Terbinafine Resistance in Trichophyton Strains Isolated from Humans and Animals: A Retrospective Cohort Study in Italy, 2016 to May 2024
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Human and Animal General Information
3.2. Mycological Examination
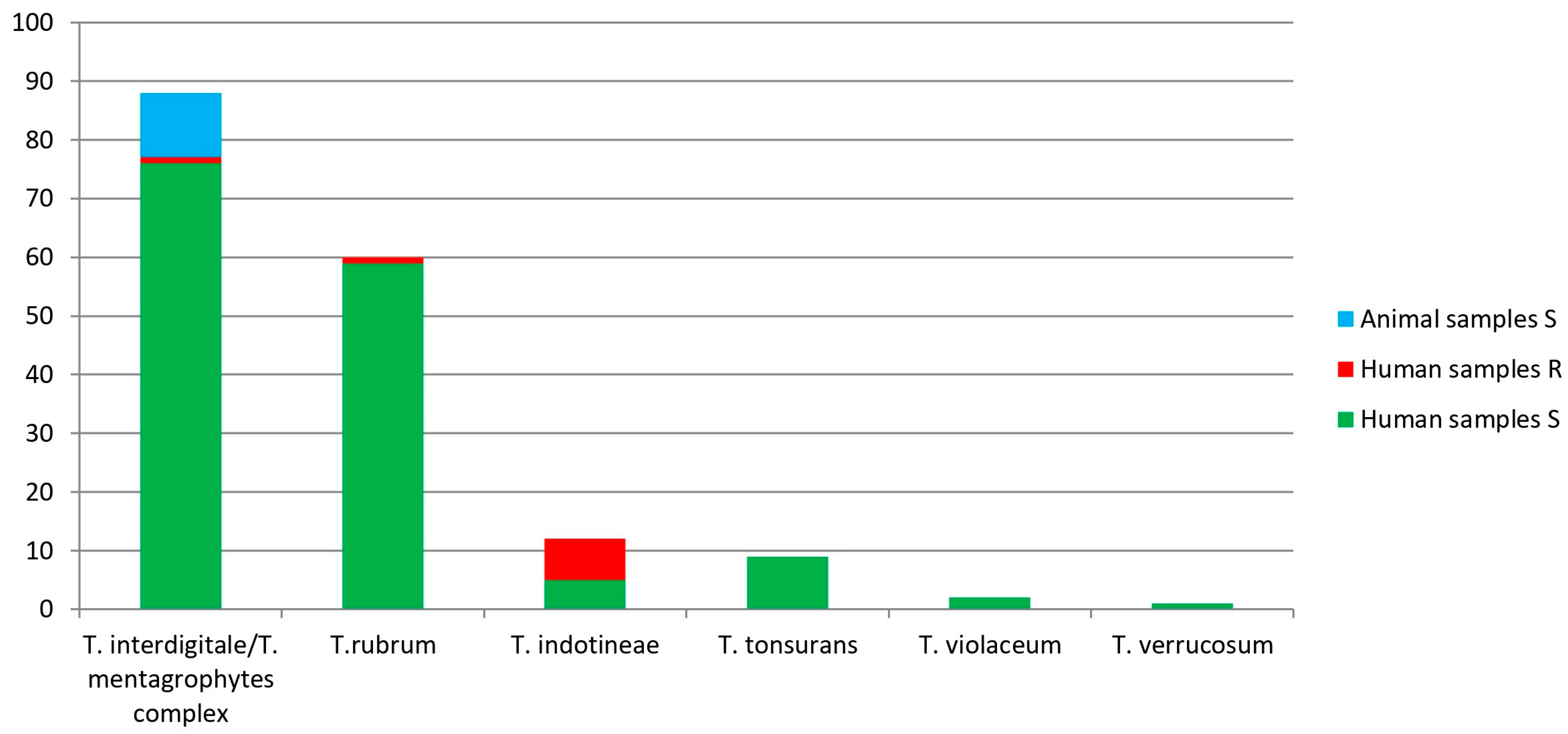
3.3. Trichophyton Species Identification, Results by Sanger Sequencing
3.4. Trichophyton Species Identification, Results by DermaGenius® Resistance Multiplex Real-Time PCR Assay
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qu, J.; Lv, X. Cryptococcal Meningitis in Apparently Immunocompetent Patients. Crit. Rev. Microbiol. 2024, 50, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Haghani, I.; Hedayati, M.T.; Shokohi, T.; Kermani, F.; Ghazanfari, M.; Javidnia, J.; Khojasteh, S.; Roohi, B.; Badali, H.; Fathi, M.; et al. Onychomycosis Due to Fusarium Species in Different Continents, Literature Review on Diagnosis and Treatment. Mycoses 2024, 67, e13652. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Alam, M.A.; Dhoundiyal, S.; Sharma, P.K. Review on Mucormycosis: Pathogenesis, Epidemiology, Microbiology and Diagnosis. Infect. Disord—Drug Targets 2024, 24, e220823220209. [Google Scholar] [CrossRef]
- Zaslavsky, K.; Grewal, P.S.; Cruz-Pimentel, M.; Qian, J.; Derzko-Dzulynsky, L.; Yan, P. Endogenous Fungal Endophthalmitis after Covid-19 Infection: Case Report and Review of Literature. Retin. Cases Brief. Rep. 2024, 18, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Uhrlaß, S.; Verma, S.B.; Gräser, Y.; Rezaei-Matehkolaei, A.; Hatami, M.; Schaller, M.; Nenoff, P. Trichophyton Indotineae—An Emerging Pathogen Causing Recalcitrant Dermatophytoses in India and Worldwide—A Multidimensional Perspective. J. Fungi 2022, 8, 757. [Google Scholar] [CrossRef]
- Kohlenberg, A.; Monnet, D.L.; Plachouras, D. Increasing Number of Cases and Outbreaks Caused by Candida Auris in the EU/EEA, 2020 to 2021. Eurosurveillance 2022, 27, 2200846. [Google Scholar] [CrossRef]
- Vitiello, A.; Ferrara, F.; Boccellino, M.; Ponzo, A.; Cimmino, C.; Comberiati, E.; Zovi, A.; Clemente, S.; Sabbatucci, M. Antifungal Drug Resistance: An Emergent Health Threat. Biomedicines 2023, 11, 1063. [Google Scholar] [CrossRef]
- Rivero-Menendez, O.; Alastruey-Izquierdo, A.; Mellado, E.; Cuenca-Estrella, M. Triazole Resistance in Aspergillus Spp.: A Worldwide Problem? J. Fungi 2016, 2, 21. [Google Scholar] [CrossRef] [PubMed]
