Assessing the Efficacy and Safety of Misoprostol Prior to Hysteroscopy in Women with Difficult Cervix: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Guideline
2.2. Inclusion Criteria
2.3. Exclusion Criteria
- If sufficient data on the study population (including history of previous delivery, and history of hormonal therapy) were not available.
- The study included patients with a previous history of cervical surgery (e.g., cone biopsy) and Mullerian anomalies.
- The study involved postmenopausal or infertile premenopausal patients who had received hormonal treatment before the operation.
- The study included patients receiving paracervical block.
2.4. Outcomes
2.5. Search Strategy
2.6. Study Selection
2.7. Data Synthesis and Extraction
2.8. Assessment of Risk Bias
2.9. Statistical Analysis
3. Results
3.1. Search Results
3.2. Synthesis of Results
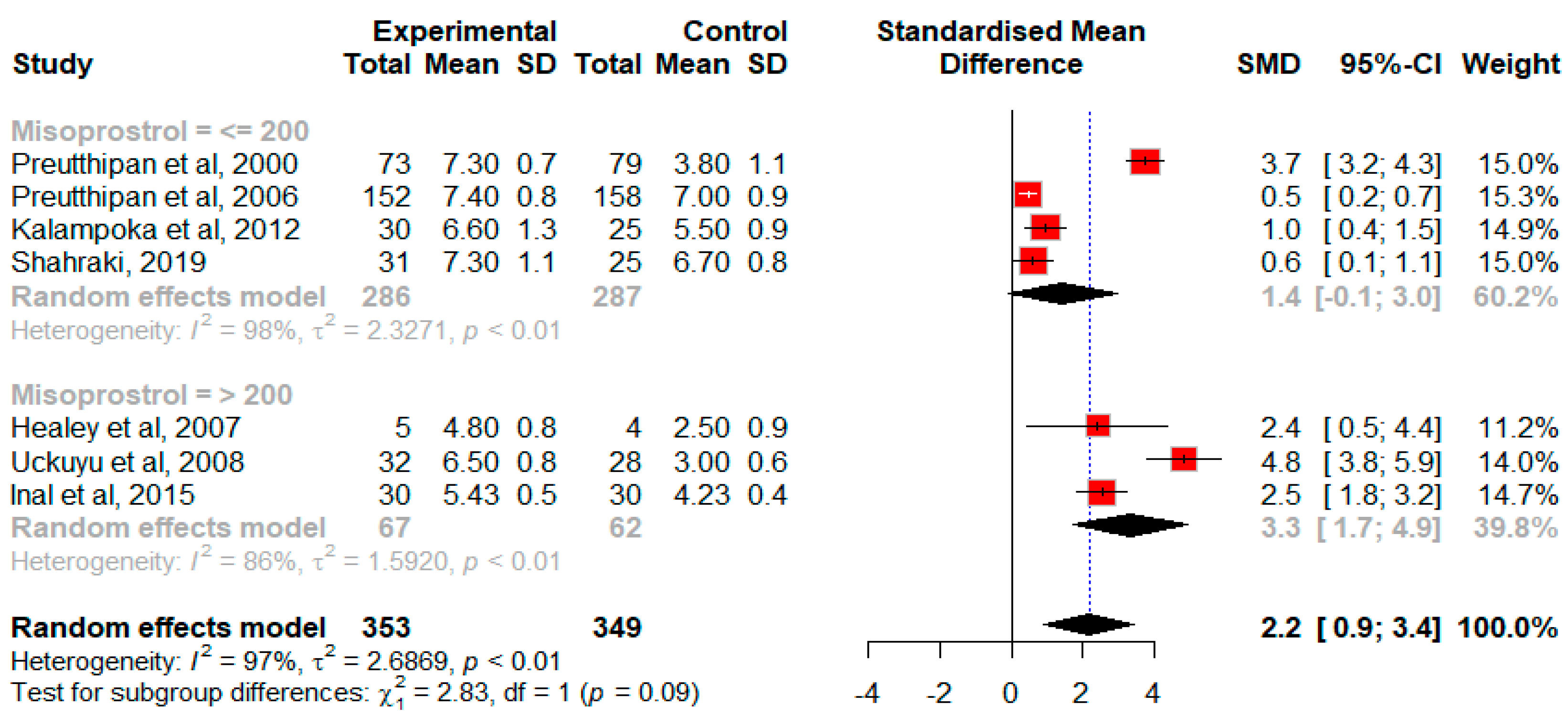
3.2.1. Efficacy of Misoprostol in Premenopausal Patients without Previous History of NVD
Effect of Misoprostol on the Cervical Width in Premenopausal Patients without Previous History of Normal Vaginal Delivery Prior to Hysteroscopy
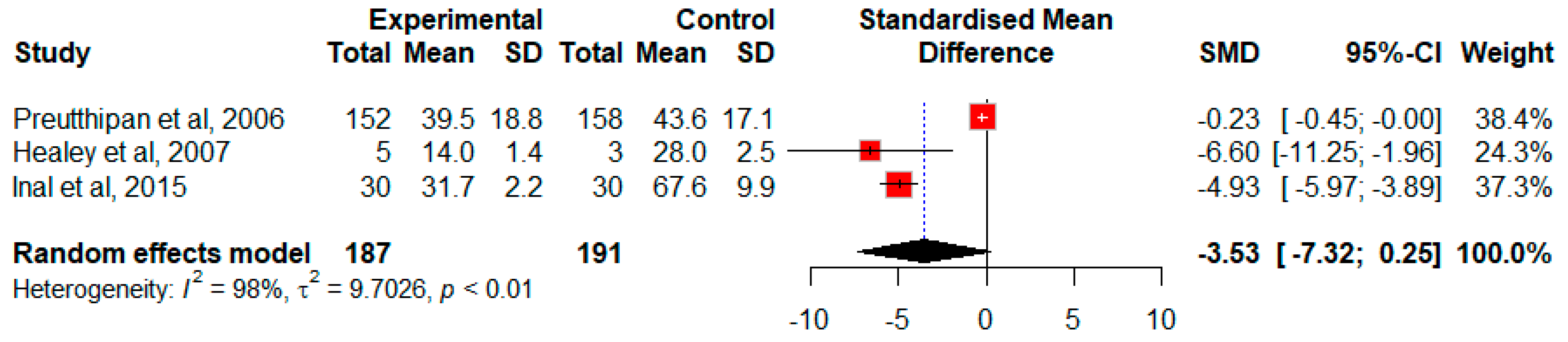
Effect of Misoprostol on the Duration of Cervical Dilation in Premenopausal Patients without Previous History of NVD
Effect of Misoprostol on the Need for Further Cervical Dilation in Premenopausal Patients without Previous History of NVD
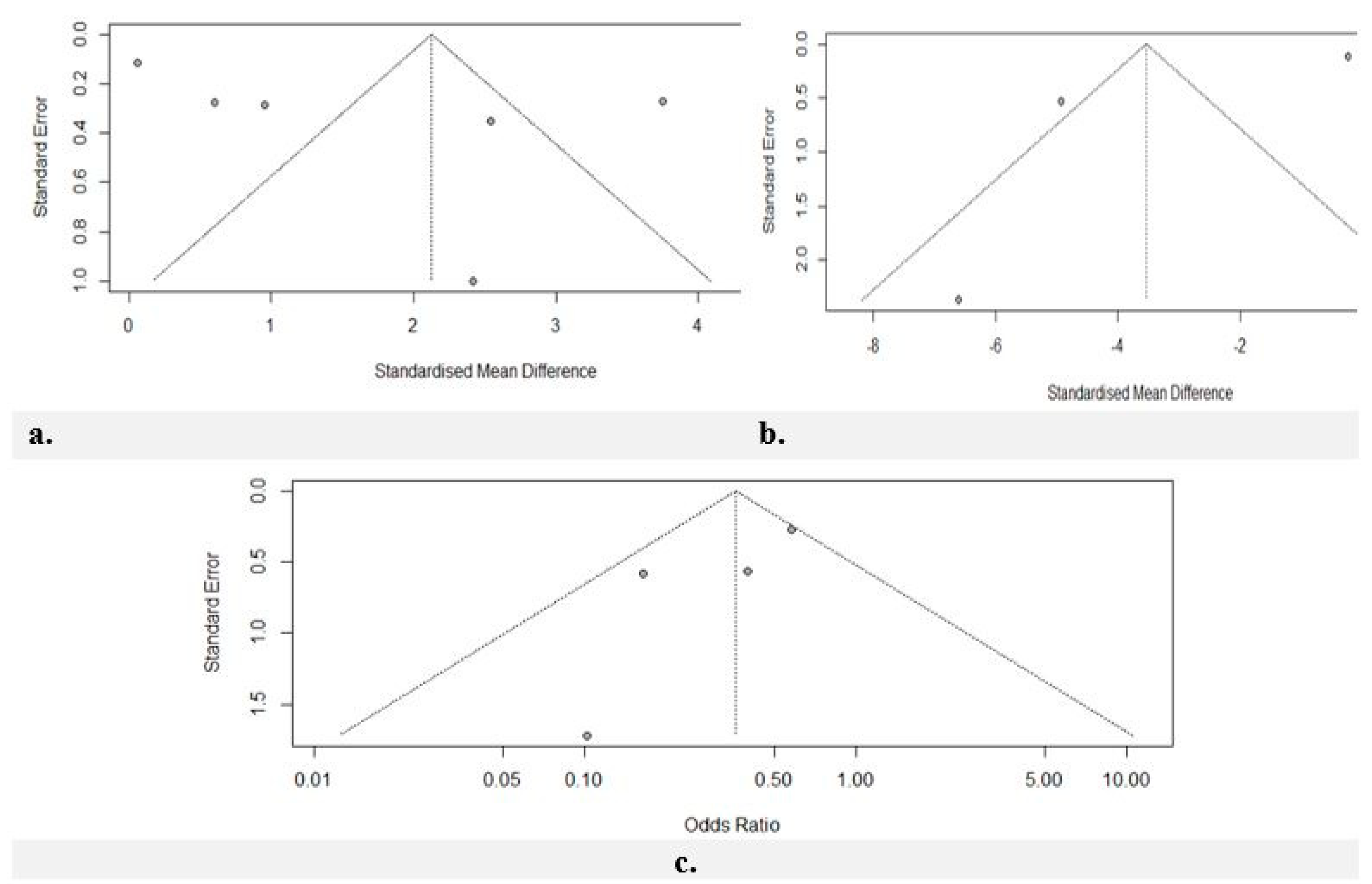
Risk of Bias Assessment
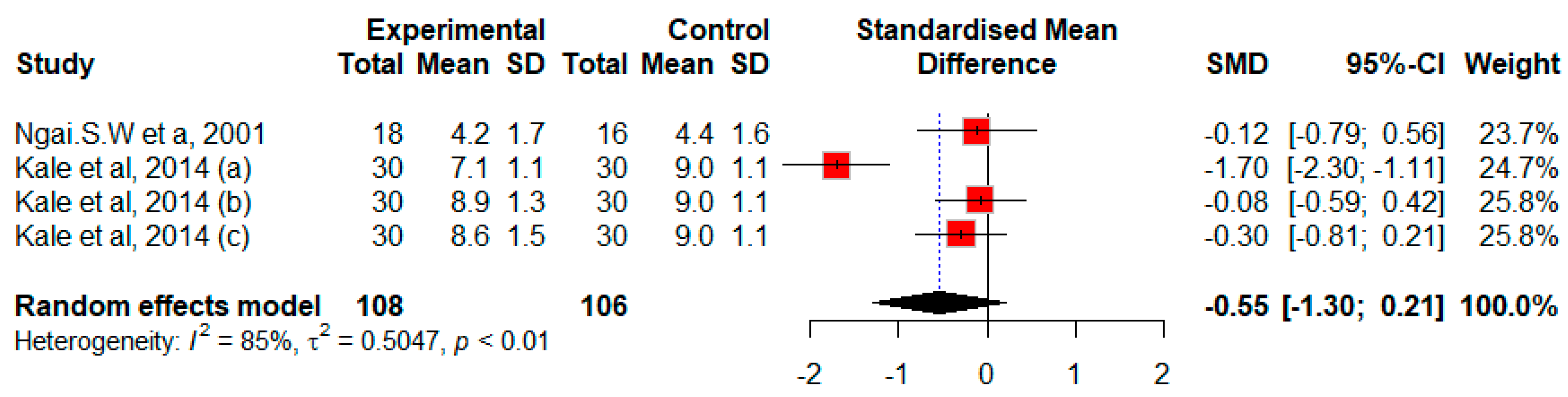
3.2.2. Efficacy of Misoprostol in Postmenopausal Patients
Efficacy of Misoprostol on Cervical Width Prior to Hysteroscopy in Postmenopausal Patients
Risk of Bias Assessment
3.2.3. The Safety of Misoprostol during Hysteroscopy
3.2.4. The Adverse Effects of Misoprostol in Patients Undergoing Hysteroscopy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nguyen, P.N.; Nguyen, V.T. Additional Value of Doppler Ultrasound to B-Mode Ultrasound in Assessing for Uterine Intracavitary Pathologies among Perimenopausal and Postmenopausal Bleeding Women: A Multicentre Prospective Observational Study in Vietnam. J. Ultrasound 2023, 26, 459–469. [Google Scholar] [CrossRef] [PubMed]
- El-Mazny, A.; Abou-Salem, N. A Double-Blind Randomized Controlled Trial of Vaginal Misoprostol for Cervical Priming before Outpatient Hysteroscopy. Fertil. Steril. 2011, 96, 962–965. [Google Scholar] [CrossRef]
