Laser Treatment for Diabetic Retinopathy: History, Mechanism, and Novel Technologies
Abstract
1. Introduction
2. Materials and Methods
3. History of Retinal Photocoagulation Therapy
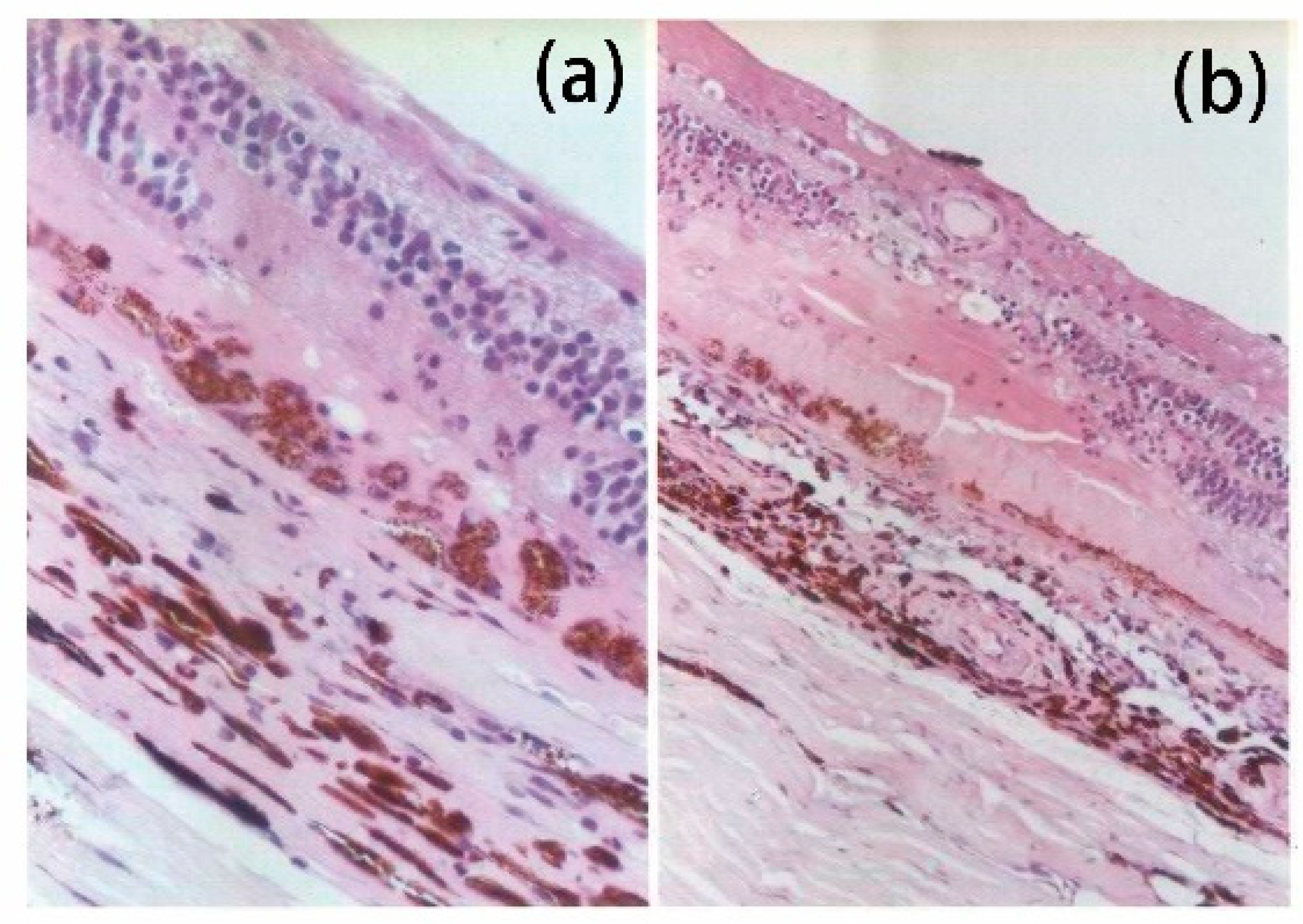
4. Mechanism of Conventional Retinal Laser Photocoagulation
5. Conventional Laser Photocoagulation
6. Pattern Scanning Laser Photocoagulation
7. Subthreshold Micropulse Laser Therapy
8. Image-Guided Navigated Laser Delivery System
9. Targeted Retinal Photocoagulation
10. Multimodal Imaging-Guided Laser Therapy
11. Retina Rejuvenation Therapy
12. Photo-Mediated Ultrasound Therapy
13. Photobiomodulation Therapy
14. Compared and Combined with Pharmacologic Therapies
15. Laser Photocoagulation in Real-World Conditions
16. Discussion
17. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhou, X.; Hohman, A.E.; Hsu, W.H.; Vujosevic, S.; Aldington, S.J.; Silva, P.; Hernández, C.; Scanlon, P.; Peto, T.; Simó, R. Screening for Diabetic Retinopathy: New Perspectives and Challenges. Lancet Diabetes Endocrinol. 2020, 8, 337–347. [Google Scholar] [CrossRef]
- Tan, T.-E.; Wong, T.Y. Diabetic Retinopathy: Looking Forward to 2030. Front. Endocrinol. 2023, 13, 1077669. [Google Scholar] [CrossRef] [PubMed]
- Antonetti, D.A.; Silva, P.S.; Stitt, A.W. Current Understanding of the Molecular and Cellular Pathology of Diabetic Retinopathy. Nat. Rev. Endocrinol. 2021, 17, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Fundus Disease Group of Ophthalmological Society of Chinese Medical Association; Fundus Disease Group of Ophthalmologist Branch of Chinese Medical Doctor Association. Evidence-based guidelines for diagnosis and treatment of diabetic retinopathy in China (2022). Chin. J. Ocul. Fundus Dis. 2023, 39, 99–124. [Google Scholar] [CrossRef]
- Diabetic Retinopathy Study Research Group. Indications for Photocoagulation Treatment of Diabetic Retinopathy: Diabetic Retinopathy Study Report no. 14. Int. Ophthalmol. Clin. 1987, 27, 239–253. [Google Scholar] [CrossRef]
- Early Treatment Diabetic Retinopathy Study Research Group. Techniques for Scatter and Local Photocoagulation Treatment of Diabetic Retinopathy: Early Treatment Diabetic Retinopathy Study Report no. 3. Int. Ophthalmol. Clin. 1987, 27, 254–264. [Google Scholar] [CrossRef]
- Tomita, Y.; Lee, D.; Tsubota, K.; Negishi, K.; Kurihara, T. Updates on the Current Treatments for Diabetic Retinopathy and Possibility of Future Oral Therapy. J. Clin. Med. 2021, 10, 4666. [Google Scholar] [CrossRef]
- Ai, X.; Yu, P.; Hou, Y.; Song, X.; Luo, J.; Li, N.; Lai, X.; Wang, X.; Meng, X. A Review of Traditional Chinese Medicine on Treatment of Diabetic Retinopathy and Involved Mechanisms. Biomed. Pharmacother. 2020, 132, 110852. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, N. Progress of Nanotechnology in Diabetic Retinopathy Treatment. Int. J. Nanomed. 2021, 16, 1391–1403. [Google Scholar] [CrossRef]
- Meyer-Schwickerath, G.R.E.; Gonçalves, I.L.; Machado das Neves, G.; Porto Kagami, L.; Eifler-Lima, V.L.; Merlo, A.A. The History of Photocoagulation. Aust. N. Z. J. Ophthalmol. 1989, 17, 427–434. [Google Scholar] [CrossRef]
- Grzybowski, A.; Markeviciute, A.; Zemaitiene, R.; MAIMAN, T.H. Stimulated Optical Radiation in Ruby. Nature 1960, 187, 493–494. [Google Scholar] [CrossRef]
- L’Espereance, F.A. The Effect of Laser Radiation on the Retinal Vasculature. Arch. Ophthalmol. 1965, 74, 752. [Google Scholar] [CrossRef] [PubMed]
- L’Esperance, F.A. An Opthalmic Argon Laser Photocoagulation System: Design, Construction, and Laboratory Investigations. Trans. Am. Ophthalmol. Soc. 1968, 66, 827–904. [Google Scholar] [PubMed]
- Bessette, F.M.; Nguyen, L.C. Laser Light: Its Nature and Its Action on the Eye. CMAJ 1989, 141, 1141–1148. [Google Scholar] [PubMed]
- Lock, J.H.; Fong, K.C.S. Retinal Laser Photocoagulation. Med. J. Malays. 2010, 65, 88–94. [Google Scholar] [PubMed]
- Trempe, C.L.; Mainster, M.A.; Pomerantzeff, O.; Avila, M.P.; Jalkh, A.E.; Weiter, J.J.; McMeel, J.W.; Schepens, C.L. Macular Photocoagulation. Ophthalmology 1982, 89, 721–728. [Google Scholar] [CrossRef]