- Bongomin, F.; Gago, S.; Oladele, R.; Denning, D. Global and Multi-National Prevalence of Fungal Diseases—Estimate Precision. J. Fungi 2017, 3, 57. [Google Scholar] [CrossRef]
- Hill, R.C.; Caplan, A.S.; Elewski, B.; Gold, J.A.W.; Lockhart, S.R.; Smith, D.J.; Lipner, S.R. Expert Panel Review of Skin and Hair Dermatophytoses in an Era of Antifungal Resistance. Am. J. Clin. Dermatol. 2024, 25, 359–389. [Google Scholar] [CrossRef]
- Saunte, D.M.L.; Pereiro-Ferreirós, M.; Rodríguez-Cerdeira, C.; Sergeev, A.Y.; Arabatzis, M.; Prohić, A.; Piraccini, B.M.; Lecerf, P.; Nenoff, P.; Kotrekhova, L.P.; et al. Emerging Antifungal Treatment Failure of Dermatophytosis in Europe: Take Care or It May Become Endemic. J. Eur. Acad. Dermatology Venereol. 2021, 35, 1582–1586. [Google Scholar] [CrossRef]
- Weitzman, I.; Summerbell, R.C. The Dermatophytes. Clin. Microbiol. Rev. 1995, 8, 240–259. [Google Scholar] [CrossRef]
- Chanyachailert, P.; Leeyaphan, C.; Bunyaratavej, S. Cutaneous Fungal Infections Caused by Dermatophytes and Non-Dermatophytes: An Updated Comprehensive Review of Epidemiology, Clinical Presentations, and Diagnostic Testing. J. Fungi 2023, 9, 669. [Google Scholar] [CrossRef] [PubMed]
- Zhan, P.; Liu, W. The Changing Face of Dermatophytic Infections Worldwide. Mycopathologia 2017, 182, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Havlickova, B.; Czaika, V.A.; Friedrich, M. Epidemiological Trends in Skin Mycoses Worldwide. Mycoses 2008, 51, 2–15. [Google Scholar] [CrossRef]
- Lee, W.J.; Kim, S.L.; Jang, Y.H.; Lee, S.-J.; Kim, D.W.; Bang, Y.J.; Jun, J.B. Increasing Prevalence of Trichophyton Rubrum Identified through an Analysis of 115,846 Cases over the Last 37 Years. J. Korean Med. Sci. 2015, 30, 639. [Google Scholar] [CrossRef] [PubMed]
- Švarcová, M.; Větrovský, T.; Kolařík, M.; Hubka, V. Defining the Relationship between Phylogeny, Clinical Manifestation, and Phenotype for Trichophyton Mentagrophytes/Interdigitale Complex; a Literature Review and Taxonomic Recommendations. Med. Mycol. 2023, 61, myad042. [Google Scholar] [CrossRef]
- Kano, R.; Kimura, U.; Kakurai, M.; Hiruma, J.; Kamata, H.; Suga, Y.; Harada, K. Trichophyton Indotineae Sp. Nov.: A New Highly Terbinafine-Resistant Anthropophilic Dermatophyte Species. Mycopathologia 2020, 185, 947–958. [Google Scholar] [CrossRef]
- Tang, C.; Ahmed, S.A.; Deng, S.; Zhang, L.; Zoll, J.; Al-Hatmi, A.M.S.; Meis, J.F.; Thakur, R.; Kang, Y.; de Hoog, G.S. Detection of Emerging Genotypes in Trichophyton Mentagrophytes Species Complex: A Proposal for Handling Biodiversity in Dermatophytes. Front. Microbiol. 2022, 13, 960190. [Google Scholar] [CrossRef]