- Uckuyu, A.; Ozcimen, E.E.; Sevinc, F.C.; Zeyneloglu, H.B. Efficacy of Vaginal Misoprostol before Hysteroscopy for Cervical Priming in Patients Who Have Undergone Cesarean Section and No Vaginal Deliveries. J. Minim. Invasive Gynecol. 2008, 15, 472–475. [Google Scholar] [CrossRef]
- Daniilidis, A.; Pantelis, A.; Dinas, K.; Tantanasis, T.; Loufopoulos, P.D.; Angioni, S.; Carcea, F. Indications of Diagnostic Hysteroscopy, a Brief Review of the Literature. Gynecol. Surg. 2012, 9, 23–28. [Google Scholar] [CrossRef]
- Kalampokas, E.; Sofoudis, C.; Antonogeorgos, G.; Panoulis, K.; Aravantinos, L.; Grigoriou, O.; Kalampokas, T. A Randomized Controlled Trial for Cervical Priming Using Vaginal Misoprostol Prior to Hysteroscopy in Women Who Have Only Undergone Cesarean Section. Arch. Gynecol. Obstet. 2012, 286, 853–857. [Google Scholar] [CrossRef]
- Zhuo, Z.; Yu, H.; Gao, L.; Jiang, X. Effectiveness of Misoprostol Administration for Cervical Ripening in Women before Operative Hysteroscopy: A Randomized, Double-Blinded Controlled Trial. Minim. Invasive Ther. Allied Technol. 2019, 28, 344–350. [Google Scholar] [CrossRef]
- Falcone, F.; Raimondo, G.; Stark, M.; Dessole, S.; Torella, M.; Raimondo, I. Balloon Catheter for Cervical Priming before Operative Hysteroscopy in Young Women: A Pilot Study. J. Investig. Surg. 2020, 33, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.K.; Subbaiah, M.; Maurya, D.K. Comparison of Efficacy of Vaginal Misoprostol Versus a Synthetic Osmotic Dilator (Dilapan-S) for Cervical Preparation before Operative Hysteroscopy: A Randomized Controlled Study. Gynecol. Minim. Invasive Ther. 2023, 12, 225–229. [Google Scholar] [CrossRef]
- Inácio, Q.A.S.; Troncon, J.K.; Valério, F.P.; Herren, H.; Nogueira, A.A.; Neto, O.B.P.; e Silva, J.C.R. Misoprostol Administration before Hysteroscopy Procedures—A Retrospective Analysis. Rev. Bras. Ginecol. Obs. 2022, 44, 1102–1109. [Google Scholar] [CrossRef]
- Mulayim, B.; Celik, N.Y.; Onalan, G.; Bagis, T.; Zeyneloglu, H.B. Sublingual Misoprostol for Cervical Ripening before Diagnostic Hysteroscopy in Premenopausal Women: A Randomized, Double Blind, Placebo-Controlled Trial. Fertil. Steril. 2010, 93, 2400–2404. [Google Scholar] [CrossRef]
- Oppegaard, K.; Nesheim, B.-I.; Istre, O.; Qvigstad, E. Comparison of Self-Administered Vaginal Misoprostol Versus Placebo for Cervical Ripening Prior to Operative Hysteroscopy Using a Sequential Trial Design. BJOG Int. J. Obstet. Gynaecol. 2008, 663, 663-e9. [Google Scholar] [CrossRef]