- Sramek, C.K.; Leung, L.-S.B.; Paulus, Y.M.; Palanker, D.V. Therapeutic Window of Retinal Photocoagulation with Green (532-Nm) and Yellow (577-Nm) Lasers. Ophthalmic Surg. Lasers Imaging 2012, 43, 341–347. [Google Scholar] [CrossRef]
- Jayadev, C.; Yadav, N.; Rajendran, A.; Nagpal, M. Recent Developments in Retinal Lasers and Delivery Systems. Indian J. Ophthalmol. 2014, 62, 50. [Google Scholar] [CrossRef]
- L’Esperance, F.A. Clinical Photocoagulation with the Frequency-Doubled Neodymium Yttrium-Aluminum-Garnet Laser. Am. J. Ophthalmol. 1971, 71, 631–638. [Google Scholar] [CrossRef]
- Lax, B. Semiconductor Lasers. Science 1963, 141, 1247–1255. [Google Scholar] [CrossRef]
- Balles, M.W.; Puliafito, C.A.; D’Amico, D.J.; Jacobson, J.J.; Birngruber, R. Semiconductor Diode Laser Photocoagulation in Retinal Vascular Disease. Ophthalmology 1990, 97, 1553–1561. [Google Scholar] [CrossRef] [PubMed]
- Dyer, D.S.; Bressler, S.B.; Bressler, N.M. The Role of Laser Wavelength in the Treatment of Vitreoretinal Diseases. Curr. Opin. Ophthalmol. 1994, 5, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Adam, M.K.; Weinstock, B.M.; Kasi, S.K.; Ehmann, D.S.; Hsu, J.; Garg, S.J.; Ho, A.C.; Chiang, A. Patient Comfort with Yellow (577 Nm) vs. Green (532 Nm) Laser Panretinal Photocoagulation for Proliferative Diabetic Retinopathy. Ophthalmol. Retin. 2018, 2, 91–95. [Google Scholar] [CrossRef]
- Gilmour, M.A. Lasers in Ophthalmology. Vet. Clin. N. Am. Small Anim. Pract. 2002, 32, 649–672. [Google Scholar] [CrossRef] [PubMed]
- Stefánsson, E. The Therapeutic Effects of Retinal Laser Treatment and Vitrectomy. A Theory Based on Oxygen and Vascular Physiology. Acta Ophthalmol. Scand. 2001, 79, 435–440. [Google Scholar] [CrossRef]
- Lu, L.; Jiang, Y.; Jaganathan, R.; Hao, Y. Current Advances in Pharmacotherapy and Technology for Diabetic Retinopathy: A Systematic Review. J. Ophthalmol. 2018, 2018, 1694187. [Google Scholar] [CrossRef]
- Stefánsson, E. The Mechanism of Retinal Photocoagulation–How Does the Laser Work? Eur. Ophthalmic Rev. 2009, 2, 76. [Google Scholar] [CrossRef]
- Ogata, N.; Tombran-Tink, J.; Jo, N.; Mrazek, D.; Matsumura, M. Upregulation of Pigment Epithelium-Derived Factor after Laser Photocoagulation. Am. J. Ophthalmol. 2001, 132, 427–429. [Google Scholar] [CrossRef]
- Frizziero, L.; Calciati, A.; Midena, G.; Torresin, T.; Parrozzani, R.; Pilotto, E.; Midena, E. Subthreshold Micropulse Laser Modulates Retinal Neuroinflammatory Biomarkers in Diabetic Macular Edema. JCM 2021, 10, 3134. [Google Scholar] [CrossRef]
- Writing Committee for the Diabetic Retinopathy Clinical Research Network; Fong, D.S.; Strauber, S.F.; Aiello, L.P.; Beck, R.W.; Callanan, D.G.; Danis, R.P.; Davis, M.D.; Feman, S.S.; Ferris, F.; et al. Comparison of the Modified Early Treatment Diabetic Retinopathy Study and Mild Macular Grid Laser Photocoagulation Strategies for Diabetic Macular Edema. Arch. Ophthalmol. 2007, 125, 469. [Google Scholar] [CrossRef]
- Everett, L.A.; Paulus, Y.M. Laser Therapy in the Treatment of Diabetic Retinopathy and Diabetic Macular Edema. Curr. Diab Rep. 2021, 21, 35. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Li, X.; Xie, J.; Zhang, L.; Zhang, G.; Zhang, A.; Chen, X.; Cui, Y.; Meng, Q. Long-Term Retinal Neurovascular and Choroidal Changes After Panretinal Photocoagulation in Diabetic Retinopathy. Front. Med. 2021, 8, 752538. [Google Scholar] [CrossRef]
- Law, N.M.; Fan, R.F. Clinical Experience with the Laser Indirect Ophthalmoscope. Ann. Acad. Med. Singap. 1991, 20, 750–754. [Google Scholar]
- Fundus Disease Group of Ophthalmological Society of Chinese Medical Association Clinical Diagnosis and Treatment Guidelines for Diabetic Retinopathy in China (2014). Chin. J. Ocul. Fundus Dis. 2014, 50, 851–865. [CrossRef]
- Reddy, S.V.; Husain, D. Panretinal Photocoagulation: A Review of Complications. Semin. Ophthalmol. 2018, 33, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Tso, M.O.M. Retinal Diseases; Lippincott Williams and Wilkins: Philadelphia, PA, USA, 1988; pp. 246–262. ISBN 978-0-397-50661-3. [Google Scholar]
- Chen, L.; Liu, Z.L. Histological observation of krypton laser photocoagulation of human retina. Chin. J. Ocul. Fundus Dis. 2002, 18, 3. [Google Scholar]