- Cañete-Gibas, C.F.; Mele, J.; Patterson, H.P.; Sanders, C.J.; Ferrer, D.; Garcia, V.; Fan, H.; David, M.; Wiederhold, N.P. Terbinafine-Resistant Dermatophytes and the Presence of Trichophyton Indotineae in North America. J. Clin. Microbiol. 2023, 61, e0056223. [Google Scholar] [CrossRef]
- Messina, F.; Santiso, G.; Romero, M.; Bonifaz, A.; Fernandez, M.; Marin, E. First Case Report of Tinea Corporis Caused by Trichophyton Indotineae in Latin America. Med. Mycol. Case Rep. 2023, 41, 48–51. [Google Scholar] [CrossRef] [PubMed]
- Posso-De Los Rios, C.J.; Tadros, E.; Summerbell, R.C.; Scott, J.A. Terbinafine Resistant Trichophyton Indotineae Isolated in Patients With Superficial Dermatophyte Infection in Canadian Patients. J. Cutan. Med. Surg. 2022, 26, 371–376. [Google Scholar] [CrossRef]
- Kong, X.; Tang, C.; Singh, A.; Ahmed, S.A.; Al-Hatmi, A.M.S.; Chowdhary, A.; Nenoff, P.; Gräser, Y.; Hainsworth, S.; Zhan, P.; et al. Antifungal Susceptibility and Mutations in the Squalene Epoxidase Gene in Dermatophytes of the Trichophyton Mentagrophytes Species Complex. Antimicrob. Agents Chemother. 2021, 65, e0005621. [Google Scholar] [CrossRef]
- Abastabar, M.; Babaei, M.; Mohammadi, R.; Valadan, R.; Javidnia, J.; Zaedi, A.; Aghili, S.R.; Haghani, I.; Khojasteh, S.; Reazaei-Matehkolaei, A.; et al. Iranian National Survey on Tinea Capitis: Antifungal Susceptibility Profile, Epidemiological Characteristics, and Report of Two Strains with a Novel Mutation in SQLE Gene with Homology Modeling. Mycopathologia 2023, 188, 449–460. [Google Scholar] [CrossRef]
- Dashti, Y.; Alobaid, K.; Al-Rashidi, S.; Dashti, M.; AbdulMoneim, M.H.; Al-Enezi, M.; Abou-Chakra, N.; Jørgensen, K.M. Autochthonous Case of Trichophyton Indotineae in Kuwait. J. Med. Mycol. 2023, 33, 101432. [Google Scholar] [CrossRef]
- Durdu, M.; Kandemir, H.; Karakoyun, A.S.; Ilkit, M.; Tang, C.; de Hoog, S. First Terbinafine-Resistant Trichophyton Indotineae Isolates with Phe397Leu and/or Thr414His Mutations in Turkey. Mycopathologia 2023, 188, 295–304. [Google Scholar] [CrossRef]