- Batukan, C.; Ozgun, M.T.; Ozcelik, B.; Aygen, E.; Sahin, Y.; Turkyilmaz, C. Cervical Ripening before Operative Hysteroscopy in Premenopausal Women: A Randomized, Double-Blind, Placebo-Controlled Comparison of Vaginal and Oral Misoprostol. Fertil. Steril. 2008, 89, 966–973. [Google Scholar] [CrossRef]
- Roberts, H.; Hickey, M. Managing the Menopause: An Update. Maturitas 2016, 86, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Kale, A.; Terzi, H.; Kale, E. Sublingual Misoprostol Is Better for Cervical Ripening Prior to Hysteroscopy in Post-Menopausal Women. Clin. Exp. Obstet. Gynecol. 2014, 41, 402–404. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, Z.; Yu, H.; Jiang, X. A Systematic Review and Meta-Analysis of Randomized Controlled Trials on the Effectiveness of Cervical Ripening with Misoprostol Administration before Hysteroscopy. Int. J. Gynecol. Obstet. 2016, 132, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A. The Cochrane Collaboration’s Tool for Assessing Risk of Bias in Randomised Trials. Bmj 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Routledge: Oxfordshire, UK, 2013. [Google Scholar]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring Inconsistency in Meta-Analyses. Bmj 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in Meta-Analysis Detected by a Simple, Graphical Test. Bmj 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Shahriyaripour, R.; Chaichian, S.; Tahermanesh, K.; Haghighi, L.; Derakhshan, R.; Sabet, B.; Rokhgireh, S. Cervical Preparation for Hysteroscopy Using Dilapan-S Three Hours before Surgery: A Randomized Controlled Trial. Clin. Exp. Obstet. Gynecol. 2024, 51, 9. [Google Scholar] [CrossRef]
- Shahraki, Z.; Ganjali, Y.; Ghajarzadeh, M. Misoprostol and Isosorbide Mononitrate for Cervical Ripening before Hysteroscopy: A Randomized Clinical Trial. Maedica 2019, 14, 260–263. [Google Scholar] [CrossRef]
- Preutthipan, S.; Herabutya, Y. A Randomized Comparison of Vaginal Misoprostol and Dinoprostone for Cervical Priming in Nulliparous Women before Operative Hysteroscopy. Fertil. Steril. 2006, 86, 990–994. [Google Scholar] [CrossRef] [PubMed]
- Preutthipan, S.; Herabutya, Y. Vaginal Misoprostol for Cervical Priming before Operative Hysteroscopy: A Randomized Controlled Trial. Obstet. Gynecol. 2000, 96, 890–894. [Google Scholar] [CrossRef]
- Ngai, S.; Chan, Y.; Ho, P. The Use of Misoprostol Prior to Hysteroscopy in Postmenopausal Women. Hum. Reprod. 2001, 16, 1486–1488. [Google Scholar] [CrossRef] [PubMed]
- Healey, S.; Butler, B.; Kum, F.N.; Dunne, J.; Hutchens, D.; Crane, J.M. A Randomized Trial of Oral Misoprostol in Premenopausal Women before Hysteroscopy. J. Obstet. Gynaecol. Can. 2007, 29, 648–652. [Google Scholar] [CrossRef]