- Fong, D.S.; Barton, F.B.; Bresnick, G.H. Impaired Color Vision Associated with Diabetic Retinopathy: Early Treatment Diabetic Retinopathy Study Report No. 1511A List of the Early Treatment Diabetic Retinopathy Study Investigators Appears at the End of Early Treatment Diabetic Retinopathy Study Report No. 7. Am. J. Ophthalmol. 1999, 128, 612–617. [Google Scholar] [CrossRef] [PubMed]
- Blondeau, P.; Pavan, P.R.; Phelps, C.D. Acute Pressure Elevation Following Panretinal Photocoagulation. Arch. Ophthalmol. 1981, 99, 1239–1241. [Google Scholar] [CrossRef]
- Royle, P.; Mistry, H.; Auguste, P.; Shyangdan, D.; Freeman, K.; Lois, N.; Waugh, N. Pan-Retinal Photocoagulation and Other Forms of Laser Treatment and Drug Therapies for Non-Proliferative Diabetic Retinopathy: Systematic Review and Economic Evaluation. Health Technol. Assess. 2015, 19, 1–248. [Google Scholar] [CrossRef]
- Shimura, M.; Yasuda, K.; Shiono, T. Posterior Sub-Tenon’s Capsule Injection of Triamcinolone Acetonide Prevents Panretinal Photocoagulation-Induced Visual Dysfunction in Patients with Severe Diabetic Retinopathy and Good Vision. Ophthalmology 2006, 113, 381–387. [Google Scholar] [CrossRef]
- Lee, S.B.; Yun, Y.J.; Kim, S.H.; Kim, J.Y. Changes in Macular Thickness after Panretinal Photocoagulation in Patients with Severe Diabetic Retinopathy and No Macular Edema. RETINA 2010, 30, 756. [Google Scholar] [CrossRef] [PubMed]
- Muqit, M.M.K.; Marcellino, G.R.; Henson, D.B.; Young, L.B.; Patton, N.; Charles, S.J.; Turner, G.S.; Stanga, P.E. Single-Session vs Multiple-Session Pattern Scanning Laser Panretinal Photocoagulation in Proliferative Diabetic Retinopathy: The Manchester Pascal Study. Arch. Ophthalmol. 2010, 128, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, D.A.; Vasquez, L.M.; Preti, R.C.; Motta, A.; Sophie, R.; Bittencourt, M.G.; Sepah, Y.J.; Monteiro, M.L.R.; Nguyen, Q.D.; Takahashi, W.Y. A Randomized Controlled Trial of Panretinal Photocoagulation with and without Intravitreal Ranibizumab in Treatment-Naive Eyes with Non-High-Risk Proliferative Diabetic Retinopathy. Retina 2015, 35, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Filho, J.A.R.; Messias, A.; Almeida, F.P.P.; Ribeiro, J.A.S.; Costa, R.A.; Scott, I.U.; Jorge, R. Panretinal Photocoagulation (PRP) versus PRP plus Intravitreal Ranibizumab for High-Risk Proliferative Diabetic Retinopathy. Acta Ophthalmol. 2011, 89, e567–e572. [Google Scholar] [CrossRef]
- The Diabetic Retinopathy Study Research Group. Photocoagulation Treatment of Proliferative Diabetic Retinopathy. Ophthalmology 1981, 88, 583–600. [Google Scholar] [CrossRef]
- Patz, A.; Smith, R.E. The ETDRS and Diabetes 2000. Ophthalmology 1991, 98, 739–740. [Google Scholar] [CrossRef]
- The Early Treatment Diabetic Retinopathy Study Research. Photocoagulation for Diabetic Macular Edema: Early Treatment Diabetic Retinopathy Study Report No. 4. Int. Ophthalmol. Clin. 1987, 27, 265–272. [Google Scholar] [CrossRef]
- Early Treatment Diabetic Retinopathy Study Research Group. Early Photocoagulation for Diabetic Retinopathy: ETDRS Report Number 9. Ophthalmology 1991, 98, 766–785. [Google Scholar] [CrossRef]
- Early Treatment Diabetic Retinopathy Study Research Group. Treatment Techniques and Clinical Guidelines for Photocoagulation of Diabetic Macular Edema. Ophthalmology 1987, 94, 761–774. [Google Scholar] [CrossRef]
- Schatz, H. Progressive Enlargement of Laser Scars Following Grid Laser Photocoagulation for Diffuse Diabetic Macular Edema. Arch. Ophthalmol. 1991, 109, 1549. [Google Scholar] [CrossRef]
- Romero-Aroca, P.; Reyes-Torres, J.; Baget-Bernaldiz, M.; Blasco-Sune, C. Laser Treatment for Diabetic Macular Edema in the 21st Century. CDR 2014, 10, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Blumenkranz, M.S.; Yellachich, D.; Andersen, D.E.; Wiltberger, M.W.; Mordaunt, D.; Marcellino, G.R.; Palanker, D. Semiautomated Patterned Scanning Laser for Retinal Photocoagulation. Retina 2006, 26, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Sanghvi, C.; McLauchlan, R.; Delgado, C.; Young, L.; Charles, S.J.; Marcellino, G.; Stanga, P.E. Initial Experience with the Pascal Photocoagulator: A Pilot Study of 75 Procedures. Br. J. Ophthalmol. 2008, 92, 1061–1064. [Google Scholar] [CrossRef]
