Abstract
Survival after lung transplantation has significantly improved during the last two decades. The refinement of the already existing extracorporeal life support (ECLS) systems, such as extracorporeal membrane oxygenation (ECMO), and the introduction of new techniques for donor lung optimization, such as ex vivo lung perfusion (EVLP), have allowed the extension of transplant indication to patients with end-stage lung failure after acute respiratory distress syndrome (ARDS) and the expansion of the donor organ pool, due to the better evaluation and optimization of extended-criteria donor (ECD) lungs and of donors after circulatory death (DCD). The close monitoring of anti-HLA donor-specific antibodies (DSAs) has allowed the early recognition of pulmonary antibody-mediated rejection (AMR), which requires a completely different treatment and has a worse prognosis than acute cellular rejection (ACR). As such, the standardization of patient selection and post-transplant management has significantly contributed to this positive trend, especially at high-volume centers. This review focuses on lung transplantation after ARDS, on the role of EVLP in lung donor expansion, on ECMO as a principal cardiopulmonary support system in lung transplantation, and on the diagnosis and therapy of pulmonary AMR.
1. Introduction
Lung transplantation is the gold standard therapy for end-stage lung diseases that are refractory to more conservative therapies. However, survival after lung transplantation is worse than survival after other solid organ transplantations. According to the 36th adult and 22nd pediatric lung and heart-lung transplant reports of the International Society for Heart and Lung Transplantation (ISHLT), unadjusted 5-year survival amounted to 55.7% for adults and 53.1% for pediatric patients [1,2,3]. The survival of lung-transplanted patients has been impaired by the development of primary graft dysfunction (PGD), acute infections, malignancies, and acute and chronic lung allograft rejection (CLAD). CLAD includes two distinct phenotypes, the Bronchiolitis Obliterans Syndrome (BOS) and the more recently identified Restrictive Allograft Syndrome (RAS) [4,5]. According to the ISHLT registry reports, 5-year freedom from BOS amounted to only 50% and 45.7% in adult and pediatric patients, respectively [1,2,3,6].
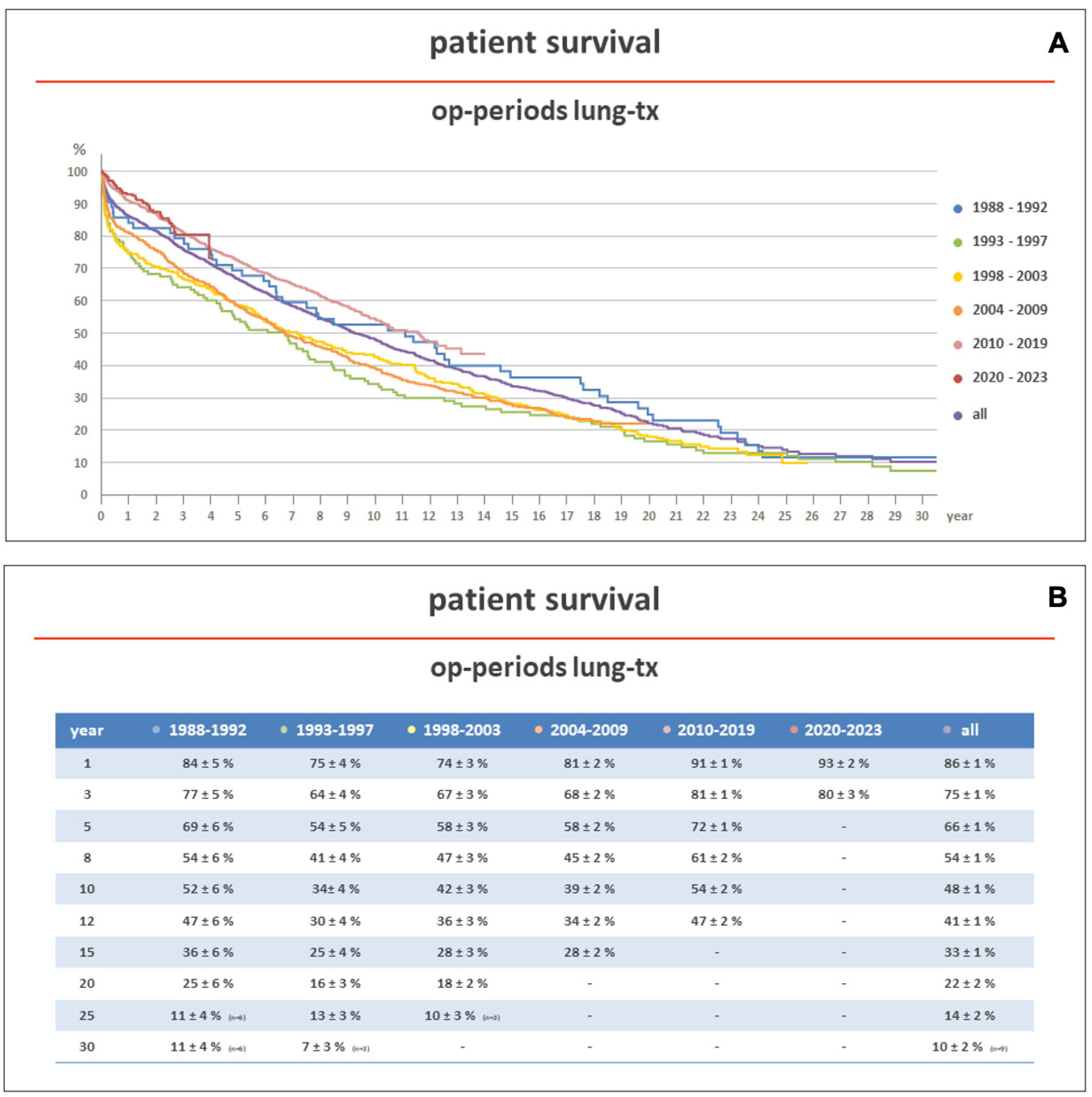
In more recent years (2010–2017), according to the same ISHLT reports, 5-year survival after lung transplantation has improved to 58.6% and 58.5% in adult and pediatric patients, respectively. This effect has been also observed at our institution, a center member of Eurotransplant (ET), where, between 1988 and 2023, 2639 patients underwent lung transplantation. The five-year unadjusted survival improved from 54% in the years between 1993 and 1997 to 72% in the years between 2010 and 2019 (Figure 1A,B).
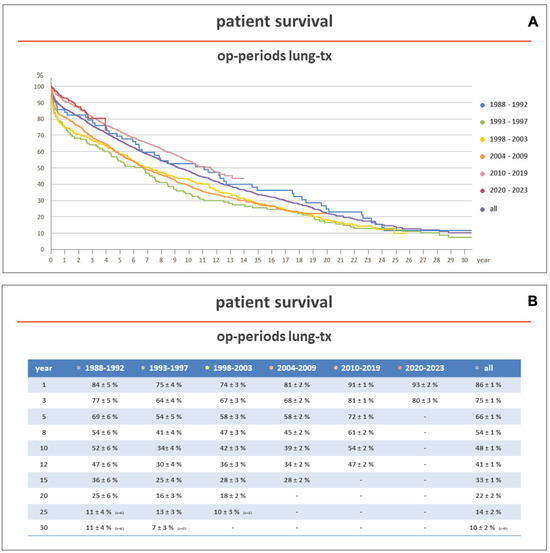
Figure 1.