- Inal, H.A.; Inal, Z.H.O.; Tonguc, E.; Var, T. Comparison of Vaginal Misoprostol and Dinoprostone for Cervical Ripening before Diagnostic Hysteroscopy in Nulliparous Women. Hum. Reprod. 2015, 103, 1326–1331. [Google Scholar] [CrossRef]
- Higgins, J. Cochrane Handbook for Systematic Reviews of Interventions; Wiley: Hoboken, NJ, USA, 2008. [Google Scholar]
- Lee, Y.Y.; Kim, T.J.; Kang, H.; Choi, C.H.; Lee, J.W.; Kim, B.G.; Bae, D.S. The Use of Misoprostol before Hysteroscopic Surgery in Non-Pregnant Premenopausal Women: A Randomized Comparison of Sublingual, Oral and Vaginal Administrations. Hum. Reprod. 2010, 25, 1942–1948. [Google Scholar] [CrossRef][Green Version]
- Socha, M.W.; Flis, W.; Wartęga, M.; Kunicka, A.; Stankiewicz, M. A Review of the Mechanism of Action and Clinical Applications of Osmotic Dilators for Cervical Ripening in the Induction of Labor and in Gynecology Procedures. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2023, 29, e940127. [Google Scholar] [CrossRef]
- Flis, W.; Socha, M.W. The Role of the Nlrp3 Inflammasome in the Molecular and Biochemical Mechanisms of Cervical Ripening: A Comprehensive Review. Cells 2024, 13, 600. [Google Scholar] [CrossRef] [PubMed]
- Kelly, R.W. Inflammatory Mediators and Cervical Ripening. J. Reprod. Immunol. 2002, 57, 217–224. [Google Scholar] [CrossRef]
- Rath, W.; Osmers, R.; Stuhlsatz, H.W.; Adelmann-Grill, B.C. Biochemical Principles of Cervix Ripening and Dilatation. Z. Geburtshilfe Perinatol. 1994, 198, 186–195. [Google Scholar]
- El-Refaey, H.; Templeton, A.; Calder, L.; Wheatley, D. Cervical Priming with Prostaglandin E1 Analogues, Misoprostol and Gemeprost. Lancet 1994, 343, 1207–1209. [Google Scholar] [CrossRef] [PubMed]
- da Costa, A.R.; Pinto-Neto, A.M.; Amorim, M.; Paiva, L.H.S.C.; Scavuzzi, A.; Schettini, J. Use of Misoprostol Prior to Hysteroscopy in Postmenopausal Women: A Randomized, Placebo-Controlled Clinical Trial. J. Minim. Invasive Gynecol. 2008, 15, 67–73. [Google Scholar] [CrossRef]
- Barcaite, E.; Bartusevicius, A.; Railaite, D.R.; Nadisauskiene, R. Vaginal Misoprostol for Cervical Priming before Hysteroscopy in Perimenopausal and Postmenopausal Women. Int. J. Gynaecol. Obstet. 2005, 91, 141–145. [Google Scholar] [CrossRef]
- Casadei, L.; Piccolo, E.; Manicuti, C.; Cardinale, S.; Collamarini, M.; Piccione, E. Role of Vaginal Estradiol Pretreatment Combined with Vaginal Misoprostol for Cervical Ripening before Operative Hysteroscopy in Postmenopausal Women. Obstet. Gynecol. Sci. 2016, 59, 220–226. [Google Scholar] [CrossRef]