- Ngo, T.M.C.; Ton Nu, P.A.; Le, C.C.; Ha, T.N.T.; Do, T.B.T.; Tran Thi, G. First Detection of Trichophyton Indotineae Causing Tinea Corporis in Central Vietnam. Med. Mycol. Case Rep. 2022, 36, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Villa-Gonzalez, J.M.; Pascual Ares, M.; López-Soria, L.M.; Gonzalez-Hermosa, M.R.; Gardeazabal García, J.; Lasa Elgezua, O. Extensive Tinea Corporis Caused by Trichophyton Indotineae: Report of a Case in Spain. J. Eur. Acad. Dermatology Venereol. 2024, 38, e22–e23. [Google Scholar] [CrossRef]
- Jabet, A.; Brun, S.; Normand, A.-C.; Imbert, S.; Akhoundi, M.; Dannaoui, E.; Audiffred, L.; Chasset, F.; Izri, A.; Laroche, L.; et al. Extensive Dermatophytosis Caused by Terbinafine-Resistant Trichophyton Indotineae, France. Emerg. Infect. Dis. 2022, 28, 229–233. [Google Scholar] [CrossRef]
- Russo, G.; Toutous Trellu, L.; Fontao, L.; Ninet, B. Towards an Early Clinical and Biological Resistance Detection in Dermatophytosis: About 2 Cases of Trichophyton Indotineae. J. Fungi 2023, 9, 733. [Google Scholar] [CrossRef]
- Verma, S.B.; Panda, S.; Nenoff, P.; Singal, A.; Rudramuruthy, S.M.; Uhrlass, S.; Das, A.; Bisherwal, K.; Shaw, D.; Vasani, R. The Unprecedented Epidemic-like Scenario of Dermatophytosis in India: I. Epidemiology, Risk Factors and Clinical Features. Indian. J. Dermatol. Venereol. Leprol. 2021, 87, 154. [Google Scholar] [CrossRef] [PubMed]
- Ebert, A.; Monod, M.; Salamin, K.; Burmester, A.; Uhrlaß, S.; Wiegand, C.; Hipler, U.; Krüger, C.; Koch, D.; Wittig, F.; et al. Alarming India-wide Phenomenon of Antifungal Resistance in Dermatophytes: A Multicentre Study. Mycoses 2020, 63, 717–728. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.B. Emergence of Recalcitrant Dermatophytosis in India. Lancet Infect. Dis. 2018, 18, 718–719. [Google Scholar] [CrossRef]
- Shen, J.J.; Arendrup, M.C.; Verma, S.; Saunte, D.M.L. The Emerging Terbinafine-Resistant Trichophyton Epidemic: What Is the Role of Antifungal Susceptibility Testing? Dermatology 2022, 238, 60–79. [Google Scholar] [CrossRef]
- McCormick, T.; Ghannoum, M. Time to Think Antifungal Resistance. Pathog. Immun. 2024, 8, 158–176. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.H.; Sardana, K.; Shenoy, M.M.; Rengasamy, M.; Khurana, A.; Ghate, S.; Venkata, C.K.; Marfatiya, Y.; Bhunia, D.; Jayaraman, J.; et al. IADVL SIG Recalcitrant Dermatophytosis Position Statement on Super Bioavailable Itraconazole. Indian Dermatol. Online J. 2024, 15, 1–7. [Google Scholar] [CrossRef]
- Ministero della Salute Modalità Di Segnalazione Delle Malattie Infettive. Available online: https://www.gazzettaufficiale.it/eli/id/2022/04/07/22A02179/sg (accessed on 16 August 2024).
