function in the Risk for Cardiovascular Disease and Mortality. Biol. Psychol. 2007, 74, 224–242. [Google Scholar] [CrossRef]
- Brateanu, A. Heart Rate Variability after Myocardial Infarction: What We Know and What We Still Need to Find out. Curr. Med. Res. Opin. 2015, 31, 1855–1860. [Google Scholar] [CrossRef]
- Song, T.; Qu, X.F.; Zhang, Y.T.; Cao, W.; Han, B.H.; Li, Y.; Piao, J.Y.; Yin, L.L.; Da Cheng, H. Usefulness of the Heart-Rate Variability Complex for Predicting Cardiac Mortality after Acute Myocardial Infarction. BMC Cardiovasc. Disord. 2014, 14, 59. [Google Scholar] [CrossRef]
- Yuda, E.; Ueda, N.; Kisohara, M.; Hayano, J. Redundancy Among Risk Predictors Derived from Heart Rate Variability and Dynamics: ALLSTAR Big Data Analysis. Ann. Noninvasive Electrocardiol. 2021, 26, e12790. [Google Scholar] [CrossRef]
- Huikuri, H.V.; Stein, P.K. Clinical Application of Heart Rate Variability after Acute Myocardial Infarction. Front. Physiol. 2012, 3, 41. [Google Scholar] [CrossRef]
- Kautzner, J.; St’ovícek, P.; Anger, Z.; Savlíková, J.; Malik, M. Utility of Short-Term Heart Rate Variability for Prediction of Sudden Cardiac Death after Acute Myocardial Infarction. Acta Univ. Palacki Olomuc Fac. Med. 1998, 141, 69–73. [Google Scholar] [PubMed]
- Perkiömäki, J.S.; Jokinen, V.; Tapanainen, J.; Airaksinen, K.E.; Huikuri, H.V. Autonomic Markers as Predictors of Nonfatal Acute Coronary Events after Myocardial Infarction. Ann. Noninvasive Electrocardiol. 2008, 13, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Sessa, F.; Anna, V.; Messina, G.; Cibelli, G.; Monda, V.; Marsala, G.; Ruberto, M.; Biondi, A.; Cascio, O.; Bertozzi, G. Heart Rate Variability as Predictive Factor for Sudden Cardiac Death. Aging 2018, 10, 166–177. [Google Scholar] [CrossRef]
- Ivan, V.M.; Georgescu, M.; Apostol, A.; Albulescu, N.; Serb, A.F.; Tatu, C.S. Trimetazidine, a Metabolic Modulator, with Cardioprotective Effects Against Myocardial Ischemia. Rev. Chim. 2018, 69, 1260–1263. [Google Scholar] [CrossRef]
- Smiseth, O.A.; Torp, H.; Opdahl, A.; Haugaa, K.H.; Urheim, S. Myocardial Strain Imaging: How Useful Is It in Clinical Decision Making? Eur. Heart J. 2016, 37, 1196–1207. [Google Scholar] [CrossRef]
- Diao, K.Y.; Yang, Z.G.; Ma, M.; He, Y.; Zhao, Q.; Liu, X.; Gao, Y.; Xie, L.J.; Guo, Y.K. The Diagnostic Value of Global Longitudinal Strain (GLS) on Myocardial Infarction Size by Echocardiography: A Systematic Review and Meta-analysis. Sci. Rep. 2017, 7, 10082. [Google Scholar] [CrossRef] [PubMed]
- Appadurai, V.; D’Elia, N.; Mew, T.; Tomlinson, S.; Chan, J.; Hamilton-Craig, C.; Scalia, G.M. Global Longitudinal Strain as a Prognostic Marker in Cardiac Resynchronisation Therapy: A Systematic Review. Int. J. Cardiol. Heart Vasc. 2021, 35, 100849. [Google Scholar] [CrossRef]