(A,B) show the survival of patients transplanted at the Hannover Medical School between 1983 and 2023, stratified according to the transplant era. Early survival was better in patients transplanted in more recent years (2010–2023). Op: operation; Tx: transplantation.
This positive trend over the last two decades is explained in part by the great standardization of patient selection for lung transplantation and in part by the recent introduction and refinement of more versatile systems for extracorporeal life support (ECLS), such as extracorporeal membrane oxygenation (ECMO), for donor lung optimization, such as ex vivo lung perfusion (EVLP), and by the introduction of therapies for the treatment of acute cellular and antibody-mediated rejection (ACR and AMR, respectively), and for the prevention and treatment of viral, bacterial and fungal infections [7].
In this review, the contributors to this positive survival trend will be thoroughly discussed, with a particular focus on ECMO, EVLP and AMR.
2. Patient Selection and Indications
In lung transplantation, patient selection is of paramount importance and has been regulated by guidelines published by the ISHLT and the national general medical associations [8]. The recipient characteristics that were considered relative or absolute contraindications to transplantation two decades ago have now been accepted by most transplant centers. For example, older age (65–70 years) is not considered a contraindication to transplantation anymore, especially if patients have a low degree of frailty.
Donor organ allocation according to urgency and the post-transplant survival benefit are the most important rules for suggesting lung transplantation. Lung allocation has been regulated by urgency scores, such as the T (transplantable) and HU (high urgency) system, and the Lung Allocation Score (LAS) and its most recent update, the Composite Allocation Score (CAS) [9]. Each country has adopted its own allocation algorithm, and this may differently impact patient outcomes [10,11]. For example, Germany and The Netherlands use the LAS, while Austria and Belgium still use the T-HU system. In France, the super urgent status was introduced to prioritize very sick recipients with pulmonary fibrosis and primary pulmonary hypertension [11]. However, the ideal allocation score still does not exist [12,13,14]. In the US, the recently introduced CAS gave additional priority points to allosensitized patients, patients with the 0 blood group and small- or large-sized patients [15].
In adults, the most frequent indications for lung transplantation are end-stage lung emphysema and pulmonary fibrosis. Historically, cystic fibrosis-associated lung disease represented the third indication for lung transplantation. However, the introduction of triple channel modulator therapy has effectively reduced the indication for lung transplantation in patients with cystic fibrosis [16]. A similar trend can be expected in the future in patients with primary pulmonary hypertension (PPH) after the introduction of the activin signalling inhibitor sotatercept [17].
During the last decade, transplant indications have changed in pediatric (<18 years old) patients too, where the most common transplant indications have become PPH and childhood interstitial lung disease (CHILD); meanwhile, similar to adults, the transplant indication for cystic fibrosis has decreased [18]. This change has significantly influenced patient peritransplant management and has posed new operative challenges. In fact, more children aged < 12 years old are transplanted than before and such patients more often require peritransplant cardiopulmonary support than older children [18].
On the contrary, the number of transplants for other indications, such as acute end-stage lung disease after acute respiratory distress syndrome (ARDS), has increased in the last 5 years [19,20].
3. Transplant Indication for ARDS
Before the COVID-19 pandemic, lung transplantation was rarely indicated for acute end-stage lung disease after ARDS, due to the following reasons. First of all, there were no criteria for defining irreversible end-stage lung damage. To make it more complicated, there was no time threshold for defining irreversibility, as some patients with prolonged venous–venous ECMO runs recovered enough lung function to be weaned off it [21,22]. Second, many ARDS patients had obviously not undergone an evaluation for lung transplantation before ARDS occurred, and many were sedated and mechanically ventilated at the time of evaluation, thus being unable to consent to transplantation. Third, long-term post-transplant survival data were not available.
The COVID-19 pandemic encouraged the revision of multicentric experience to get more insights into lung transplantation for ARDS. In these patients, the survival results were encouraging, but they were usually obtained in highly selected patients [23,24,25,26]. Criteria for lung transplantation in patients with irreversible lung failure after ARDS have been recently published. The following “rules” were proposed [19,27]:
- Patients should be younger than 65 years, since the results of ECMO as a bridge to transplantation (BTT) have been worse in older patients. However, not only chronological age but also patient frailty should be considered.
- Patients should preferably only have single-organ dysfunction. However, patients requiring temporary hemodialysis have been successfully transplanted too. Concomitant liver dysfunction or left heart failure should be considered a contraindication to lung transplantation. The development of right heart failure due to secondary pulmonary hypertension should not be considered a contraindication to transplantation. In this case, veno-venous (v-v) ECMO should be upgraded to veno-arterial (v-a) or veno-arterial-venous (v-a-v) ECMO.
- Enough time should be allowed for lung recovery, usually 4–6 weeks after the initial event. Evaluation for lung transplantation may be considered earlier than 4 weeks, in case of potentially lethal pulmonary complications that cannot be managed medically or through the use of ECMO.
- There should be radiological evidence of irreversible lung disease, such as extensive honeycombing, cystic changes, reticular opacities, and traction bronchiectasis, >80% lung involvement, and a right atrium to left atrium ratio > 1; these are associated with an extended period of static respiratory mechanics or ECMO parameters [19]. The presence of secondary pulmonary arterial hypertension should be also considered a sign of irreversibility.
- Patients should be awake and able to discuss transplantation. The advantages of “awake” ECMO as a bridge to lung transplantation are well known. However, in some patients, who are otherwise good candidates for transplantation, spontaneous breathing is not possible. In this case, a patient’s next-of-kin may consent to transplantation.
- Patients should be able to participate in physical rehabilitation while on the transplantation waiting list.
- Patients should fulfil the remaining typical criteria for transplantation; these include, for example, an adequate body mass index and an absence of other notable conditions, such as severe coronary artery disease or a history of active smoking.
- Patients should have a negative SARS-CoV-2 virology status.
- Transplantation for ARDS should be performed only in high-volume centers that have substantial experience with ECMO as a bridge to transplantation.
- The transplant center should have access to a broad donor pool and low waiting list mortality. The clinical condition of patients with irreversible lung failure after ARDS may rapidly worsen, thus precluding transplantation. Continuous patient re-evaluation is therefore needed.
