The Benefits of Cognitive Therapeutic Exercise in Symptomatic Arnold–Chiari Syndrome Type I: A Case Report on Gait, Balance, and Pain Management
Abstract
1. Introduction
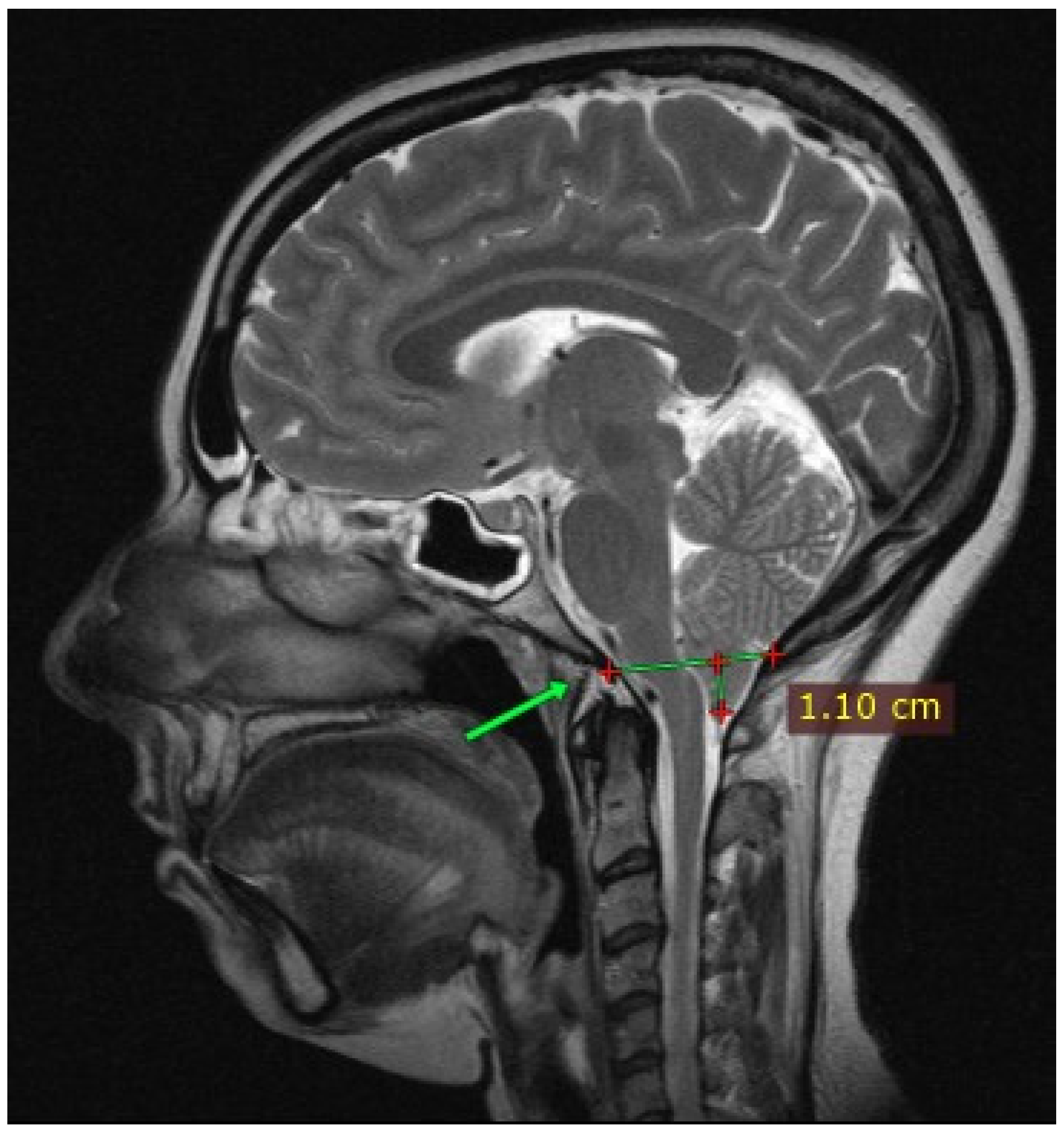
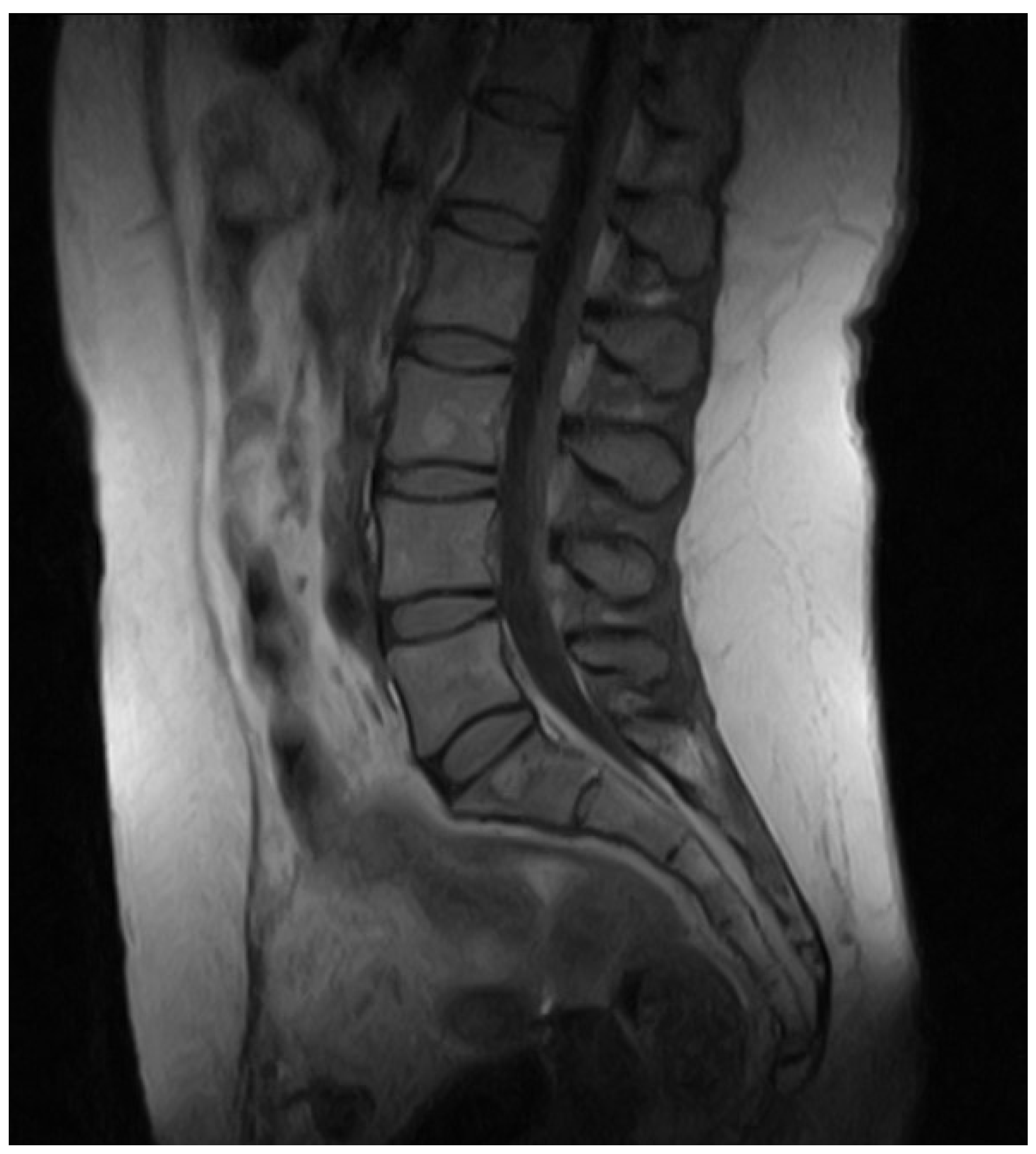
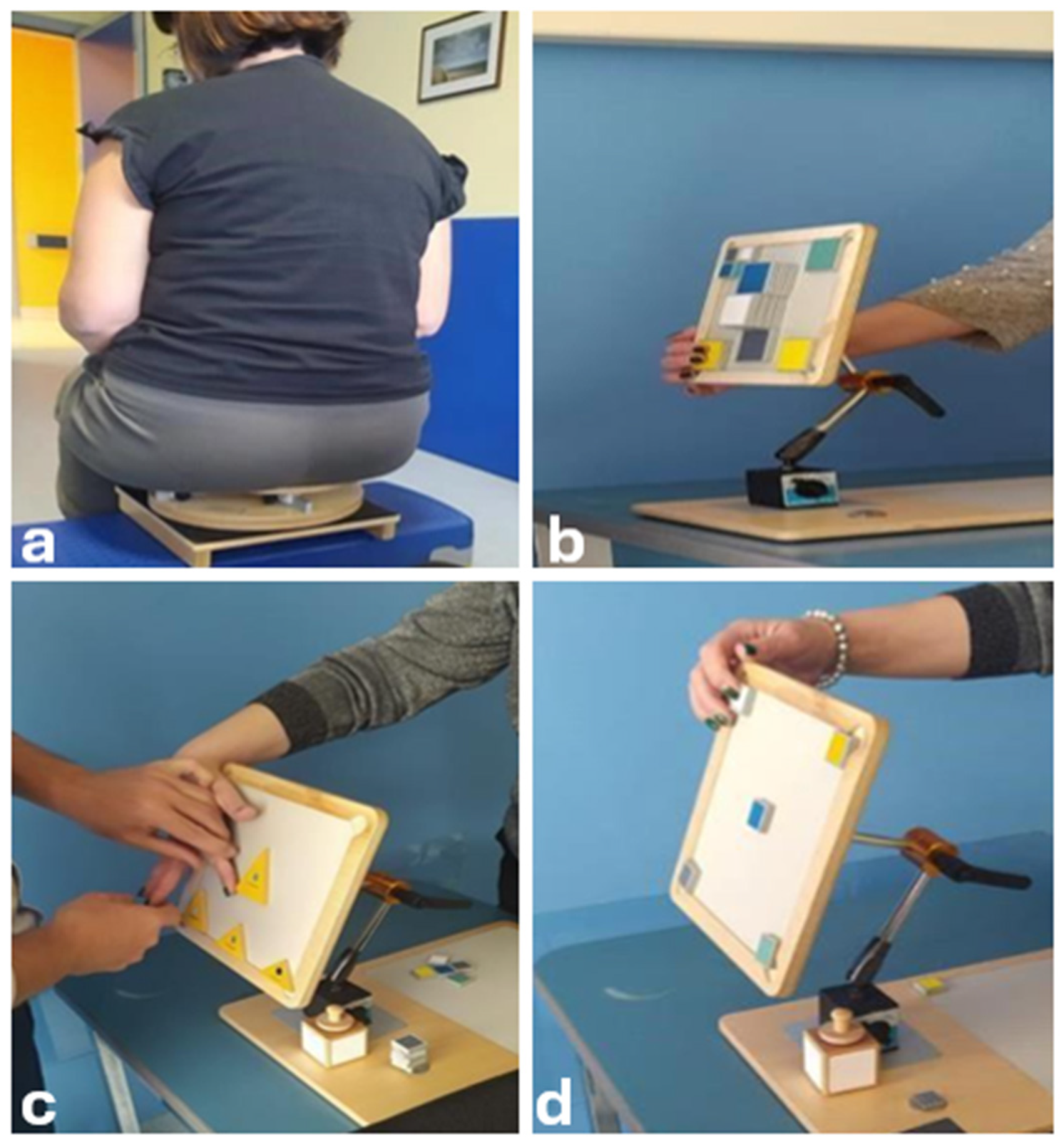
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ciaramitaro, P.; Massimi, L.; Bertuccio, A.; Solari, A.; Farinotti, M.; Peretta, P.; Saletti, V.; Chiapparini, L.; Barbanera, A.; Garbossa, D.; et al. Diagnosis and treatment of Chiari malformation and syringomyelia in adults: International consensus document. Neurol. Sci. 2022, 43, 1327–1342. [Google Scholar] [CrossRef] [PubMed]
- Ciaramitaro, P.; Garbossa, D.; Peretta, P.; Piatelli, G.; Massimi, L.; Valentini, L.; Migliaretti, G.; Baldovino, S.; Roccatello, D.; Kodra, Y.; et al. Syringomyelia and Chiari Syndrome Registry: Advances in epidemiology, clinical phenotypes and natural history based on a North Western Italy cohort. Ann. Ist. Super. Sanita 2020, 56, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Blanque, R.; Almazán-Soto, C.; Piqueras-Sola, B.; Sánchez-García, J.C.; Reinoso-Cobo, A.; Menor-Rodríguez, M.J.; Cortés-Martín, J. Chiari Syndrome: Advances in Epidemiology and Pathogenesis: A Systematic Review. J. Clin. Med. 2023, 12, 6694. [Google Scholar] [CrossRef] [PubMed]
- Arora, R. Imaging spectrum of cerebellar pathologies: A pictorial essay. Pol. J. Radiol. 2015, 80, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Alotaibi, N.M.; Samuel, N.; Ibrahim, G.M.; Fallah, A.; Cusimano, M.D. Acquired Chiari Malformation and Syringomyelia Secondary to Space-Occupying Lesions: A Systematic Review. World Neurosurg. 2017, 98, 800–808.e802. [Google Scholar] [CrossRef] [PubMed]
- Zisakis, A.; Sun, R.; Pepper, J.; Tsermoulas, G. Chiari Malformation Type 1 in Adults. Adv. Tech. Stand. Neurosurg. 2023, 46, 149–173. [Google Scholar] [CrossRef]