- Graziani, C.; Duranti, A.; Morelli, A.; Busani, L.; Pezzotti, P. Zoonosi in Italia Nel Periodo 2009–2013. 2016. Available online: https://www.iss.it/rapporti-istisan/-/asset_publisher/Ga8fOpve0fNN/content/16-1-zoonosi-in-italia-nel-periodo-2009-2013.caterina-graziani-anna-duranti-alessandra-morelli-luca-busani-patrizio-pezzotti2016-72-p (accessed on 16 August 2024).
- Servizio Regionale per l’epidemiologia, Sorveglianza e Controllo Delle Malattie Infettive. Sistema Di Notifica Delle Malattie Infettive (Premal) Bollettino 2020–2022—Regione Lazio. Available online: https://www.inmi.it/wp-content/uploads/2024/03/Bollettino-PREMAL-2020-2022.pdf (accessed on 16 August 2024).
- Facciolà, A.; Visalli, G.; D’Andrea, G.; Laganà, A.; Varvarà, M.; Spataro, P.; Di Pietro, A. The Italian Mandatory Notification System: An Important Public Health Tool For Continuous Monitoring Of Infectious Diseases. New Microbiol. 2022, 45, 115–123. [Google Scholar]
- Crotti, S.; Cruciani, D.; Spina, S.; Piscioneri, V.; Natalini, Y.; Pezzotti, G.; Sabbatucci, M.; Papini, M. A Terbinafine Sensitive Trichophyton Indotineae Strain in Italy: The First Clinical Case of Tinea Corporis and Onychomycosis. J. Fungi 2023, 9, 865. [Google Scholar] [CrossRef]
- Mackenzie, D.W.R. “Hairbrush Diagnosis” in Detection and Eradication of Non-Fluorescent Scalp Ringworm. BMJ 1963, 2, 363–365. [Google Scholar] [CrossRef]
- Singh, A.; Singh, P.; Dingemans, G.; Meis, J.F.; Chowdhary, A. Evaluation of DermaGenius ® Resistance Real-time Polymerase Chain Reaction for Rapid Detection of Terbinafine-resistant Trichophyton Species. Mycoses 2021, 64, 721–726. [Google Scholar] [CrossRef]
- Kane, J.; Summerbell, R.; Sigler, L.; Krajden, S.; Land, G. (Eds.) Laboratory Handbook of Dermatophytes: A Clinical Guide and Laboratory Manual of Dermatophytes and Other Filamentous Fungi from Skin, Hair, and Nails, 1st ed.; Star Pub Co: Singapore, 1999. [Google Scholar]
- The University of Adelaide. Available online: https://www.Adelaide.Edu.Au (accessed on 20 August 2024).
- Eurostat: Statistics Explained Migration and Migrant Population Statistics. Available online: https://ec.europa.eu/eurostat/web/interactive-publications/migration-2023 (accessed on 20 August 2024).