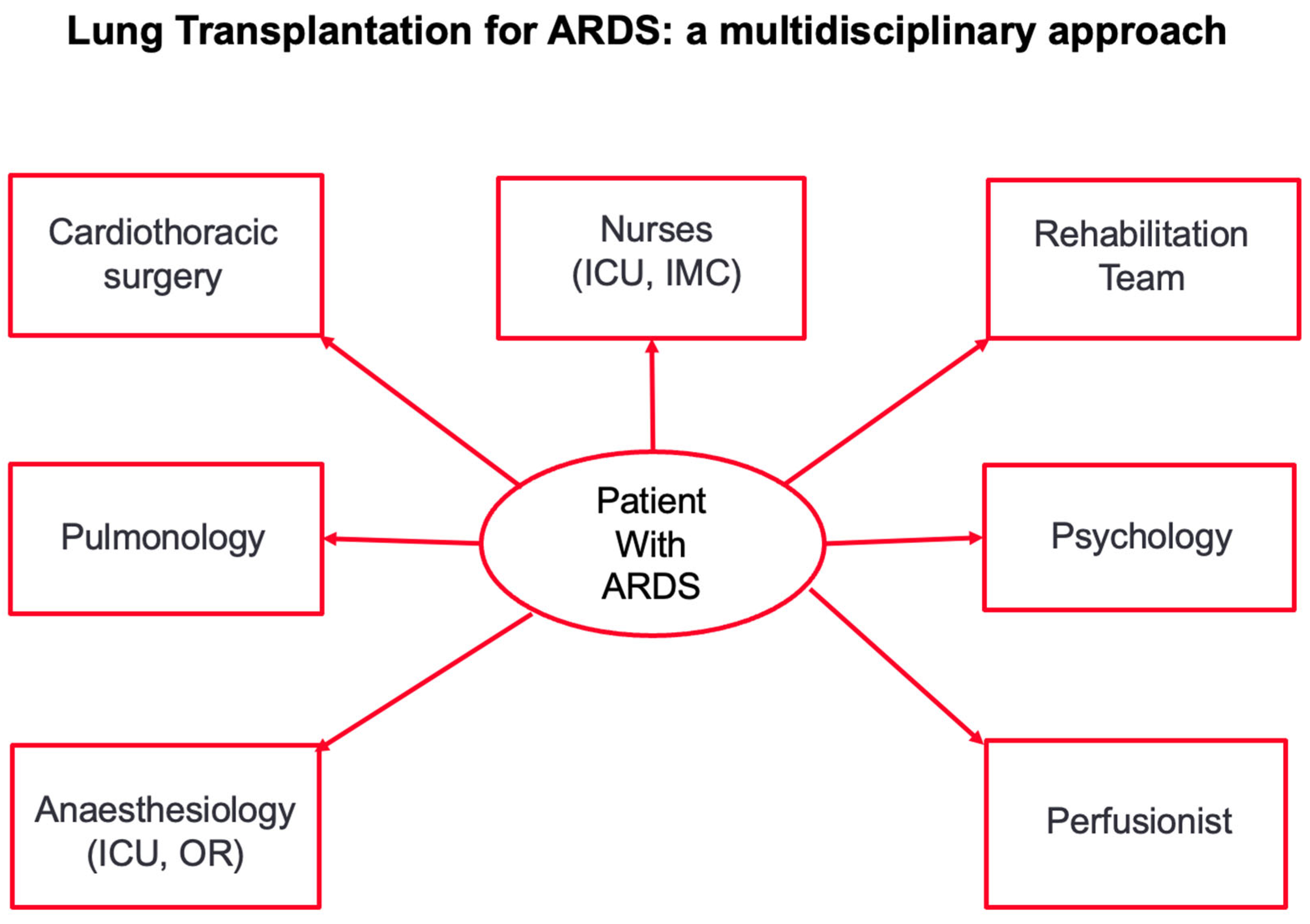
- These criteria and the indication for transplantation should be evaluated, discussed and approved by the multidisciplinary transplant conference. A multidisciplinary approach is of paramount importance in successfully transplanting patients after irreversible lung damage due to ARDS (Figure 2).
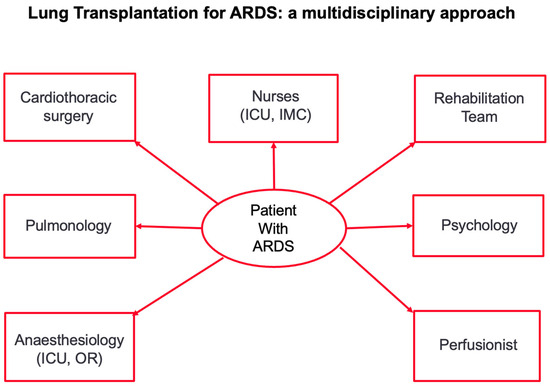 Figure 2. Figure 2 shows the multidisciplinary approach to transplantation in patients with irreversible lung damage after ARDS. ICU: intensive care unit; IMC: intermediate care station.
Figure 2. Figure 2 shows the multidisciplinary approach to transplantation in patients with irreversible lung damage after ARDS. ICU: intensive care unit; IMC: intermediate care station.
In the last 3 years, several case series on lung transplantation in highly selected patients after COVID-19 ARDS have been published [28,29,30,31,32,33]. In 2021, Bharat et al. published the first multicentric experience with lung transplantation in 12 patients with COVID-19 ARDS. All patients were on mechanical ventilation (MV, median time, 70 days; interquartile range, IQR, 49–80 days) and 11 patients were on ECMO (median time, 55 days; IQR, 44–79 days) before transplantation. After a waiting time of 6 days, the patients were transplanted using a clamshell approach and switching v-v to veno-arterial (v-a) ECMO. All the explanted lungs showed massive fibrosis. After transplantation, the median MV time was 16 (4–21) days, the median intensive care unit (ICU) time was 20 (13–24) days, and the median hospital stay was 37 (27–42) days; 30-day survival was 100% [28].
In a study based on the United Network for Organ Sharing (UNOS) database, Roach et al. showed that, between August 2020 and September 2021, 214 (7.0%) transplantations were performed for COVID-19-related respiratory failure (65% after ARDS, 35% for pulmonary fibrosis after COVID-19 ARDS). The 30-day mortality was 2.2%, and the 3-month survival was 95.6% [29].
In 19 (18%) out of 106 patients referred for transplantation between January 2020 and May 2021, Lang et al. reported a median (IQR) time on ECMO as a bridge to transplantation (BTT) of 41 (2–66) days. All patients underwent bilateral lung transplantation on v-a ECMO support. The median MV time was 24 (4–82) days, the median ICU time was 36 (12–98) days, and the median hospital stay was 64 (47–450) days; the 30-day survival was 100%. The 3-month survival did not differ between patients transplanted for COVID-19 ARDS and patients transplanted for other indications [30].
A propensity-matched analysis of 11 patients transplanted for COVID-19 and 163 hospitalized patients transplanted for other indications demonstrated comparable rates of intrathoracic adhesions, a comparable posttransplant duration of MV, PGD 3 at 72 h, and delayed chest closure between groups. However, patients transplanted for COVID-19 ARDS had significantly longer posttransplant hospital stays (48 vs. 23.5 days), a higher prevalence of acute rejection ≥ A2 (40% vs. 0%) and donor-specific antibodies > 2000 MFI (70% vs. 0%), but comparable 1-year survival rates [31].
Another analysis from the UNOS database showed that the 6-month survival did not differ between matched patients (n = 227) transplanted for COVID-19 respiratory failure and patients (n = 454) transplanted for other lung diseases (94.4% vs. 88.1%, p = 0.26) [32].
Finally, Bermudez et al. showed that the 1-year survival was similar between 188 patients transplanted for COVID-19 ARDS and 117 patients transplanted for post-COVID pulmonary fibrosis (87.3% vs. 86.7%). Among these patients, the 1-year survival did not differ between patients who required pretransplant ECMO and patients who did not require it (84.8% vs. 90.9%, p = 0.2). Additionally, the 1-year survival was similar in recipients requiring ECMO for COVID-19 lung failure and recipients requiring ECMO for non-COVID restrictive lung failure (84.8% vs. 78.0%, p = 0.1) [33].
4. Donor Optimization and EVLP
Standard cold preservation is the gold standard for donor lung preservation. However, once the donor lungs have been perfused and stored in the transport box, no other intervention or evaluation is possible, and the cold ischemic time is running until organ reperfusion. Thus, one of the first rules in standard cold preservation is to keep the ischemic time as short as possible to avoid posttransplant PGD [34,35,36].