- Thunstedt, D.C.; Schmutzer, M.; Fabritius, M.P.; Thorsteinsdottir, J.; Kunz, M.; Ruscheweyh, R.; Straube, A. Headache characteristics and postoperative course in Chiari I malformation. Cephalalgia 2022, 42, 879–887. [Google Scholar] [CrossRef]
- Shaikh, A.G.; Ghasia, F.F. Neuro-ophthalmology of type 1 Chiari malformation. Expert. Rev. Ophthalmol. 2015, 10, 351–357. [Google Scholar] [CrossRef]
- Almotairi, F.S.; Andersson, M.; Andersson, O.; Skoglund, T.; Tisell, M. Swallowing Dysfunction in Adult Patients with Chiari I Malformation. J. Neurol. Surg. B Skull Base 2018, 79, 606–613. [Google Scholar] [CrossRef]
- Vale, J.M.; Silva, E.; Pereira, I.G.; Marques, C.; Sanchez-Serrano, A.; Torres, A.S. Chiari malformation and central sleep apnea syndrome: Efficacy of treatment with adaptive servo-ventilation. J. Bras. Pneumol. 2014, 40, 574–578. [Google Scholar] [CrossRef][Green Version]
- Luzzi, S.; Giotta Lucifero, A.; Elsawaf, Y.; Elbabaa, S.K.; Del Maestro, M.; Savioli, G.; Galzio, R.; Gragnaniello, C. Pulsatile cerebrospinal fluid dynamics in Chiari I malformation syringomyelia: Predictive value in posterior fossa decompression and insights into the syringogenesis. J. Craniovertebral Junction Spine 2021, 12, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Toader, C.; Ples, H.; Covache-Busuioc, R.-A.; Costin, H.P.; Bratu, B.-G.; Dumitrascu, D.-I.; Glavan, L.A.; Ciurea, A.V. Decoding Chiari Malformation and Syringomyelia: From Epidemiology and Genetics to Advanced Diagnosis and Management Strategies. Brain Sci. 2023, 13, 1658. [Google Scholar] [CrossRef] [PubMed]
- Famili, H.P.; Zalewski, C.K.; Ibrahimy, A.; Mack, J.; Cantor, F.; Heiss, J.D.; Brewer, C.C. Audiovestibular Findings in a Cohort of Patients with Chiari Malformation Type I and Dizziness. J. Clin. Med. 2023, 12, 2767. [Google Scholar] [CrossRef] [PubMed]
- Biswas, D.; Eppelheimer, M.S.; Houston, J.R.; Ibrahimy, A.; Bapuraj, J.R.; Labuda, R.; Allen, P.A.; Frim, D.; Loth, F. Quantification of Cerebellar Crowding in Type I Chiari Malformation. Ann. Biomed. Eng. 2019, 47, 731–743. [Google Scholar] [CrossRef] [PubMed]