- Nair, V.G.; Roy, K.K.; Rai, R.; Das, A.; Bharti, J.; Zangmo, R. Effectiveness of Misoprostol in Office Hysteroscopy in Premenopausal Nulliparous Women: A Prospective Randomized Double-Blind Placebo-Controlled Trial. J. Hum. Reprod. Sci. 2020, 13, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Fouda, U.M.; Elsetohy, K.A.; Elshaer, H.S.; Hammad, B.E.M.; Shaban, M.M.; Youssef, M.A.; Hashem, A.T.; Attia, A.H. Misoprostol Prior to Diagnostic Office Hysteroscopy in the Subgroup of Patients with No Risk Factors for Cervical Stenosis: A Randomized Double Blind Placebo-Controlled Trial. Gynecol. Obstet. Investig. 2018, 83, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Hua, Y.; Zhang, W.; Hu, X.; Yang, A. The Use of Misoprostol for Cervical Priming Prior to Hysteroscopy: A Systematic Review and Analysis. Drug Des. Dev. Ther. 2016, 10, 2789–2801. [Google Scholar] [CrossRef] [PubMed]
- Preutthipan, S.; Herabutya, Y. A Randomized Controlled Trial of Vaginal Misoprostol for Cervical Priming before Hysteroscopy. Obstet. Gynecol. 1999, 94, 427–430. [Google Scholar]
- Thomas, J.A.; Leyland, N.; Durand, N.; Windrim, R.C. The Use of Oral Misoprostol as a Cervical Ripening Agent in Operative Hysteroscopy: A Double-Blind, Placebo-Controlled Trial. Am. J. Obstet. Gynecol. 2002, 186, 876–879. [Google Scholar] [CrossRef]
- Fung, T.M.; Lam, M.H.; Wong, S.F.; Ho, L.C. A Randomised Placebo-Controlled Trial of Vaginal Misoprostol for Cervical Priming before Hysteroscopy in Postmenopausal Women. BJOG Int. J. Obstet. Gynaecol. 2002, 109, 561–565. [Google Scholar]
- Kant, A.; Divyakumar; Priyambada, U. A Randomized Trial of Vaginal Misoprostol for Cervical Priming before Hysteroscopy in Postmenopausal Women. J. Mid-Life Health 2011, 2, 25–27. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Ghosh, B.; Naha, M.; Mittal, S. Vaginal Misoprostol for Cervical Priming Prior to Diagnostic Hysteroscopy–Efficacy, Safety and Patient Satisfaction: A Randomized Controlled Trial. Arch. Gynecol. Obstet. 2009, 279, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Mathlouthi, N.; Saodi, O.; Ben Temime, R.; Makhlouf, T.; Attia, L.; Chachia, A. Sublingual Misoprostol for Cervical Ripening before Diagnostic Hysteroscopy: A Randomized and Prospective Study About 108 Cases. Tunis. Medicale 2011, 89, 825–829. [Google Scholar]