First applied in 2001, ex vivo lung perfusion (EVLP) represents one of the most important recent innovations in lung transplantation [37,38,39,40,41,42,43]. The technique of EVLP comprises the perfusion of donor organs outside the body and has been developed to preserve explanted donor lungs in a perfused, ventilated and normothermic condition, thus decreasing tissue ischemia and lung inflammation, while allowing for extended evaluation and potential reconditioning prior to transplantation.
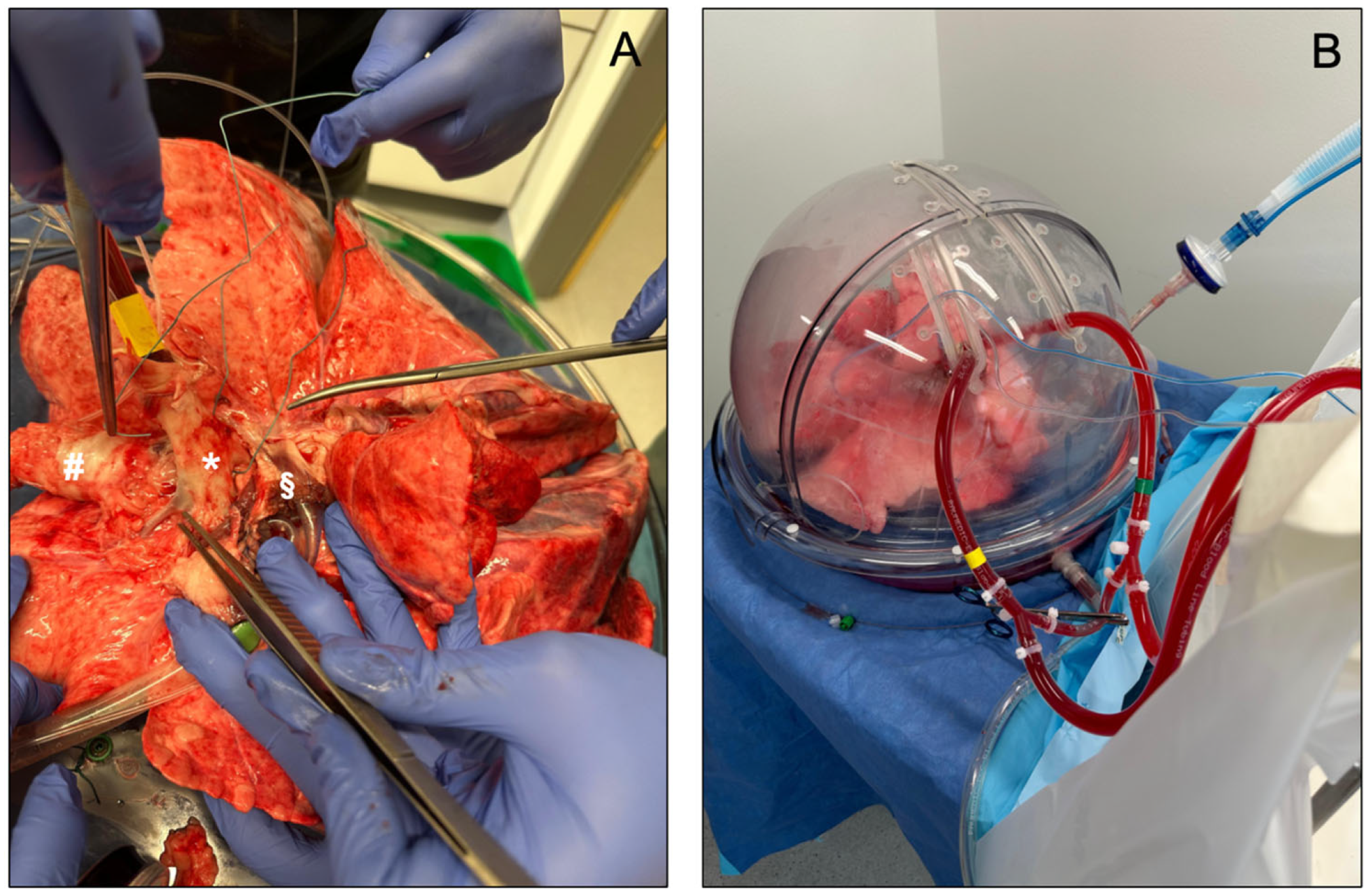
To date, three distinct EVLP devices have been approved for clinical use: the Organ Care System Lung (OCSTM Lung, TransMedics, Andover, MA, USA), the Vivoline LS1 system (Vivoline Medical, Lund, Sweden) and the XPS XVIVO Perfusion AB system (XVIVO Perfusion, Gothenburg, Sweden, Figure 3). The three systems have a common set-up, including an organ chamber, a reservoir for collecting the perfusion fluid, a pump (roller, centrifugal, or piston-driven), a ventilator, and an oxygenator. Interventions such as bronchoscopy are possible in all three systems.
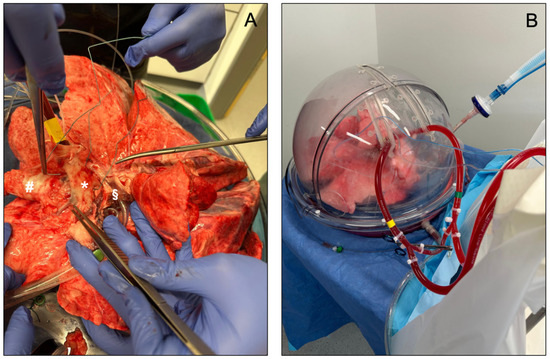
Figure 3.
(A,B) show an EVLP set-up using the XPS XVIVO Perfusion AB system (XVIVO Perfusion, Gothenburg, Sweden). In (A), * identifies the pulmonary artery, # the trachea, and § the left atrium.
However, in clinical application, the EVLP systems differ in some of their technical characteristics and perfusate properties (Table 1). The OCSTM system uses OCSTM solution with red blood cells (RBCs, cellular perfusate) to target a hematocrit between 15% and 25%, an open left atrium (LA) and a pulmonary artery flow of 2–3 L/min. The Vivoline LS1 system uses the Steen solution added with RBCs to target a hematocrit between 10% and 15% (the so-called Lund protocol). The LA is left open. A pulmonary arterial flow of 100% cardiac output (CO) is used. The XPS system utilizes Steen solution but without RBCs (acellular perfusate, the so-called Toronto protocol). The LA is left closed by suturing the LA draining cannula, so that the LA pressure reaches 3 to 5 mmHg. The pulmonary artery flow is kept at 40% of CO.

Table 1.
Table 1 shows the differences between the actually used EVLP protocols.
The OCSTM Lung is the only EVLP portable system, meaning that EVLP is initiated at the donor hospital and, thus, the cold ischemic time is minimized. The other two EVLP systems are static, implying that EVLP initiation occurs at the recipient hospital and, therefore, that the cold ischemic time is significatively longer than the cold ischemic time in the OCS-driven EVLP runs.