- Paulus, Y.M.; Kaur, K.; Egbert, P.R.; Blumenkranz, M.S.; Moshfeghi, D.M. Human Histopathology of PASCAL Laser Burns. Eye 2013, 27, 995–996. [Google Scholar] [CrossRef]
- Wu, Z.-Q. Efficacy of Conbercept Combined with Multi-Wavelength Multi-Point Scanning Laser in Treatment of Early PDR. Int. Eye Sci. 2019, 805–808. [Google Scholar]
- Nagpal, M.; Marlecha, S.; Nagpal, K. Comparison of Laser Photocoagulation for Diabetic Retinopathy Using 532-nm Standard Laser versus Multispot Pattern Scan Laser. Retina 2010, 30, 452–458. [Google Scholar] [CrossRef]
- Chappelow, A.V.; Tan, K.; Waheed, N.K.; Kaiser, P.K. Panretinal Photocoagulation for Proliferative Diabetic Retinopathy: Pattern Scan Laser versus Argon Laser. Am. J. Ophthalmol. 2012, 153, 137–142.e2. [Google Scholar] [CrossRef] [PubMed]
- Muqit, M.M.K.; Gray, J.C.B.; Marcellino, G.R.; Henson, D.B.; Young, L.B.; Patton, N.; Charles, S.J.; Turner, G.S.; Stanga, P.E. Barely Visible 10-Millisecond Pascal Laser Photocoagulation for Diabetic Macular Edema: Observations of Clinical Effect and Burn Localization. Am. J. Ophthalmol. 2010, 149, 979–986.e2. [Google Scholar] [CrossRef]
- Mahgoub, M.M.; Macky, T.A. The Effect of Laser Panretinal Photocoagulation on Diabetic Macular Edema Using the Pascal® Photocoagulator versus the Conventional Argon Laser Photocoagulator. Ophthalmologica 2016, 235, 137–140. [Google Scholar] [CrossRef]
- Friberg, T.R.; Karatza, E.C. The Treatment of Macular Disease Using a Micropulsed and Continuous Wave 810-nm Diode Laser. Ophthalmology 1997, 104, 2030–2038. [Google Scholar] [CrossRef]
- Sivaprasad, S.; Elagouz, M.; McHugh, D.; Shona, O.; Dorin, G. Micropulsed Diode Laser Therapy: Evolution and Clinical Applications. Surv. Ophthalmol. 2010, 55, 516–530. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, N.; Matsumoto, M.; Shimizu, H.; Mandai, M.; Hata, Y.; Ishibashi, T. Photocoagulated Human Retinal Pigment Epithelial Cells Produce an Inhibitor of Vascular Endothelial Cell Proliferation. Investig. Ophthalmol. Vis. Sci. 1995, 36, 1686–1691. [Google Scholar]
- Flaxel, C.; Bradle, J.; Acott, T.; Samples, J.R. Retinal Pigment Epithelium Produces Matrix Metalloproteinases after Laser Treatment. Retina 2007, 27, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Roider, J. Subthreshold (Retinal Pigment Epithelium) Photocoagulation in Macular Diseases: A Pilot Study. Br. J. Ophthalmol. 2000, 84, 40–47. [Google Scholar] [CrossRef]
- Brinkmann, R.; Roider, J.; Birngruber, R. Selective Retina Therapy (SRT): A Review on Methods, Techniques, Preclinical and First Clinical Results. Bull. Soc. Belge. Ophtalmol. 2006, 51–69. [Google Scholar]
- Roider, J.; Liew, S.H.M.; Klatt, C.; Elsner, H.; Poerksen, E.; Hillenkamp, J.; Brinkmann, R.; Birngruber, R. Selective Retina Therapy (SRT) for Clinically Significant Diabetic Macular Edema. Graefes Arch. Clin. Exp. Ophthalmol. 2010, 248, 1263–1272. [Google Scholar] [CrossRef]
- Jorge, E.C.; Jorge, E.N.; Botelho, M.; Farat, J.G.; Virgili, G.; El Dib, R. Monotherapy Laser Photocoagulation for Diabetic Macular Oedema. Cochrane Database Syst. Rev. 2018, 2018, CD010859. [Google Scholar] [CrossRef]
- Wu, L.; Zas, M.; Cotic, M.; Wu, M.; Wu, A. Macular Laser Photocoagulation in the Management of Diabetic Macular Edema: Still Relevant in 2020? Taiwan J. Ophthalmol. 2020, 10, 87. [Google Scholar] [CrossRef]
- Sabal, B.; Teper, S.; Wylęgała, E. Subthreshold Micropulse Laser for Diabetic Macular Edema: A Review. J. Clin. Med. 2022, 12, 274. [Google Scholar] [CrossRef]
- Su, D.; Hubschman, J.-P. A Review of Subthreshold Micropulse Laser and Recent Advances in Retinal Laser Technology. Ophthalmol. Ther. 2017, 6, 1–6. [Google Scholar] [CrossRef][Green Version]
- Lavinsky, D.; Cardillo, J.A.; Melo, L.A.S.; Dare, A.; Farah, M.E.; Belfort, R. Randomized Clinical Trial Evaluating mETDRS versus Normal or High-Density Micropulse Photocoagulation for Diabetic Macular Edema. Investig. Ophthalmol. Vis. Sci. 2011, 52, 4314. [Google Scholar] [CrossRef] [PubMed]