- Avellaneda Fernández, A.; Guerrero, A.; Martínez, M.; Vázquez, M.; Fernández, J.; Octavio, E.; Labrado, J.; Silva, M.; Araoz, M.; García-Ramos, R.; et al. Malformations of the craniocervical junction (Chiari type I and syringomyelia: Classification, diagnosis and treatment). BMC Musculoskelet. Disord. 2009, 10 (Suppl. S1), S1. [Google Scholar] [CrossRef]
- Abdallah, A.; Rakip, U. Conservative Treatment of Chiari Malformation Type I Based on the Phase-Contrast Magnetic Resonance Imaging: A Retrospective Study. World Neurosurg. 2022, 163, e323–e334. [Google Scholar] [CrossRef]
- Lee, S.; Bae, S.; Jeon, D.; Kim, K.Y. The effects of cognitive exercise therapy on chronic stroke patients’ upper limb functions, activities of daily living and quality of life. J. Phys. Ther. Sci. 2015, 27, 2787–2791. [Google Scholar] [CrossRef]
- Nguyen, P.T.; Chou, L.W.; Hsieh, Y.L. Proprioceptive Neuromuscular Facilitation-Based Physical Therapy on the Improvement of Balance and Gait in Patients with Chronic Stroke: A Systematic Review and Meta-Analysis. Life 2022, 12, 882. [Google Scholar] [CrossRef]
- Marques, S.; Vaughan-Graham, J.; Costa, R.; Figueiredo, D. The Bobath concept (NDT) in adult neurorehabilitation: A scoping review of conceptual literature. Disabil. Rehabil. 2024, 10, 1–12. [Google Scholar] [CrossRef]
- Chanubol, R.; Wongphaet, P.; Chavanich, N.; Werner, C.; Hesse, S.; Bardeleben, A.; Merholz, J. A randomized controlled trial of Cognitive Sensory Motor Training Therapy on the recovery of arm function in acute stroke patients. Clin. Rehabil. 2012, 26, 1096–1104. [Google Scholar] [CrossRef]
- Van de Winckel, A.; De Patre, D.; Rigoni, M.; Fiecas, M.; Hendrickson, T.J.; Larson, M.; Jagadeesan, B.D.; Mueller, B.A.; Elvendahl, W.; Streib, C.; et al. Exploratory study of how Cognitive Multisensory Rehabilitation restores parietal operculum connectivity and improves upper limb movements in chronic stroke. Sci. Rep. 2020, 10, 20278. [Google Scholar] [CrossRef] [PubMed]