The EVLP benefits/added values are the following: (1) a reduction in the cold ischemic time and the risk of ischemia–reperfusion injury (IRI), which clinically manifests as PGD after transplantation. PGD is a well-known risk factor for the development of CLAD [44]; (2) the expansion of the lung donor pool, by allowing the evaluation and reconditioning of extended-criteria (marginal) donors (ECD), which represent 40% of the donor pool, and of lungs from donors after circulatory death (DCD). This last option is not available in Germany, where, in 2022, only 242 of the 459 (53%) offered donor lungs were finally transplanted; (3) the application of therapies during EVLP. In fact, antibiotic treatment in donor lungs with known bacterial infections, antiviral treatment using ultraviolet irradiation in lungs from HCV-infected donors, and thrombolysis in lungs from donors who died of acute pulmonary embolism can be safely started and performed while the lungs are being perfused; and (4) it is an ideal research platform to investigate new treatments (translational research from bench to the bedside), such as cell-based therapies with the infusion of mesenchymal stromal cells and regulatory T cells, anti-inflammatory therapies using alpha1 antitrypsin, and the lavage of donor lungs after aspiration using surfactant [38,39,41,43].
Clinical studies performed during the last 13 years have shown the non-inferiority of EVLP in comparison to standard cold preservation [45,46,47,48]. A single-center, randomized controlled study, including 80 patients, demonstrated a lower % of PGD > 1 at all time points in patients whose donor lungs were perfused using EVLP, but it did not reach statistical significance due to an exiguous PGD Grade 3 prevalence in the control group [47]. The INSPIRE multicenter, randomized controlled study showed a decreased % PGD grade 3 at 72 h after transplantation and a tendency for shorter hospitalization in patients whose lung grafts were preserved using the OCSTM Lung. Those effects did not translate into a beneficial effect on the 12-month survival altogether [48].
Other studies showed the EVLP potential in expanding the use of ECD lungs, which had not otherwise been accepted for transplantation [49,50,51,52]. In one of the first published studies on EVLP, the Toronto group was able to transplant 20 out of 23 (87%) high-risk donor lungs, which showed a lower prevalence of PGD than non-ECD lungs (15% vs. 30%) [49]. In the EXPAND multicenter, single-arm, pivotal trial, 87% of the OCS-perfused ECD lungs were successfully transplanted, with a 91% patient survival at 1 year, notwithstanding there being 44% of PGD grade 3 at 72 h after transplantation [51]. On the contrary, in the DEVELOP-UK multicenter, observational study, only 18 out of 53 (34%) ECD lungs were successfully transplanted after EVLP perfusion using the Lund protocol. Patients transplanted with ECD lungs showed a more complicated posttransplant course than patients transplanted with standard lungs [50].
Notwithstanding the versatility and potential benefits of EVLP, a United Network Organ Sharing (UNOS) survey showed that only 4.7–6.5% of lung transplantations were performed using EVLP [39]. Moreover, the results after EVLP use vary according to the transplant center volume and experience [53,54]. Logistics and EVLP costs are of major concern, impeding the widespread use of EVLP, such as in Germany, where the EVLP costs are still not covered by health insurance. However, recent studies from the US have demonstrated the cost effectiveness of EVLP in lung transplantation [55,56].
Recently, our colleagues in Toronto investigated the role of static lung storage at 10 °C instead of the standard 4 °C for prolonging the graft cold ischemic time. The rationale behind this new cold storage strategy lies in the better mitochondrial protection achieved at 10 °C than 4 °C. In 2021, Ali et al. presented their first experience with prolonged lung graft preservation at 10 °C in five patients. The median preservation time amounted to 10.4 h for the first implanted lung and 12.1 h for the second implanted lung. All patients survived without any PGD grade 3 at 72 h after transplantation [57]. The authors confirmed these results in a prospective, multicenter, nonrandomized trial, comparing outcomes between 70 patients, whose grafts were preserved at 10 °C, and 140 matched controls. For the 70 case patients, the lung grafts were retrieved and transported to the recipient hospital at 4 °C and then preserved at 10 °C in a specific incubator for a median of 7.48 h. Thus, the median ischemic time for the first implanted lung amounted to 12.28 h, and amount to 14.08 h for the second implanted lung. The prevalence of PGD grade 3 at 72 h was 5.7% in the study group and 9.3% in the control group. The need for postoperative ECMO support, a stay in the ICU and hospital, and the 1-year survival did not differ between groups [58].
A new portable lung preservation device has been recently introduced for controlled hypothermic storage (CHS); this is the LUNGguard (Paragonix Technologies, Waltham, MA, USA), which allows CHS at 4–8 °C. In a European prospective multicenter observational study, Provoost et al. used the LUNGguard for CHS in 36 patients undergoing lung transplantation. The CHS temperature was 6.5 °C (3.7–9.3 °C) and the ischemic times were 13.38 h and 15.41 h for the first and second implanted lung. The prevalence of PGD grade 3 at 72 h amounted to 2.8%. Only one patient died in the hospital [59].
By safely prolonging the cold ischemic time, CHS may improve lung transplantation logistics and performance, allowing day-time transplantation [58,59].
5. ECLS or the Holy Grail in Lung Transplantation
In many patients, lung transplantation is possible without the use of extracorporeal life support (ECLS) [60].
Among the available ECLS systems, ECMO is by far the most employed in lung transplantation. Moreover, in comparison to heart transplantation, there is still no long-term bridging assist device in lung transplantation. Extracorporeal carbon dioxide removal (ECOO2R) allows only partial decarboxylation. The central or peripheral Novalung (Novalung GmbH, Hechingen, Germany) system requires a full sternotomy or the cannulation of the femoral vessels, respectively, and depend on the cardiac function of the patient for pumping blood through the system [61,62,63,64,65,66,67].
On the contrary, ECMO versatility allows for pre-transplant support, which can be directly continued intraoperatively and post-transplant. The paramount role of ECMO in lung transplantation has been confirmed by numerous retrospective studies, without any randomized trial available altogether [60].
ECMO has been successfully used as BTT, especially in its veno-venous (v-v) configuration that may be upgraded to a veno-arterial venous (v-a-v) configuration in patients developing right heart failure or hemodynamic instability before transplantation, by implanting an additional arterial cannula in a femoral artery. The survival results of ECMO as a BTT have been improving in recent years due to better patient selection, the introduction of awake ECMO protocols and an increase in center transplant volume and experience, as aforementioned for lung transplantation in patients with ARDS [68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87]. In 2008, the “awake” strategy was introduced in the management of pre-transplant patients at our institution [73]. Since then, many other case series have validated the benefit of spontaneous breathing and mobilization during ECMO support, thus reducing patient muscular deconditioning due to immobilization and mechanical ventilation [68,69,70,71,78,81,85,88,89]. Moreover, patient rehabilitation during ECMO support has been improved by the introduction of new cannulas and implant techniques [90,91,92,93]. In our experience, the overall graft survival did not differ between BTT and non-BTT patients (79% vs. 90% and 61% vs. 68% at 1 and 5 years, respectively, p = 0.13) and ECMO as BTT was not a risk factor for mortality at the multivariate analysis [70]. Finally, another ECMO configuration that has recently been introduced for supporting right heart function and may have a promising role in lung transplant candidates as a BTT when v-v ECMO fails is right atrium (RA) to pulmonary artery (PA) bypass (RA-PA bypass). This system uses an ECMO circuit connected to a dual site single-lumen ProtekDuo Cannula (ProtekDuoTM Kit, LivaNova, Munich, Germany) that is implanted percutaneously through the right internal jugular vein and advanced under radiologic or echocardiographic control until the PA. This system allows early patient mobilization and combines respiratory and hemodynamic support [94,95].