- Figueira, J.; Khan, J.; Nunes, S.; Sivaprasad, S.; Rosa, A.; de Abreu, J.F.; Cunha-Vaz, J.G.; Chong, N.V. Prospective Randomised Controlled Trial Comparing Sub-Threshold Micropulse Diode Laser Photocoagulation and Conventional Green Laser for Clinically Significant Diabetic Macular Oedema. Br. J. Ophthalmol. 2009, 93, 1341–1344. [Google Scholar] [CrossRef] [PubMed]
- Sivaprasad, S.; Sandhu, R.; Tandon, A.; Sayed-Ahmed, K.; McHugh, D.A. Subthreshold Micropulse Diode Laser Photocoagulation for Clinically Significant Diabetic Macular Oedema: A Three-year Follow Up. Clin. Exp. Ophthalmol. 2007, 35, 640–644. [Google Scholar] [CrossRef]
- Blumenkranz, M.S.; Li, J. Advances in Retinal Laser Therapy. Int. J. Ophthalmic Res. 2018, 4, 259–264. [Google Scholar] [CrossRef]
- Lavinsky, D.; Sramek, C.; Wang, J.; Huie, P.; Dalal, R.; Mandel, Y.; Palanker, D. Subvisible Retinal Laser Therapy. Retina 2014, 34, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Hamada, M.; Ohkoshi, K.; Inagaki, K.; Ebihara, N.; Murakami, A.; Lai, K.; Zhao, H.; Zhou, L.; Huang, C.; Zhong, X.; et al. Subthreshold Pan-Retinal Photocoagulation Using Endpoint Management Algorithm for Severe Nonproliferative Diabetic Retinopathy: A Paired Controlled Pilot Prospective Study. Ophthalmic Res. 2021, 64, 648–655. [Google Scholar] [CrossRef]
- Hamada, M.; Ohkoshi, K.; Inagaki, K.; Ebihara, N.; Murakami, A. Subthreshold Photocoagulation Using Endpoint Management in the PASCAL® System for Diffuse Diabetic Macular Edema. Int. J. Retin. Vitr. 2022, 2018, 7465794. [Google Scholar] [CrossRef]
- Hamada, M.; Ohkoshi, K.; Inagaki, K.; Ebihara, N.; Murakami, A.; Lai, K.; Zhao, H.; Zhou, L.; Huang, C.; Zhong, X.; et al. Pascal Short-Pulse plus Subthreshold Endpoint Management Laser Therapy for Diabetic Macular Edema: The “Sandwich Technique”. Int. J. Retin. Vitr. 2022, 8, 32. [Google Scholar] [CrossRef]
- Kernt, M.; Cheuteu, R.; Vounotrypidis, E.; Haritoglou, C.; Kampik, A.; Ulbig, M.W.; Neubauer, A.S. Focal and Panretinal Photocoagulation with a Navigated Laser (NAVILAS®). Acta Ophthalmol. 2011, 89, e662–e664. [Google Scholar] [CrossRef]
- Neubauer, A.S.; Kernt, M.; Haritoglou, C.; Ulbig, M.; Kampik, A.; Kozak, I.; Oster, S.F.; Hartmann, K.; Kim, J.; Freeman, W.R. Image Quality of a Novel Navigated Retina Laser (NAVILAS®). Investig. Ophthalmol. Vis. Sci. 2010, 51, 1639. [Google Scholar]
- Kozak, I.; Kim, J.S.; Oster, S.F.; Chhablani, J.; Freeman, W.R. Focal Navigated Laser Photocoagulation in Retinovascular Disease. Retina 2012, 32, 930–935. [Google Scholar] [CrossRef] [PubMed]
- Herold, T.R.; Langer, J.; Vounotrypidis, E.; Kernt, M.; Liegl, R.; Priglinger, S.G.; Sivaprasad, S.; Sandhu, R.; Tandon, A.; Sayed-Ahmed, K.; et al. 3-Year-Data of Combined Navigated Laser Photocoagulation (Navilas) and Intravitreal Ranibizumab Compared to Ranibizumab Monotherapy in DME Patients. PLoS ONE 2018, 13, e0202483. [Google Scholar] [CrossRef]
- Ober, M.D.; Kernt, M.; Cortes, M.A.; Kozak, I. Time Required for Navigated Macular Laser Photocoagulation Treatment with the Navilas®. Graefes Arch. Clin. Exp. Ophthalmol. 2013, 251, 1049–1053. [Google Scholar] [CrossRef]
- Chhablani, J.; Mathai, A.; Rani, P.; Gupta, V.; Arevalo, J.F.; Kozak, I. Comparison of Conventional Pattern and Novel Navigated Panretinal Photocoagulation in Proliferative Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2014, 55, 3432. [Google Scholar] [CrossRef][Green Version]
- Reddy, S.; Hu, A.; Schwartz, S.D. Ultra Wide Field Fluorescein Angiography Guided Targeted Retinal Photocoagulation (TRP). Semin. Ophthalmol. 2009, 24, 9–14. [Google Scholar] [CrossRef]
- Lin, Z.; Deng, A.; Hou, N.; Gao, L.; Zhi, X. Advances in Targeted Retinal Photocoagulation in the Treatment of Diabetic Retinopathy. Front. Endocrinol. 2023, 14, 1108394. [Google Scholar] [CrossRef]
- Muqit, M.M.K.; Marcellino, G.R.; Henson, D.B.; Young, L.B.; Patton, N.; Charles, S.J.; Turner, G.S.; Stanga, P.E. Optos-Guided Pattern Scan Laser (Pascal)-Targeted Retinal Photocoagulation in Proliferative Diabetic Retinopathy. Acta Ophthalmol. 2013, 91, 251–258. [Google Scholar] [CrossRef]