- Moiety, F.M.S.; Azzam, A. Prostaglandins Prior to Hysteroscopy. Gynecol. Surg. 2012, 9, 169–173. [Google Scholar] [CrossRef]



| Study, Date, and Country | Participants | Sample Size (Number) | Agents | Root | Dose | Time of Drug Administration before Hysteroscopy (Hours) | Frequency of Agent | Duration of Follow-Up (Hours) | Quality of the Article |
|---|---|---|---|---|---|---|---|---|---|
| Preutthipan, S., 2000, Thailand [23] | Premenopausal, nulliparous women | 73 vs. 79 | Misoprostol vs. Placebo | vaginal | 200 μg | 9–10 | 1 dose | Some concern | |
| Preutthipan, S., 2006, Thailand [22] | Premenopausal, nulliparous women | 152 vs. 158 | Misoprostol vs. Dinoprostone | vaginal | 200 μg vs. 3 mg | 9–10 | 1 dose | Some concern | |
| Healey, S., 2007, Canada [25] | Premenopausal, nulliparous women | 7 vs. 4 | Misoprostol vs. Placebo | oral | 400 μg | 12 | 1 dose | Low risk | |
| Uckuyu, A., 2007, Turkey [3] | Premenopausal women with a history of c/s and no previous NVD | 32 vs. 28 | Misoprostol vs. Placebo | vaginal | 400 μg | 12 and 6 | 2 doses | 8 | Low risk |
| Kalampokas, E., 2012, Greece [5] | Premenopausal women with a history of c/s and no previous NVD | 30 vs. 25 | Misoprostol vs. No treatment | vaginal | 200 μg | 12 | 1 dose | 6 | High risk |
| Inal, H., 2015, Turkey [26] | Premenopausal, nulliparous women | 30 vs. 30 vs. 30 | Misoprostol vs. Dinoprostone vs. Placebo | vaginal | 400 μg vs. 10 mg vs. placebo | 6–8 | 1 dose | 2 | Low risk |
| Shahraki, Z., 2019, Iran [21] | Premenopausal, nulliparous women | 31 vs. 25 | Misoprostol vs. IMN | vaginal | 25 μg vs. 40 μg | 16 h every 4 h vs. 12 h every 6 h | 4 doses vs. 2 doses | High risk |
| Study | Instrument | Effectiveness | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cervical Width | Mean ± SD (mm) | Need for Further Dilation | n (%) | Duration of Dilation | Mean ± SD (Seconds) | ||||||||
| Group 1 | Group 2 | Group 3 | Group 1 | Group 2 | Group 3 | Group 1 | Group 2 | Group 3 | |||||
| Preutthipan, S., 2000, Thailand [23] | 5.5 mm diagnostic hysteroscope/7 mm operative hysteroscope or 9 mm resectoscope | Misoprostol: 7.3 ± 0.7 * | Placebo: 3.8 ± 1.1 | Misoprostol: 55 (75.3) * | Placebo: 75 (94.9) | Misoprostol: median: 40 * | Placebo: median: 120 | ||||||
| Preutthipan, S. 2006 Thailand [22] | 5.5 mm diagnostic hysteroscope/7 mm operative hysteroscope or 9 mm resectoscope | Misoprostol: 7.4 ± 0.8 | Dinoprostone: 7.0 ± 0.9 | Misoprostol: 107 (70.4) * | Dinoprostone: 127 (80.4) | Misoprostol: 39.5 ± 18.8 * | Dinoprostone: 43.6 ± 17.1 | ||||||