In many transplant centers like ours, starting from the early 2010s, ECMO in its veno-arterial configuration has replaced the use of cardiopulmonary bypass (CPB) for intraoperative support. Intraoperative ECMO allows for continuing support in patients who may not be weaned from it and the possibility of performing major cardiac surgery; for example, coronary artery bypass grafting (CABG) requires a lower amount of heparin and priming volume in comparison to CPB and causes a lower degree of systemic inflammatory reaction (SIRS) than CPB [96,97,98,99,100,101,102,103,104,105]. In two recent publications, our group has demonstrated that survival does not differ between patients who require intraoperative ECMO and those who do not, and that the intraoperative use of ECMO does not emerge as a risk factor for in-hospital mortality or mortality after hospital discharge [106,107]. Moreover, our group has developed a decision algorithm that helps in identifying those patients at risk of requiring intraoperative ECMO support in order to avoid ECMO implant under urgent/emergent conditions [106].
Coronary artery bypass grafting (CABG) can be performed safely under v-a ECMO support, with or without cardioplegic arrest. We have recently demonstrated that this procedure is feasible, and that patients undergoing concomitant CABG and lung transplantation show survival equivalent to that of patients without severe coronary artery disease (p = 0.556). At our institution, CABG is usually performed directly after lung transplantation [108].
CPB still has a limited role in lung transplantation, particularly in patients requiring concomitant cardiac surgical procedures other than CABG, in small infants, and in patients where unexpected massive blood loss due to injury of the pulmonary artery or of the left atrium occurs during intraoperative dissection [106,107].
After transplantation, ECMO has an important role in rescuing grafts that develop severe graft dysfunction due to PGD, acute rejection and pneumonia, refractory to more conservative therapies. However, graft function and survival are worse in patients who require secondary ECMO [109,110,111,112,113,114,115,116,117]. Thus, the early recognition of incoming graft dysfunction with prompt ECMO implant has improved graft prognosis [109]. In patients with PPH, the postoperative direct extension of intraoperative ECMO support has effectively reduced the prevalence of PGD [88,89,118]. In these patients, we and our colleagues in Vienna have recently demonstrated that the primary mechanism causing PGD might be more likely related due to a diastolic dysfunction of the left ventricle. In fact, the long-standing underfilling of the left ventricle in the presence of reduced cardiac output secondary to very high pulmonary vascular resistance may result in left ventricular deconditioning, rendering the left ventricle incapable of handling a normal preload during the early postoperative period. The characteristic pulmonary oedema of PGD is a consequence of a sudden increase in the left ventricular end diastolic pressure and in the left atrial pressures soon after graft reperfusion, aggravated further after weaning from mechanical ventilation. Postoperative v-a ECMO use provides time for gradual adaption of the left ventricle to the new hemodynamic situation after transplantation [88].
6. Antibody-Mediated Rejection in Lung Transplantation
Antibody-mediated rejection (AMR) has emerged as a relevant clinical and prognostic complication later in lung transplantation than in other solid organ transplantations, such as kidney transplantation [119,120,121,122,123]. Consequently, its definition, monitoring and treatment have been literally borrowed from kidney transplantation. However, in comparison to acute cellular rejection (ACR) [124,125], the definition of AMR in lung transplantation is still debated [126,127,128,129].
The development of anti-HLA DSAs after transplantation, also defined as allosensitization or alloimmunisation, is a major risk factor for AMR. Among the mismatched antigens between the donor and recipient, the HLA antigens play an important role in the processing and recognition of self vs. non-self antigens. Class I HLA antigens are expressed on the surface of all nucleated cells, and present foreign antigens to cytotoxic T lymphocytes. Contrarily, class II HLA antigens are expressed predominantly on the surface of antigen-presenting cells (APC) [130]. Recently, antibodies against non-HLA antigens (non-HLA DSAs) have been also associated with AMR and CLAD, acting synergically with anti-HLA DSAs [131,132,133]. The mechanisms of allograft injury and the treatment of anti-HLA and non-HLA DSAs are similar [134,135,136].
Anti-HLA DSAs are antibodies of IgG class (IgG1, IgG2, IgG3, and IgG4). The IgG1 and IgG3 classes bind the complement, but, at a high titer, all IgG classes may acquire this characteristic. DSAs are pre-formed, i.e., they exist before lung transplantation, if the recipient has already been exposed to HLA antigens; this is as a consequence of blood transfusions, previous lung transplantation and major surgery, and pregnancies. Pre-formed DSA are of prognostic importance, because they can cause hyperacute rejection. Therefore, every transplant recipient undergoes a screening for anti-HLA antibodies before listing. Moreover, a crossmatch, which is obtained by mixing donor lymphocytes and recipient serum, is performed before (prospective crossmatch) or soon after (retrospective crossmatch) transplantation. Since donor lungs are not offered to recipients that show antibodies against donor HLA antigens (forbidden antigens), highly sensitized patients face a longer waiting list time and increased waiting list mortality [136,137,138,139,140]. DSAs developing after lung transplantation are called de-novo DSAs. Patients may develop de-novo DSAs early, during the first weeks after transplantation, or later, usually just before or concomitantly with CLAD development.
The increase in DSA prevalence has paralleled the use of the more specific and sensitive Luminex assay [141]. In retrospective case series published over the last ten years [131,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165], the prevalence of DSAs, either preformed or de novo, ranges between 4.3% in the study of Witt et al. [149], where only patients with AMR were considered, and 64.1% in the study of Roux et al. [155], yielding an overall a mean prevalence of 30.4%. DSAs were more often antibodies against class II HLA antigens, especially against the DQ antigens. Two studies showed a prevalence of complement-binding DSAs, at 42.9% and 39.7% [142,143,166,167]. The median time to DSA detection ranged from as early as 14 and 30 days after transplantation in the studies published by Le Pavec et al. [158] and Ius et al. [157] to a median of 537 days in the study published by Kim et al. [151]. Moreover, a failure to detects DSAs in the serum of patients with presumed AMR does not mean that DSAs are not produced at all, as they may be bound to the graft (intragraft DSA) [156,168,169].
In the prospective HLA antibodies after lung transplantation (HALT) study, 43 out of the 119 (36%) included patients showed DSAs after transplantation, being against class I HLA antigens in 14% of patients, against class II HLA antigens in 53% of patients, and against both HLA classes in 33% of patients. DSA specificity was most frequent for HLA-DQ antigens. DSAs were detected within the first 30 days of transplantation in 60% of patients, and thereafter in the remaining 40%. DSAs were pre-formed in 10% of patients [165].
In comparison to acute cellular rejection (ACR) [124,125], the definition of AMR in lung transplantation is debated and derives from the Banff definition of AMR in kidney transplantation [127,128,129]. In 2016, the ISHLT issued a consensus statement for the diagnosis of AMR [126]. The diagnostic criteria to be fulfilled were the following: the presence of DSAs; the presence of graft dysfunction; the exclusion of other causes of graft dysfunction, such as infection; the presence of a specific histology during the transbronchial biopsies, such as capillary neutrophilic infiltration and endotheliitis, diffuse alveolar damage (DAD), or the presence of acute fibrinous and organizing pneumonia (AFOP); and the presence of C4d deposits during the immunohistochemistry. Graft dysfunction distinguishes between the presence of clinical and subclinical AMR. The diagnosis of clinical AMR may be definite if all the previous criteria are present; probable if at least four criteria are fulfilled; and possible if at least three criteria are present. Similarly, the diagnosis of subclinical AMR may be definite if all criteria (DSA, histology and C4d) are present; probable if at least two criteria are fulfilled; and possible if at least one criterion is present [126].