- Toscano, L.; Messias, A.; Messias, K.; de Cenço Lopes, R.; Ribeiro, J.A.S.; Scott, I.U.; Jorge, R. Proliferative Diabetic Retinopathy Treated with Intravitreal Ranibizumab and Photocoagulation Directed at Ischemic Retinal Areas—A Randomized Study. Doc. Ophthalmol. 2021, 143, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Takamura, Y.; Matsumura, T.; Arimura, S.; Gozawa, M.; Morioka, M.; YutakaYamada, U.; Inatani, M. Direct Photocoagulation Guided by Merged Retinal Images for the Treatment of Focal Diabetic Macular Edema. Int. J. Endocrinol. 2018, 2018, e2401094. [Google Scholar] [CrossRef]
- Nozaki, M.; Ando, R.; Kimura, T.; Kato, F.; Yasukawa, T. The Role of Laser Photocoagulation in Treating Diabetic Macular Edema in the Era of Intravitreal Drug Administration: A Descriptive Review. Medicina 2023, 59, 1319. [Google Scholar] [CrossRef]
- Vessey, K.A.; Ho, T.; Jobling, A.I.; Mills, S.A.; Tran, M.X.; Brandli, A.; Lam, J.; Guymer, R.H.; Fletcher, E.L. Nanosecond Laser Treatment for Age-Related Macular Degeneration Does Not Induce Focal Vision Loss or New Vessel Growth in the Retina. Investig. Ophthalmol. Vis. Sci. 2018, 59, 731–745. [Google Scholar] [CrossRef] [PubMed]
- Pelosini, L.; Hamilton, R.; Mohamed, M.; Hamilton, A.P.; Marshall, J. Retina Rejuvenation Therapy for Diabetic Macular Edema: A Pilot Study. RETINA 2013, 33, 548. [Google Scholar] [CrossRef]
- Wang, M.; Nguyen, V.P.; Singh, R.; Mossallam, B.; Yang, X.; Wang, X.; Paulus, Y.M. Choroidal Neovascularization Removal with Photo-mediated Ultrasound Therapy. Med. Phys. 2023, 50, 3661–3670. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Cai, J.; Li, X.; Hu, Z.; Zhang, H.; Mordovanakis, A.; Paulus, Y.M.; Liu, Q.; Wang, X.; Yang, X.; et al. High-Precision, Non-Invasive Anti-Microvascular Approach via Concurrent Ultrasound and Laser Irradiation. Sci. Rep. 2019, 7, 40243. [Google Scholar] [CrossRef]
- Tang, J.; Herda, A.A.; Kern, T.S. Photobiomodulation in the Treatment of Patients with Non-Center-Involving Diabetic Macular Oedema. Br. J. Ophthalmol. 2014, 98, 1013–1015. [Google Scholar] [CrossRef]
- Kim, J.E.; Glassman, A.R.; Josic, K.; Melia, M.; Aiello, L.P.; Baker, C.; Eells, J.T.; Jampol, L.M.; Kern, T.S.; Marcus, D.; et al. A Randomized Trial of Photobiomodulation Therapy for Center-Involved Diabetic Macular Edema with Good Visual Acuity (Protocol AE). Ophthalmol. Retin. 2022, 6, 298–307. [Google Scholar] [CrossRef]
- Zhang, W.; Geng, J.; Sang, A. Effectiveness of Panretinal Photocoagulation Plus Intravitreal Anti-VEGF Treatment against PRP Alone for Diabetic Retinopathy: A Systematic Review With Meta-Analysis. Front. Endocrinol. 2022, 13, 807687. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chen, Z.; Wang, X. Clinical Efficacy and Acceptability of Panretinal Photocoagulation Combined with Conbercept for Patients with Proliferative Diabetic Retinopathy. Medicine 2021, 100, e25611. [Google Scholar] [CrossRef]
- Sun, J.K.; Jampol, L.M. The Diabetic Retinopathy Clinical Research Network (DRCR.Net) and Its Contributions to the Treatment of Diabetic Retinopathy. Ophthalmic Res. 2019, 62, 225–230. [Google Scholar] [CrossRef]
- Elman, M.J.; Raden, R.Z.; Sloan, M.D. Diabetic Retinopathy Clinical Research Network. A Randomized Trial Comparing Intravitreal Triamcinolone Acetonide and Focal/Grid Photocoagulation for Diabetic Macular Edema. Ophthalmology 2008, 115, 1447–1449.e10. [Google Scholar] [CrossRef]
- Diabetic Retinopathy Clinical Research Network; Scott, I.U.; Edwards, A.R.; Beck, R.W.; Bressler, N.M.; Chan, C.K.; Elman, M.J.; Friedman, S.M.; Greven, C.M.; Maturi, R.K.; et al. A Phase II Randomized Clinical Trial of Intravitreal Bevacizumab for Diabetic Macular Edema. Ophthalmology 2007, 114, 1860–1867. [Google Scholar] [CrossRef] [PubMed]