- Sticchi, C.; Raso, R.; Ferrara, L.; Vecchi, E.; Ferrero, L.; Filippi, D.; Finotto, G.; Frassinelli, E.; Silvestre, C.; Zozzoli, S.; et al. Increasing Number of Cases Due to Candida Auris in North Italy, July 2019–December 2022. J. Clin. Med. 2023, 12, 1912. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Fungal Priority Pathogens List to Guide Research, Development and Public Health Action; WHO: Geneva, Switzerland, 2022. [Google Scholar]
- Global AMR R&D Hub. The Dynamic Dashboard. Available online: https://globalamrhub.org/dynamic-dashboard/ (accessed on 20 August 2024).
| Case Number 1 | Year | Sex | Age | Native Country | Clinical Manifestation | Terbinafine S/R 2 | Accession Number | Reference |
|---|---|---|---|---|---|---|---|---|
| 105 | 2023 | F | 42 | India | Tinea corporis and onychomycosis | S | OR192943 | Crotti et al. 2023 [41] |
| 115 | 2023 | F | 33 | Sri Lanka | Tinea corporis and tinea unguium | R | OR880561 | This study |
| 147 | 2024 | F | 44 | Peru | Tinea pedis | S | PP898430 | This study |
| 148 | 2024 | M | 16 | Peru | Tinea pedis | S | PP898431 | This study |
| 149 | 2024 | M | 18 | Bangladesh | Tinea corporis | R | PP898432 | This study |
| 150 | 2024 | M | 38 | Bangladesh | Tinea corporis | R | PP898433 | This study |
| 152 | 2024 | M | 47 | Bangladesh | Tinea cruris | S | PP898434 | This study |
| 153 | 2024 | F | 39 | Bangladesh | Tinea corporis | R | PP898435 | This study |
| 156 | 2024 | M | 20 | Bangladesh | Tinea faciei | R | PP898436 | This study |
| 159 | 2024 | M | 38 | Bangladesh | Tinea corporis | S | PP898437 | This study |
| 170 | 2024 | M | 28 | Bangladesh | Tinea cruris | R | PP898438 | This study |
| 171 | 2024 | M | 36 | Italy | Tinea cruris | R | PP898439 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crotti, S.; Cruciani, D.; Sabbatucci, M.; Spina, S.; Piscioneri, V.; Torricelli, M.; Calcaterra, R.; Farina, C.; Pisano, L.; Papini, M. Terbinafine Resistance in Trichophyton Strains Isolated from Humans and Animals: A Retrospective Cohort Study in Italy, 2016 to May 2024. J. Clin. Med. 2024, 13, 5493. https://doi.org/10.3390/jcm13185493
Crotti S, Cruciani D, Sabbatucci M, Spina S, Piscioneri V, Torricelli M, Calcaterra R, Farina C, Pisano L, Papini M. Terbinafine Resistance in Trichophyton Strains Isolated from Humans and Animals: A Retrospective Cohort Study in Italy, 2016 to May 2024. Journal of Clinical Medicine. 2024; 13(18):5493. https://doi.org/10.3390/jcm13185493
Chicago/Turabian StyleCrotti, Silvia, Deborah Cruciani, Michela Sabbatucci, Sara Spina, Vincenzo Piscioneri, Martina Torricelli, Roberta Calcaterra, Claudio Farina, Luigi Pisano, and Manuela Papini. 2024. "Terbinafine Resistance in Trichophyton Strains Isolated from Humans and Animals: A Retrospective Cohort Study in Italy, 2016 to May 2024" Journal of Clinical Medicine 13, no. 18: 5493. https://doi.org/10.3390/jcm13185493
APA StyleCrotti, S., Cruciani, D., Sabbatucci, M., Spina, S., Piscioneri, V., Torricelli, M., Calcaterra, R., Farina, C., Pisano, L., & Papini, M. (2024). Terbinafine Resistance in Trichophyton Strains Isolated from Humans and Animals: A Retrospective Cohort Study in Italy, 2016 to May 2024. Journal of Clinical Medicine, 13(18), 5493. https://doi.org/10.3390/jcm13185493