- Gherbesi, E.; Gianstefani, S.; Angeli, F.; Ryabenko, K.; Bergamaschi, L.; Armillotta, M.; Guerra, E.; Tuttolomondo, D.; Gaibazzi, N.; Squeri, A.; et al. Myocardial Strain of the Left Ventricle by Speckle Tracking Echocardiography: From Physics to Clinical Practice. Echocardiography 2024, 41, e15753. [Google Scholar] [CrossRef] [PubMed]
- Schuster, A.; Backhaus, S.J.; Stiermaier, T.; Eitel, I. Prognostic Utility of Global Longitudinal Strain in Myocardial Infarction. World J. Cardiol. 2018, 10, 35–37. [Google Scholar] [CrossRef]
- Biering-Sørensen, T.; Biering-Sørensen, S.R.; Olsen, F.J.; Sengeløv, M.; Jørgensen, P.G.; Mogelvang, R.; Shah, A.M.; Jensen, J.S. Global Longitudinal Strain by Echocardiography Predicts Long-Term Risk of Cardiovascular Morbidity and Mortality in a Low-Risk General Population. Circ. Cardiovasc. Imaging 2017, 10, e005521. [Google Scholar] [CrossRef]
- Ben Driss, A.; Ben Driss Lepage, C.; Sfaxi, A.; Hakim, M.; Elhadad, S.; Tabet, J.Y.; Salhi, A.; Brandao Carreira, V.; Hattab, M.; Meurin, P.; et al. Strain Predicts Left Ventricular Functional Recovery after Acute Myocardial Infarction with Systolic Dysfunction. Int. J. Cardiol. 2020, 307, 1–7. [Google Scholar] [CrossRef]
- Iwahashi, N.; Kirigaya, J.; Abe, T.; Horii, M.; Toya, N.; Hanajima, Y.; Takahashi, H.; Akiyama, E.; Okada, K.; Matsuzawa, Y.; et al. Impact of Three-Dimensional Global Longitudinal Strain for Patients with Acute Myocardial Infarction. Eur. Heart J. Cardiovasc. Imaging 2020, 22, 1413–1424. [Google Scholar] [CrossRef] [PubMed]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D. Fourth Universal Definition of Myocardial Infarction. Circulation 2018, 138, e618–e651. [Google Scholar] [CrossRef]
- Lawton, J.S.; Tamis-Holland, J.E.; Bangalore, S.; Bates, E.R.; Beckie, T.M.; Bischoff, J.M.; Bittl, J.A.; Cohen, M.G.; DiMaio, J.M.; Don, C.W.; et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e4–e17. [Google Scholar] [CrossRef] [PubMed]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the Management of Acute Myocardial Infarction in Patients Presenting with ST-Segment Elevation: The Task Force for the Management of Acute Myocardial Infarction in Patients Presenting with ST-Segment Elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [CrossRef] [PubMed]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the Management of Acute Coronary Syndromes: Developed by the Task Force on the Management of Acute Coronary Syndromes of the European Society of Cardiology (ESC). Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef]
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Front. Public Health 2017, 5, 258. [Google Scholar] [CrossRef]
- Sade, L.E.; Joshi, S.S.; Cameli, M.; Cosyns, B.; Delgado, V.; Donal, E.; Edvardsen, T.; Carvalho, R.F.; Manka, R.; Podlesnikar, T.; et al. Current Clinical Use of Speckle-Tracking Strain Imaging: Insights from a Worldwide Survey from the European Association of Cardiovascular Imaging (EACVI). Eur. Heart J. Cardiovasc. Imaging 2023, 24, 1583–1592. [Google Scholar] [CrossRef]