These guidelines represent a significant improvement in the diagnosis of AMR in lung transplantation. However, this classification shows several limitations and a new consensus statement is going to be published by ISHLT in the near future. According to the authors of the ISHLT consensus statement, the main challenges in the diagnosis and grading of AMR in lung transplantation are the lack of specific diagnostic features and the variable relationship between DSA and the presence of graft damage and dysfunction. Therefore, a secure diagnosis of AMR mandates a multidisciplinary approach that integrates the clinical presentation with available immunologic and pathologic diagnostic tools [126]. The histologic criteria and the C4d deposition are the Achilles’ heel of this classification, because the histopathologic findings in AMR are non-specific patterns of injury that can be seen in a variety of disorders, including severe acute cellular rejection, infection (especially bacterial and viral), graft preservation injury and drug reactions [170]. Moreover, it is not always possible to evaluate all proposed criteria. For example, transbronchial biopsies may be a high-risk procedure in patients with severe graft dysfunction who would instead benefit from prompt therapy against AMR. This classification does not also clearly define the severity of AMR, and criteria for defining chronic AMR still have to be developed. Finally, other parameters may be considered in the future to help with the diagnosis of AMR, such as the detection of intragraft DSAs and of donor-derived cell-free DNA [171,172].
The development of DSAs and AMR have been associated with worse survival and an increased risk of CLAD. However, most of the evidence is based on retrospective, single-center studies [142,143,144,146,147,148,149,150,151,152,153,154,155,156,157,159,160,161,162,163,173,174]. The complement binding capacity, DSA persistency, anti-HLA DQ specificity, high MFI values and antibody titers, and evidence of intragraft DSAs have been invariably associated with mortality and CLAD. The knowledge of the mechanisms through which DSA provoke graft damage is of paramount importance for planning treatment and developing new drugs [175,176,177]. First of all, complement-binding DSAs activate the classical complement cascade. This reaction is initiated by the binding of DSAs with the complement factor C1q, and causes cellular damage directly through the formation of the membrane attack complex, and indirectly through the production of the anaphylatoxins C3a and C5a; in turn, these increase vascular permeability, the expression of adhesion molecules and inflammatory cell infiltration. DSAs also mediate antibody-dependent cell-mediated cytotoxicity (ADCC). Upon the binding of DSAs with the corresponding HLA antigens, the antigen/antibody complex is recognized by effector cells, such as natural killer (NK) cells, cytotoxic effector T cells, macrophages and neutrophilic granulocytes via the Fc receptor (FcR). As a consequence, cell lysis ensues. The DSA-induced cross-linking of HLA class I and class II antigens on the cell surface, for example, on the surface of endothelial cells, activates the expression of leukocyte adhesion molecules and chemokines, which allows the infiltration of neutrophilic granulocytes and monocytes, thus starting graft damage.
Over the last two decades, several new drugs and interventions have been developed to treat DSAs and AMR in lung transplantation [136,175,176,177,178,179,180,181,182,183]. The available drugs target specific mechanisms of DSA production, such as Belatacept (Bristol-Myers Squibb GmbH, München, Germany), which blocks the CTLA4 costimulatory pathway in the germinal center; Tocilizumab (Roche Pharma AG, Grenzach-Wyhlen, Germany), an antagonist of the IL-6 receptor [184,185,186,187,188,189]; Clazakizumab (CSLBehring, Marburg, Germany), an antagonist of IL-6; Rituximab (Roche Pharma AG, Grenzach-Wyhlen, Germany), an anti-CD20 chimeric monoclonal antibody that targets B-cell production [190]; and the proteasome inhibitors Bortezomib (Ratiopharm GmbH, Ulm, Germany) and Carfilzomib (AMGEN GmbH, München, Germany) [191,192,193], and the anti-CD38 Daratumumab (Janssen-Cilag International NV, Beerse, Belgium), which inhibit DSA production by plasma cells. Other drugs target the mechanisms yielding to graft damage by inhibiting the complement cascade, such as Eculizumab (Alexion Europe SAS, Levallois-Perret, France), an inhibitor of the complement factor C5 [194,195,196], and Berinert (CSL Behring GmbH, Marburg, Germany), a C1 esterase inhibitor.
Extracorporeal photopheresis [197,198], therapeutic plasmapheresis (tPE) [199,200], immunoabsorption and Imlifidase (IdeS), a recombinant cysteine protease derived from Streptococcus pyogenes that rapidly cleaves IgG [201], remove DSAs from the bloodstream.
While the previous drugs and interventions act at a precise point of the immunologic cascade yielding to DSA production and graft damage, the human intravenous immunoglobulins (IVIG), which may contain only IgG or be enriched with IgA and IgM immunoglobulins (IgGAM, Pentaglobin, Biotest AG, Dreieich, Germany), have pleiotropic effects. They neutralize DSAs in the periphery before antigen recognition, scavenge activated complement, inhibit the activation of ADCC effector cells by FcR blocking and the tissue migration of activated neutrophilic granulocytes and monocytes, and activate T regulatory cells [202,203,204,205,206,207,208,209,210,211,212,213,214].
The complexity of the immunologic cascade yielding to DSA production and AMR in lung transplantation and in other solid organ transplantations requires the treatment protocols to preferably include more than one drug or intervention, such as several sessions of tPE and immunoabsorption, IVIG, and Rituximab. This is particularly important in patients with preformed DSA who are at a high risk of hyperacute rejection if they were deliberately transplanted with a positive virtual crossmatch [215,216,217,218,219,220].
The treatment of de novo DSAs has been suggested only for patients who concomitantly show graft dysfunction [174,221]. However, the treatment of clinical AMR has shown disappointing results [148,149,222,223,224,225], especially considering that the diagnosis of clinical AMR is complex and time-consuming. Therefore, some authors have recently proposed that DSA treatment should be performed pre-emptively, before graft dysfunction ensues, as in patients with subclinical AMR [146,164].
7. Conclusions and Future Directions
Over the last two decades, the introduction of new techniques and therapies and the refinement of those already existing have significantly improved survival in lung transplantation. At our institution (Figure 1), the 5-year unadjusted survival of lung-transplanted recipients has now reached the 5-year survival of other solid organ transplant recipients, as in heart transplantation, where the 5-year unadjusted survival is 72.2% [226].
There is still room to push the survival bar further higher. The development of new primary immunosuppressive drugs other than the available calcineurin and m-TOR inhibitors, as well as cell-cycle inhibitors, is highly needed, with special attention paid to reducing the adverse side effects of drugs. The use of DCD donors, which is unfortunately not allowed in Germany, may reduce the gap between donor and recipient numbers, thus reducing waiting list mortality [227,228]. Transplantation across the HLA barrier may also reduce the waiting list mortality [215,216,217,218,219,220]. The availability of new therapies against AMR may open the door to ABO-incompatible lung transplantation, which shares many immunologic mechanisms with AMR itself [229,230]. Finally, the introduction of cell-based therapies, such as those based on regulatory T cells (Tregs), may improve graft tolerance, thus reducing the incidence of CLAD and immunosuppressive drug toxicity [231].