| Healey, S., 2007, Canada [25] | A 6 mm diagnostic hysteroscope | Misoprostol: 4.8 | Placebo: 2.5 | Misoprostol: 2/5 (40) | Placebo: 3/3 (100) | Placebo: 14 | Placebo: 28 | ||||||
| Uckuyu, A., 2007, Turkey [3] | A rigid 10 mm resectoscope + a 30-degree forward-oblique lens | Misoprostol: 6.5 ± 0.8 * | Placebo: 3.0 ± 0.6 | ||||||||||
| Kalampokas, E., 2012, Greece [5] | A 12 mm resectoscope + 15-degree oblique lens | Misoprostol: 6.6 ± 1.3 * | No treatment: 5.5 ± 0.9 | ||||||||||
| Inal, H., 2015, Turkey [26] | A rigid 5.5 mm hysteroscope + 30-degree viewing angle | Misoprostol: 5.43 ± 0.50 ** | Dinoprostone: 5.83 ± 0.64 | Placebo: 4.23 ± 0.43 | Misoprostol: 17 (56.7) ** | Dinoprostone: 9 (30) | Placebo: 23 (76.7) | Misoprostol: 31.7 ± 2.23 ** | Dinoprostone: 26.93 ± 1.92 | Placebo: 67.56 ± 9.89 | |||
| Shahraki, Z., 2019, Iran [21] | Misoprostol: 7.3 ± 1.1 | IMN: 6.7 ± 0.8 | |||||||||||
| Study, Date, and Country | Participants | Sample Size (Number) | Agents | Root | Dose | Time of Drug Administration before Hysteroscopy (Hours) | Frequency of Agent | Duration of Follow-Up (Hours) | Quality of the Article |
|---|---|---|---|---|---|---|---|---|---|
| Ngai et al., 2001, China [24] | Postmenopausal women | 18 vs. 16 | Misoprostol vs. Placebo | Oral | 400 μg vs. Placebo | 12 | 1 dose | 6 | High risk |
| Kale et al., 2014, Turkey [14] | Postmenopausal women | 30 vs. 30 vs. 30 vs. 30 | Misoprostol | Sublingual vs. vaginal vs. rectal vs. Placebo | 200 μg | 6 and 12 | 2 doses | 6 | High risk |
| Shahriyaripour et al., 2024, Iran [20] | Postmenopausal women | 5 vs. 4 | Misoprostol vs. Dilapan_S | vaginal | 400 μg vs. Dilapan-S | 3 | 1 dose | Some concern |
| Study | Instrument | Effectiveness | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Cervical Width Mean ± SD (mm) | Duration of Dilation Mean ± SD (Seconds) | ||||||||
| Group 1 | Group 2 | Group 3 | Group 4 | Group 1 | Group 2 | Group 3 | Group 4 | ||
| Ngai et al., 2001, China [24] | Misoprostol: 4.2 ± 1.7 | Placebo: 4.4 ± 1.6 | |||||||
| Kale et al., 2014, Turkey [14] | A 10 mm + 15-degree optical system | Sublingual misoprostol: 7.1 ± 1.1 ** | Vaginal misoprostol: 8.9 ± 1.3 | Rectal misoprostol: 8.6 ± 1.5 | Placebo: 9.0 ± 1.1 | ||||
| Shahriyaripour et al., 2024, Iran [20] | A 9 mm resectoscope + 12-degree lens | Misoprostol: 84 ± 25 | Dilapan-S: 42.7 ± 5.2 | ||||||
| Study | Complications | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group 1 | Group 2 | Group 3 | Group 4 | |||||||||||||||||