- Palmiero, G.; Cesaro, A.; Galiero, R.; Loffredo, G.; Caturano, A.; Vetrano, E.; Rinaldi, L.; Salvatore, T.; Ruggiero, R.; Di Palo, M.R.; et al. Impact of gliflozins on cardiac remodeling in patients with type 2 diabetes mellitus & reduced ejection fraction heart failure: A pilot prospective study. GLISCAR study. Diabetes Res. Clin. Pract. 2023, 200, 110686. [Google Scholar] [CrossRef] [PubMed]
- Erdogan, A.; Coch, M.; Bilgin, M.; Parahuleva, M.; Tillmanns, H.; Waldecker, B.; Soydan, N. Prognostic Value of Heart Rate Variability after Acute Myocardial Infarction in the Era of Immediate Reperfusion. Herzschrittmacherther Elektrophysiol. 2008, 19, 161–168. [Google Scholar] [CrossRef]
- Stein, P.K. Assessing Heart Rate Variability from Real-World Holter Reports. Card. Electrophysiol. Rev. 2002, 6, 239–244. [Google Scholar] [CrossRef]
- Tiwari, R.; Kumar, R.; Malik, S.; Raj, T.; Kumar, P. Analysis of Heart Rate Variability and Implication of Different Factors on Heart Rate Variability. Curr. Cardiol. Rev. 2021, 17, e160721189770. [Google Scholar] [CrossRef] [PubMed]
- Kleiger, R.E.; Miller, J.P.; Bigger, J.T., Jr.; Moss, A.J. Decreased Heart Rate Variability and Its Association with Increased Mortality after Acute Myocardial Infarction. Am. J. Cardiol. 1987, 59, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Dyab, R.; Zuccarella-Hackl, C.; Princip, M.; Sivakumar, S.; Meister-Langraf, R.E.; Znoj, H.; Schmid, J.P.; Barth, J.; Schnyder, U.; von Känel, R.; et al. Role of Heart Rate Variability in the Association between Myocardial Infarction Severity and Post-Myocardial Infarction Distress. Life 2023, 13, 2266. [Google Scholar] [CrossRef]
- Goldenberg, I.; Goldkorn, R.; Shlomo, N.; Einhorn, M.; Levitan, J.; Kuperstein, R.; Klempfner, R.; Johnson, B. Heart Rate Variability for Risk Assessment of Myocardial Ischemia in Patients without Known Coronary Artery Disease: The HRV-DETECT (Heart Rate Variability for the Detection of Myocardial Ischemia) Study. J. Am. Heart Assoc. 2019, 8, e014540. [Google Scholar] [CrossRef]
- Buccelletti, E.; Gilardi, E.; Scaini, E.; Galiuto, L.; Persiani, R.; Biondi, A.; Basile, F.; Silveri, N.G. Heart Rate Variability and Myocardial Infarction: Systematic Literature Review and Metanalysis. Eur. Rev. Med. Pharmacol. Sci. 2009, 13, 299–307. [Google Scholar] [PubMed]
- Thomsen, P.E.B.; Jons, C.; Raatikainen, M.P.; Joergensen, R.M.; Hartikainen, J.; Virtanen, V.; Boland, J.; Anttonen, O.; Gang, U.J.; Hoest, N.; et al. Long-Term Recording of Cardiac Arrhythmias with an Implantable Cardiac Monitor in Patients with Reduced Ejection Fraction after Acute Myocardial Infarction. Circulation 2010, 122, 1258–1264. [Google Scholar] [CrossRef] [PubMed]