- Morreale, M.; Marchione, P.; Pili, A.; Lauta, A.; Castiglia, S.F.; Spallone, A.; Pierelli, F.; Giacomini, P. Early versus delayed rehabilitation treatment in hemiplegic patients with ischemic stroke: Proprioceptive or cognitive approach? Eur. J. Phys. Rehabil. Med. 2016, 52, 81–89. [Google Scholar] [PubMed]
- Gonzalez-Santos, J.; Soto-Camara, R.; Rodriguez-Fernández, P.; Jimenez-Barrios, M.; Gonzalez-Bernal, J.; Collazo-Riobo, C.; Jahouh, M.; Bravo-Anguiano, Y.; Trejo-Gabriel-Galan, J.M. Effects of home-based mirror therapy and cognitive therapeutic exercise on the improvement of the upper extremity functions in patients with severe hemiparesis after a stroke: A protocol for a pilot randomised clinical trial. BMJ Open 2020, 10, e035768. [Google Scholar] [CrossRef] [PubMed]
- Gürbüz, M.S.; Berkman, M.Z.; Ünal, E.; Akpınar, E.; Gök, Ş.; Orakdöğen, M.; Aydın, S. Foramen Magnum Decompression and Duraplasty is Superior to Only Foramen Magnum Decompression in Chiari Malformation Type 1 Associated with Syringomyelia in Adults. Asian Spine J. 2015, 9, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Milano, J.B.; Barcelos, A.; Onishi, F.J.; Daniel, J.W.; Botelho, R.V.; Dantas, F.R.; Neto, E.R.; de Freitas Bertolini, E.; Mudo, M.L.; Brock, R.S.; et al. The effect of filum terminale sectioning for Chiari 1 malformation treatment: Systematic review. Neurol. Sci. 2020, 41, 249–256. [Google Scholar] [CrossRef]
- Royo-Salvador, M.B.; Fiallos-Rivera, M.V.; Villavicencio, P. Neuro-cranio-vertebral syndrome related to coccygeal dislocation: A preliminary study. World Neurosurg. X 2024, 21, 100252. [Google Scholar] [CrossRef]
- Riley, D.S.; Barber, M.S.; Kienle, G.S.; Aronson, J.K.; von Schoen-Angerer, T.; Tugwell, P.; Kiene, H.; Helfand, M.; Altman, D.G.; Sox, H.; et al. CARE guidelines for case reports: Explanation and elaboration document. J. Clin. Epidemiol. 2017, 89, 218–235. [Google Scholar] [CrossRef]
- Ware, J.E., Jr.; Sherbourne, C.D. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med. Care 1992, 30, 473–483. [Google Scholar] [CrossRef]