| Participants (n) | Root/Agent | Uterine Laceration | False Tract | Uterine Perforation | Participants (n) | Root/Agent | Uterine Laceration | False Tract | Uterine Perforation | Participants (n) | Root/Agent | Uterine Laceration | False Tract | Uterine Perforation | Participants (n) | Root/Agent | Uterine Laceration | False Tract | Uterine Perforation | |
| Uckuyu, A. et al. [3] | 32 | Vaginal misoprostol | 1 | 1 | 0 | 28 | Placebo | 4 | 3 | 1 | ||||||||||
| Kalampokas, E. et al. [5] | 30 | Vaginal misoprostol | 1 | 1 | 25 | No treatment | 2 | 1 | ||||||||||||
| Kale, A. et al. [14] | 30 | Sublingual Misoprostol | 0 | 30 | Vaginal misoprostol | 1 | 30 | Rectal misoprostol | 2 | 30 | Placebo | 0 | ||||||||
| Preutthipan, S. et al. [23] | 73 | Vaginal misoprostol | 1 * | 1 | 0 | 79 | Placebo | 9 | 5 | 2 | ||||||||||
| Preutthipan, S. et al. [22] | 152 | Vaginal misoprostol | 3 * | 3 | 0 | 158 | Vaginal dinoprostone | 12 | 4 | 2 | ||||||||||
| Inal, H. et al. [26] | 30 | Vaginal misoprostol | 4 | 1 | 0 | 30 | Vaginal dinoprostone | 1 | 0 | 0 | 30 | Placebo | 7 | 2 | 1 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karimzadeh, A.; Allahqoli, L.; Salehiniya, H.; Hanjani, S.; Namavari, G.; Fazel Anvari-Yazdi, A.; Tahermanesh, K.; Alkatout, I. Assessing the Efficacy and Safety of Misoprostol Prior to Hysteroscopy in Women with Difficult Cervix: A Systematic Review and Meta-Analysis. J. Clin. Med. 2024, 13, 5494. https://doi.org/10.3390/jcm13185494
Karimzadeh A, Allahqoli L, Salehiniya H, Hanjani S, Namavari G, Fazel Anvari-Yazdi A, Tahermanesh K, Alkatout I. Assessing the Efficacy and Safety of Misoprostol Prior to Hysteroscopy in Women with Difficult Cervix: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2024; 13(18):5494. https://doi.org/10.3390/jcm13185494
Chicago/Turabian StyleKarimzadeh, Atieh, Leila Allahqoli, Hamid Salehiniya, Soheil Hanjani, Ghazal Namavari, Abbas Fazel Anvari-Yazdi, Kobra Tahermanesh, and Ibrahim Alkatout. 2024. "Assessing the Efficacy and Safety of Misoprostol Prior to Hysteroscopy in Women with Difficult Cervix: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 13, no. 18: 5494. https://doi.org/10.3390/jcm13185494
APA StyleKarimzadeh, A., Allahqoli, L., Salehiniya, H., Hanjani, S., Namavari, G., Fazel Anvari-Yazdi, A., Tahermanesh, K., & Alkatout, I. (2024). Assessing the Efficacy and Safety of Misoprostol Prior to Hysteroscopy in Women with Difficult Cervix: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 13(18), 5494. https://doi.org/10.3390/jcm13185494