- Gross, J.G.; Glassman, A.R.; Liu, D.; Sun, J.K.; Antoszyk, A.N.; Baker, C.W.; Bressler, N.M.; Elman, M.J.; Ferris, F.L., III; Gardner, T.W.; et al. Five-Year Outcomes of Panretinal Photocoagulation vs Intravitreous Ranibizumab for Proliferative Diabetic Retinopathy: A Randomized Clinical Trial. JAMA Ophthalmol. 2018, 136, 1138–1148. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.M.; Emanuelli, A.; Bandello, F.; Barranco, J.J.E.; Figueira, J.; Souied, E.; Wolf, S.; Gupta, V.; Ngah, N.F.; Liew, G.; et al. KESTREL and KITE: 52-Week Results from Two Phase III Pivotal Trials of Brolucizumab for Diabetic Macular Edema. Am. J. Ophthalmol. 2022, 238, 157–172. [Google Scholar] [CrossRef]
- Chauhan, M.Z.; Rather, P.A.; Samarah, S.M.; Elhusseiny, A.M.; Sallam, A.B. Current and Novel Therapeutic Approaches for Treatment of Diabetic Macular Edema. Cells 2022, 11, 1950. [Google Scholar] [CrossRef]
- Figueira, J.; Fletcher, E.; Massin, P.; Silva, R.; Bandello, F.; Midena, E.; Varano, M.; Sivaprasad, S.; Eleftheriadis, H.; Menon, G.; et al. Ranibizumab Plus Panretinal Photocoagulation versus Panretinal Photocoagulation Alone for High-Risk Proliferative Diabetic Retinopathy (PROTEUS Study). Ophthalmology 2018, 125, 691–700. [Google Scholar] [CrossRef] [PubMed]
- Lang, G.E.; Stahl, A.; Voegeler, J.; Quiering, C.; Lorenz, K.; Spital, G.; Liakopoulos, S. Efficacy and Safety of Ranibizumab with or without Panretinal Laser Photocoagulation versus Laser Photocoagulation Alone in Proliferative Diabetic Retinopathy-the PRIDE Study. Acta Ophthalmol. 2020, 98, e530–e539. [Google Scholar] [CrossRef]
- Liu, K.; Wang, H.; He, W.; Ye, J.; Song, Y.; Wang, Y.; Liu, X.; Wu, Z.; Chen, S.; Fan, K.; et al. Intravitreal Conbercept for Diabetic Macular Oedema: 2-Year Results from a Randomised Controlled Trial and Open-Label Extension Study. Br. J. Ophthalmol. 2022, 106, 1436–1443. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, J.; Zhang, C.; Zhang, J.; Gu, L.; Luo, D.; Qiu, Q. Diabetic Macular Edema: Current Understanding, Molecular Mechanisms and Therapeutic Implications. Cells 2022, 11, 3362. [Google Scholar] [CrossRef]
- Virgili, G.; Parravano, M.; Evans, J.R.; Gordon, I.; Lucenteforte, E. Anti-Vascular Endothelial Growth Factor for Diabetic Macular Oedema: A Network Meta-Analysis. Cochrane Database Syst. Rev. 2017, 6, CD007419. [Google Scholar] [CrossRef]
- Gawęcki, M.; Kiciński, K.; Bianco, L.; Battaglia Parodi, M. Regression of Neovascularization after Panretinal Photocoagulation Combined with Anti-VEGF Injection for Proliferative Diabetic Retinopathy—A Review. Diagnostics 2023, 14, 31. [Google Scholar] [CrossRef]
- Blindbæk, S.L.; Peto, T.; Grauslund, J. How Do We Evaluate the Role of Focal/Grid Photocoagulation in the Treatment of Diabetic Macular Edema? Acta Ophthalmol. 2019, 97, 339–346. [Google Scholar] [CrossRef]
- Patil, N.S.; Mihalache, A.; Hatamnejad, A.; Popovic, M.M.; Kertes, P.J.; Muni, R.H. Intravitreal Steroids Compared with Anti-VEGF Treatment for Diabetic Macular Edema. Ophthalmol. Retin. 2023, 7, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Iovino, C.; Mastropasqua, R.; Lupidi, M.; Bacherini, D.; Pellegrini, M.; Bernabei, F.; Borrelli, E.; Sacconi, R.; Carnevali, A.; D’Aloisio, R.; et al. Intravitreal Dexamethasone Implant as a Sustained Release Drug Delivery Device for the Treatment of Ocular Diseases: A Comprehensive Review of the Literature. Pharmaceutics 2020, 12, 703. [Google Scholar] [CrossRef]
- Patel, D.; Patel, S.N.; Chaudhary, V.; Garg, S.J. Complications of Intravitreal Injections: 2022. Curr. Opin. Ophthalmol. 2022, 33, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Nomoto, H.; Lavinsky, D.; Paulus, Y.M.; Leung, L.-S.; Dalal, R.; Blumenkranz, M.S.; Palanker, D. Effect of Intravitreal Triamcinolone Acetonide on Healing of Retinal Photocoagulation Lesions. Retina 2013, 33, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhou, Z.; Zhang, B.; Wang, D. Efficacy and Safety of Various Treatments for Proliferative Diabetic Retinopathy: A Systematic Review and Network Meta-Analysis. Front. Pharmacol. 2021, 12, 709501. [Google Scholar] [CrossRef] [PubMed]
- Erbahçeci, İ.E.; Örnek, K. To the Editor. Retina 2012, 32, 1228. [Google Scholar] [CrossRef] [PubMed]