- Haefeli, M.; Elfering, A. Pain assessment. Eur. Spine J. 2006, 15 (Suppl. 1), S17–S24. [Google Scholar] [CrossRef]
- Aliaga, L.; Hekman, K.E.; Yassari, R.; Straus, D.; Luther, G.; Chen, J.; Sampat, A.; Frim, D. A novel scoring system for assessing Chiari malformation type I treatment outcomes. Neurosurgery 2012, 70, 656–664; discussion 664–655. [Google Scholar] [CrossRef]
- Benedetto, L.; Oró, J.; Mueller, D.; Panarello, D.; Turco, E.; Caruso, G.; Germanò, A.; Ingrassia, M.; Mueller, J.; Turco, L. Psychological symptoms and Quality of Life in adults with Chiari malformation type I: An Assessment by the Italian version of Chiari Symptom Profile. Mediterr. J. Clin. Psychol. 2022, 10, 114–136. [Google Scholar] [CrossRef]
- Ma, Y.; Fan, Z.; Gao, W.; Yu, Z.; Ren, M.; Ma, Q.; Song, D.; Zhang, L.; Mi, L. Cognitive therapeutic exercise in early proprioception recovery after knee osteoarthritis surgery. Front. Rehabil. Sci. 2022, 3, 915010. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Solana, J.; Álvarez-Pardo, S.; Moreno-Villanueva, A.; Santamaría-Peláez, M.; González-Bernal, J.J.; Vélez-Santamaría, R.; González-Santos, J. Efficacy of a Rehabilitation Program Using Mirror Therapy and Cognitive Therapeutic Exercise on Upper Limb Functionality in Patients with Acute Stroke. Healthcare 2024, 12, 569. [Google Scholar] [CrossRef] [PubMed]
- Cavallaro, F.; Portaro, S.; Pintaudi, T.; Ceccio, M.; Alito, A. Remote Cognitive Therapeutic Exercise in Facial Nerve Palsy Rehabilitation: Pandemic Tips and Tricks. Innov. Clin. Neurosci. 2023, 20, 10–12. [Google Scholar] [PubMed]
- Sallés, L.; Martín-Casas, P.; Gironès, X.; Durà, M.J.; Lafuente, J.V.; Perfetti, C. A neurocognitive approach for recovering upper extremity movement following subacute stroke: A randomized controlled pilot study. J. Phys. Ther. Sci. 2017, 29, 665–672. [Google Scholar] [CrossRef]