- Chattipakorn, N.; Incharoen, T.; Kanlop, N.; Chattipakorn, S. Heart Rate Variability in Myocardial Infarction and Heart Failure. Int. J. Cardiol. 2007, 120, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Brinza, C.; Floria, M.; Covic, A.; Covic, A.; Scripcariu, D.-V.; Burlacu, A. The Usefulness of Assessing Heart Rate Variability in Patients with Acute Myocardial Infarction (HeaRt-V-AMI). Sensors 2022, 22, 3571. [Google Scholar] [CrossRef]
- Kim, H.G.; Cheon, E.J.; Bai, D.S.; Lee, Y.H.; Koo, B.H. Stress and Heart Rate Variability: A Meta-Analysis and Review of the Literature. Psychiatry Investig. 2018, 15, 235–245. [Google Scholar] [CrossRef]


| Characteristics | Values: % (n = 108) |
|---|---|
| Age (years) * | 67.10 (8.46) * |
| Sex | M: 62.03% F: 37.94% |
| Hypertension | 62.96% |
| Familial history of CVD | 51.85% |
| Personal history of CVD | 56.48% |
| Smokers | 44.44% |
| Dyslipidemia | 73.14% |
| Obesity | 41.66% |
| Diabetes | 43.51% |
| COPD | 10.18% |
| History of CVA | 6.48% |
| Creatinine > 1.5 mg% | 28.70% |
| Arrhythmias | 37.96% |
| Medication at discharge | |
| Beta-blockers | 87.03% |
| Amiodarone | 13.88% |
| Ivabradine | 10.18% |
| ACEi/ARB | 63.88% |
| ARNi | 11.11% |
| SGLT2i | 23.14% |
| MRA | 48.14% |
| Parameter | Baseline Median (Q1–Q3) | 1 Month Median (Q1–Q3) | 6 Months Median (Q1–Q3) | Statistic * | p-Value (p) |
|---|---|---|---|---|---|
| SDNN | 75.3 (48.2–100) | 87 (55.7–111) | 94.2 (67.6–118) | 157.53 | p < 0.001 |
| RMSSD | 27 (22–33) | 30.5 (27.0–38.0) | 35 (30–42) | 190.75 | |
| PNN50 | 10.6 (8.98–12.7) | 11.1 (10.0–12.8) | 12.1 (9.7–16.8) | 33.47 | |
| HF | 292 (266–321) | 321 (293–348) | 359 (320–384) | 131.98 | |
| LF | 959 (866–1043) | 932 (840–1024) | 806 (688–916) | 54.54 | |
| LF/HF | 3.21 (2.89–3.56) | 2.86 (2.54–3.17) | 2.17 (1.87–2.63) | 190.90 |
| Parameter (P) | Comparison of P Time Point | Compared with P Time Point | Statistic * | p-Value (p) |
|---|---|---|---|---|
| SDNN | Baseline Baseline 1 Month | 1 Month 6 Months 6 Months | 174 144 732 | <0.001 |
| RMSSD | Baseline Baseline 1 Month | 1 Month 6 Months 6 Months | 275 19.5 150 | <0.001 |
| PNN50 | Baseline Baseline 1 Month | 1 Month 6 Months 6 Months | 2070 928 1266 | 0.016 <0.001 <0.001 |
| HF | Baseline Baseline 1 Month | 1 Month 6 Months 6 Months | 167 240 1188 | <0.001 |
| LF | Baseline Baseline 1 Month | 1 Month 6 Months 6 Months | 2338 960 658 | 0.540 <0.001 <0.001 |
| LF/HF | Baseline Baseline 1 Month | 1 Month 6 Months 6 Months | 5886 5843 5622 | <0.001 |
| Variable | SDNN Group (OC, EC) | Chi-Square Statistic * | p-Value (p) | ||
|---|---|---|---|---|---|
| Group 1 | Group 2 | Group 3 | |||
| Arrhythmias | 24, 13.67 | 12, 16.32 | 5, 11.01 | 6.52 | 0.0384 |
| Hypertension | 23, 22.67 | 25, 27.07 | 20, 18.26 | 0.89 | 0.6407 |