- Obeid, A.; Su, D.; Patel, S.N.; Uhr, J.H.; Borkar, D.; Gao, X.; Fineman, M.S.; Regillo, C.D.; Maguire, J.I.; Garg, S.J.; et al. Outcomes of Eyes Lost to Follow-up with Proliferative Diabetic Retinopathy That Received Panretinal Photocoagulation versus Intravitreal Anti–Vascular Endothelial Growth Factor. Ophthalmology 2019, 126, 407–413. [Google Scholar] [CrossRef]
- Teo, Z.L.; Tham, Y.-C.; Yu, M.; Chee, M.L.; Rim, T.H.; Cheung, N.; Bikbov, M.M.; Wang, Y.X.; Tang, Y.; Lu, Y.; et al. Patient Perceptions of SARS-CoV-2 Exposure Risk and Association with Continuity of Ophthalmic Care. JAMA Ophthalmol. 2021, 139, 508. [Google Scholar] [CrossRef]
- Ha, M.; Choi, S.Y.; Kim, M.; Na, J.K.; Park, Y.-H. Diabetic Nephropathy in Type 2 Diabetic Retinopathy Requiring Panretinal Photocoagulation. Korean J. Ophthalmol. 2019, 33, 46. [Google Scholar] [CrossRef]
| Disease | Stage | Fundus Pathology |
|---|---|---|
| DR | I | Only microaneurysms. |
| II | Severity more than I but less than III. | |
| III | One of the following findings: (1) ≥20 intraretinal hemorrhages in each quadrant; (2) Venous beading in ≥2 quadrants; (3) IRMA in ≥1 quadrant. | |
| IV | NVE or NVD. | |
| V | Retinal fibrosis, with or without preretinal or vitreous hemorrhage. | |
| VI | TRD combined with FVM. | |
| DME | NCI-DME | Retinal thickening not within 1 mm diameter from the fovea. |
| CI-DME | Retinal thickening within 1 mm diameter from the fovea. |
| Laser Grade | Histologically Change within 24 h | Clinical Appearance | Clinical Application |
|---|---|---|---|
| I | RPE swollen and vacuole formation | Faint, grayish-white discs | Leakage lesion like CSCR and CME |
| II | RPE, photoreceptor layer and ONL necrosis; choriocapillaris occluded by thrombi | A grayish ring around a denser whitish center | No therapeutic value |
| III | RPE, photoreceptor layer, ONL and INL necrosis | Two distinct grayish rings surrounding a white center | Ischemic, proliferative retinopathy like DR, RVO |
| Mild | Slight damage in INL | A soft white center | |
| Moderate | Moderate damage in INL | A dense white center | |
| Severe | Severe damage in INL | An even dense white center | |
| IV | Full-thickness retinal necrosis | White rings surrounding a dense white center | Chorioretinal tumor |
| Laser Techniques | Duration (ms) | Spot Size (μm) | Indications | Advantages | Limitations |
|---|---|---|---|---|---|
| PRP | 100 to 300 | 200 to 500 | DR | Mature technology, long-term efficacy | More side effects |
| Focal/Grid | 50 to 100 | 50 to 200 | DME | Repeatable treatment | Limit indication, unexpected injury to the macula |
| Pattern Scanning | 10 to 1000 | 100 to 400 | PDR/DME | Short treatment time and less pain | Unascertained long-term efficacy, without eye tracking |
| SMPL/SRT | DME | Limit the damage in RPE | Invisible laser spots | ||
| Pulsed mode | 2 × 10−4 to 1 × 10−3 | 160 | |||
| Scanning mode | CW (6.6 or 10.6 m/s) | 18 or 66 | |||
| SDM | 0.01 to 0.03 | 75 | |||
| Navigated | 10 to 1000 | 100 to 400 | PDR/DME | Short treatment time, eye tracking | Without stereoscopic view |
| TRP | 100 to 300 | 200 to 500 | DR | Less tissue damage | May require further laser supplementation |
| Multimodal Imaging-Guided | 100 to 300 | 200 to 500 | DR | More precise and safe | Manually incorporate images |
| 2RT | 3 × 10−6 | 400 | DME | Less tissue damage | No treatment protocols |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Hua, R.; Zhao, Y.; Liu, L. Laser Treatment for Diabetic Retinopathy: History, Mechanism, and Novel Technologies. J. Clin. Med. 2024, 13, 5439. https://doi.org/10.3390/jcm13185439
Wang S, Hua R, Zhao Y, Liu L. Laser Treatment for Diabetic Retinopathy: History, Mechanism, and Novel Technologies. Journal of Clinical Medicine. 2024; 13(18):5439. https://doi.org/10.3390/jcm13185439
Chicago/Turabian StyleWang, Siyu, Rui Hua, Yuqi Zhao, and Limin Liu. 2024. "Laser Treatment for Diabetic Retinopathy: History, Mechanism, and Novel Technologies" Journal of Clinical Medicine 13, no. 18: 5439. https://doi.org/10.3390/jcm13185439
APA StyleWang, S., Hua, R., Zhao, Y., & Liu, L. (2024). Laser Treatment for Diabetic Retinopathy: History, Mechanism, and Novel Technologies. Journal of Clinical Medicine, 13(18), 5439. https://doi.org/10.3390/jcm13185439