- Cappellino, F.; Paolucci, T.; Zangrando, F.; Iosa, M.; Adriani, E.; Mancini, P.; Bellelli, A.; Saraceni, V.M. Neurocognitive rehabilitative approach effectiveness after anterior cruciate ligament reconstruction with patellar tendon. A randomized controlled trial. Eur. J. Phys. Rehabil. Med. 2012, 48, 17–30. [Google Scholar]
- Celletti, C.; Paolucci, T.; Maggi, L.; Volpi, G.; Billi, M.; Mollica, R.; Camerota, F. Pain Management through Neurocognitive Therapeutic Exercises in Hypermobile Ehlers–Danlos Syndrome Patients with Chronic Low Back Pain. BioMed Res. Int. 2021, 2021, 6664864. [Google Scholar] [CrossRef]
- Alito, A.; Cifalinò, M.E.; Fontana, J.M.; Verme, F.; Piterà, P.; Capodaglio, P. Tackling Kinesiophobia in Chronic Shoulder Pain: A Case Report on the Combined Effect of Pain Education and Whole-Body Cryostimulation. J. Clin. Med. 2024, 13, 2094. [Google Scholar] [CrossRef]
- Lim, J.A.; Choi, S.H.; Lee, W.J.; Jang, J.H.; Moon, J.Y.; Kim, Y.C.; Kang, D.H. Cognitive-behavioral therapy for patients with chronic pain: Implications of gender differences in empathy. Medicine 2018, 97, e10867. [Google Scholar] [CrossRef]
- Mateos-Aparicio, P.; Rodríguez-Moreno, A. The Impact of Studying Brain Plasticity. Front. Cell Neurosci. 2019, 13, 66. [Google Scholar] [CrossRef]
- Bertino, S.; Basile, G.A.; Bramanti, A.; Ciurleo, R.; Tisano, A.; Anastasi, G.P.; Milardi, D.; Cacciola, A. Ventral intermediate nucleus structural connectivity-derived segmentation: Anatomical reliability and variability. Neuroimage 2021, 243, 118519. [Google Scholar] [CrossRef] [PubMed]
- Dayan, E.; Cohen, L.G. Neuroplasticity subserving motor skill learning. Neuron 2011, 72, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Ingale, G.; Zade, R.; Timothy, R. Physiotherapeutic Intervention in a 19-Year-Old Female Patient With Syringohydromyelia: A Case Report. Cureus 2023, 15, e50419. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, P.R.; Zade, R.J.; Samal, S.S. The Originality of Neuro Rehabilitation Protocols in a Definitive Case of Syringomyelia Related to Chiari I Malformation. Cureus 2023, 15, e45157. [Google Scholar] [CrossRef]




| T0 | T1 | T2 | |