| Personal history of CVD | 18, 20.33 | 26, 24.29 | 17, 16.38 | 0.94 | 0.6229 |
| Familial history of CVD | 15, 18.67 | 24, 22.3 | 17, 15.04 | 2.29 | 0.3169 |
| Smokers | 15, 16 | 20, 19.11 | 13, 12.89 | 0.18 | 0.91 |
| Dyslipidemia | 25, 26.33 | 31, 31.45 | 23, 21.21 | 0.83 | 0.6582 |
| Obesity | 10, 15 | 18, 17.92 | 17, 12.08 | 6.28 | 0.052 |
| Diabetes | 15, 15.67 | 20, 18.71 | 12,12.62 | 0.26 | 0.8777 |
| Parameter | Group | Baseline Median (IQR) | 1 Month Median (IQR) | 6 Months Median (IQR) | Kruskal–Wallis Chi-Squared | p-Value (p) |
|---|---|---|---|---|---|---|
| SDNN | Deceased | 48.55 (24.15) | 64.2 (44.07) | 70.8 (29.5) | 6.86 | 0.00878 |
| Survivors | 76.95 (53.82) | 89.4 (54.22) | 99.05 (50.87) | 6.53 | 0.01057 | |
| 11.57 | <0.001 | |||||
| RMSSD | Deceased | 19.5 (4.75) | 23 (8.5) | 26.5 (5.75) | 17.55 | <0.001 |
| Survivors | 29 (10) | 33.5 (11) | 38 (10) | 26.27 | <0.001 | |
| 32.43 | <0.001 | |||||
| PNN50 | Deceased | 8.1 (2.4) | 9.1 (2.45) | 9.45 (2.1) | 22.36 | <0.001 |
| Survivors | 11 (3.375) | 11.6 (2.65) | 13.05 (6.325) | 19.68 | <0.001 | |
| 20.26 | <0.001 | |||||
| HF | Deceased | 211 (53 | 238 (51) | 216.5 (40.5) | 31.44 | <0.001 |
| Survivors | 301 (49.5) | 329.5 (50.25) | 364.5 (46.75) | 33.42 | <0.001 | |
| 36.22 | <0.001 | |||||
| LF | Deceased | 1109 (153.75) | 1112 (147) | 1081 (89.25) | 23.99 | <0.001 |
| Survivors | 937.5 (172.75) | 903 (169) | 788 (170.75) | 32.10 | <0.001 | |
| 36.22 | <0.001 | |||||
| LF/HF | Deceased | 5.28 (0.68) | 4.64 (0.46) | 4.87 (0.57) | 36.11 | <0.001 |
| Survivors | 3.115 (0.61) | 2.75 (0.52) | 2.07 (0.67) | 36.17 | <0.001 | |
| 36.22 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bogdan, C.; Apostol, A.; Ivan, V.M.; Sandu, O.E.; Petre, I.; Suciu, O.; Marc, L.-E.; Maralescu, F.-M.; Lighezan, D.F. Heart Rate Variability and Global Longitudinal Strain for Prognostic Evaluation and Recovery Assessment in Conservatively Managed Post-Myocardial Infarction Patients. J. Clin. Med. 2024, 13, 5435. https://doi.org/10.3390/jcm13185435
Bogdan C, Apostol A, Ivan VM, Sandu OE, Petre I, Suciu O, Marc L-E, Maralescu F-M, Lighezan DF. Heart Rate Variability and Global Longitudinal Strain for Prognostic Evaluation and Recovery Assessment in Conservatively Managed Post-Myocardial Infarction Patients. Journal of Clinical Medicine. 2024; 13(18):5435. https://doi.org/10.3390/jcm13185435
Chicago/Turabian StyleBogdan, Carina, Adrian Apostol, Viviana Mihaela Ivan, Oana Elena Sandu, Ion Petre, Oana Suciu, Luciana-Elena Marc, Felix-Mihai Maralescu, and Daniel Florin Lighezan. 2024. "Heart Rate Variability and Global Longitudinal Strain for Prognostic Evaluation and Recovery Assessment in Conservatively Managed Post-Myocardial Infarction Patients" Journal of Clinical Medicine 13, no. 18: 5435. https://doi.org/10.3390/jcm13185435