|---|---|---|---|
| SF-36 (%) | |||
| Physical functioning | 30 | 75 | 90 |
| Role limitations due to physical health | 0 | 75 | 100 |
| Role limitations due to emotional problems | 66.7 | 100 | 100 |
| Energy/fatigue | 15 | 60 | 70 |
| Emotional well-being | 44 | 84 | 84 |
| Social functioning | 37.5 | 75 | 87.5 |
| Physical pain | 0 | 67.5 | 100 |
| General health | 30 | 50 | 60 |
| Health change | 0 | 75 | 100 |
| NRS (0–10) | 8 | 3 | 1 |
| CCOS (4–16) | 9 | 10 | 14 |
| CSP (0–228) | 138 | 58 | 19 |
| VAS (0–10) (dysperception) | 8 | 3 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tisano, A.; Alito, A.; Ragonese de Gregorio, R.; Campo, A.; Santoro, G.; Milardi, D.; Cavallaro, F.; Cucinotta, F. The Benefits of Cognitive Therapeutic Exercise in Symptomatic Arnold–Chiari Syndrome Type I: A Case Report on Gait, Balance, and Pain Management. J. Clin. Med. 2024, 13, 5502. https://doi.org/10.3390/jcm13185502
Tisano A, Alito A, Ragonese de Gregorio R, Campo A, Santoro G, Milardi D, Cavallaro F, Cucinotta F. The Benefits of Cognitive Therapeutic Exercise in Symptomatic Arnold–Chiari Syndrome Type I: A Case Report on Gait, Balance, and Pain Management. Journal of Clinical Medicine. 2024; 13(18):5502. https://doi.org/10.3390/jcm13185502
Chicago/Turabian StyleTisano, Adriana, Angelo Alito, Rita Ragonese de Gregorio, Adele Campo, Giuseppe Santoro, Demetrio Milardi, Filippo Cavallaro, and Francesca Cucinotta. 2024. "The Benefits of Cognitive Therapeutic Exercise in Symptomatic Arnold–Chiari Syndrome Type I: A Case Report on Gait, Balance, and Pain Management" Journal of Clinical Medicine 13, no. 18: 5502. https://doi.org/10.3390/jcm13185502
APA StyleTisano, A., Alito, A., Ragonese de Gregorio, R., Campo, A., Santoro, G., Milardi, D., Cavallaro, F., & Cucinotta, F. (2024). The Benefits of Cognitive Therapeutic Exercise in Symptomatic Arnold–Chiari Syndrome Type I: A Case Report on Gait, Balance, and Pain Management. Journal of Clinical Medicine, 13(18), 5502. https://doi.org/10.3390/jcm13185502