Author Contributions
Conceptualization: K.A. and F.I.; methodology: K.A., F.I. and N.D.d.M.; software: F.I.; validation: C.K., J.S. and M.G.; formal analysis: F.I.; investigation: K.A. and F.I.; resources: F.I.; data curation: K.A., N.D.d.M. and F.I.; writing—original draft: F.I.; writing—review and editing: K.A. and F.I.; visualization: K.A., N.D.d.M., C.K., J.S., M.G. and F.I.; supervision: C.K., J.S. and M.G.; project administration: F.I.; funding acquisition: none required. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
In accordance with local German protocols, study approval by the institutional ethical review board was waived, since the manuscript is a review of already published studies.
Informed Consent Statement
All patients included in the survival analysis reported in Figure 1 signed a written informed consent regarding the use of their personal clinical data for research purposes at the time of listing for lung transplantation.
Data Availability Statement
The survival data reported in Figure 1 are available upon request.
Conflicts of Interest
F.I. reports that the study grants, congress fees and consultancy fees were paid by Biotest AG. The remaining authors do not report any conflicts of interest.
List of Abbreviations
| ARDS | acute respiratory distress syndrome |
| BOS | bronchiolitis obliterans syndrome |
| BTT | bridge to transplantation |
| CABG | coronary artery bypass grafting |
| CBP | cardiopulmonary bypass |
| CLAD | chronic lung allograft dysfunction |
| CO | cardiac output |
| DCD | donation after circulatory death |
| eDSA | early donor-specific anti-HLA antibodies |
| ECD | extended-criteria donor |
| ECLS | extracorporeal life support |
| ECMO | extracorporeal membrane oxygenation |
| ET | Eurotransplant |
| EVLP | ex vivo lung perfusion |
| ISHLT | International Society of Heart and Lung Transplantation |
| LAS | lung allocation score |
| MFI | mean fluorescence intensity |
| PGD | primary graft dysfunction |
| RBCs | red blood cells |
| RAS | restrictive allograft syndrome |
References
- Chambers, D.S.; Cherikh, W.S.; Harhay, M.O.; Hayes, D., Jr.; Hsich, E.; Khush, K.K.; Meiser, B.; Potena, L.; Rossano, J.W.; Toll, A.E.; et al. The international thoracic organ transplant registry of the International Society for Heart and Lung Transplantation: Thirty-sixth adult lung and heart-lung transplantation report–2019; focus theme: Donor and recipient size match. J. Heart Lung Transplant. 2019, 38, 1042–1055. [Google Scholar] [CrossRef] [PubMed]
- Goldfarb, S.B.; Hayes, D., Jr.; Levvey, B.J.; Cherikh, W.S.; Chambers, D.C.; Khush, K.K.; Kucheryavaya, A.Y.; Meiser, B.; Rossano, J.W.; Stehlik, J.; et al. The Registry of the International Society for Heart and Lung Transplantation: Twenty-First Pediatric Lung and Heart-Lung Transplantation Report–2018; Focus Theme: Multiorgan Transplantation. J. Heart Lung Transplant. 2018, 37, 1196–1206. [Google Scholar] [CrossRef] [PubMed]
- Hayes, D.; Cherikh, W.; Chambers, D.; Harhay, M.O.; Khush, K.K.; Lehman, R.R.; Meiser, B.; Rossano, J.W.; Hsich, E.; Potena, L.; et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Twenty-second pediatric lung and heart-lung transplantation report-2019; Focus theme: Donor and recipient size match. J. Heart Lung Transplant. 2019, 38, 1015–1027. [Google Scholar] [CrossRef] [PubMed]
- Verleden, S.E.; Todd, J.L.; Sato, M.; Palmer, S.M.; Martinu, T.; Pavlisko, E.N.; Vos, R.; Neyrinck, A.; Van Raemdonck, D.; Saito, T.; et al. Impact of CLAD phenotype on survival after lung re-transplantation: A multicenter study. Am. J. Transplant. 2015, 15, 2223–2230. [Google Scholar] [CrossRef]
- DerHovanessian, A.; Wallace, W.D.; Lynch, J.P., III; Belperio, J.A.; Weigt, S.S. Chronic lung allograft dysfunction: Evolving concepts and therapies. Semin. Respir. Crit. Care Med. 2018, 39, 155–171. [Google Scholar] [CrossRef]
- Kulkarni, H.S.; Cherikh, W.S.; Chambers, D.C.; Garcia, V.C.; Hachem, R.R.; Kreisel, D.; Puri, V.; Kozower, B.D.; Byers, D.E.; Witt, C.A.; et al. Bronchiolitis obliterans syndrome-free survival after lung transplantation: An International Society for Heart and Lung Transplantation Thoracic Transplant Registry analysis. J. Heart Lung Transplant. 2019, 38, 5–16. [Google Scholar] [CrossRef]
- van der Mark, S.C.; Hoek, R.A.S.; Hellemons, M.E. Developments in lung transplantation over the past decade. Eur. Respir. Rev. 2020, 29, 190132. [Google Scholar] [CrossRef]
- Weill, D.; Benden, C.; Corris, P.A.; Dark, J.H.; Davis, R.D.; Keshavjee, S.; Lederer, D.J.; Mulligan, M.J.; Patterson, G.A.; Singer, L.G.; et al. A consensus document for the selection of lung transplant candidates: 2014—An update from the Pulmonary Transplantation Council of the International Society for Heart and Lung Transplantation. J. Heart Lung Transplant. 2015, 34, 1–15. [Google Scholar] [CrossRef]
- Gottlieb, J.; Greer, M.; Sommerwerck, U.; Deuse, T.; Witt, C.; Schramm, R.; Hagl, C.; Strueber, M.; Smits, J.M. Introduction of the lung allocation score in Germany. Am. J. Transplant. 2014, 14, 1318–1327. [Google Scholar] [CrossRef]
- Tsuang, W.M.; Snyder, L.D.; Budev, M.M. Perspectives on donor lung allocation from both sides of the Atlantic: The United States. Clin. Transplant. 2020, 34, e13873. [Google Scholar] [CrossRef]
- Holm, A.M.; Immer, F.; Benden, C. Lung allocation for transplant: The European perspective. Clin. Transplant. 2020, 34, e13883. [Google Scholar] [CrossRef] [PubMed]
- Parker, W.F.; Dussault, N.E.; Jablonski, R.; Garrity, E.R.; Churpek, M.M. Assessing the accuracy of the lung allocation score. J. Heart Lung Transplant. 2022, 41, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Brahmbhatt, J.M.; Hee Wai, T.; Goss, C.H.; Lease, E.D.; Merlo, C.A.; Kapnadak, S.G.; Ramos, K.J. The lung allocation score and other available models lack predictive accuracy for post-lung transplant survival. J. Heart Lung Transplant. 2022, 41, 1063–1074. [Google Scholar] [CrossRef] [PubMed]
- Kolaitis, N.A.; Chen, H.; Calabrese, D.R.; Kumar, K.; Obata, J.; Bach, C.; Golden, J.A.; Simon, M.A.; Kukreja, J.; Hays, S.R.; et al. The Lung Allocation Score Remains Inequitable for Patients with Pulmonary Arterial Hypertension, Even after the 2015 Revision. Am. J. Respir. Crit. Care Med. 2023, 207, 300–311. [Google Scholar] [CrossRef]
- Valapour, M.; Lehr, C.J.; Wey, A.; Skeans, M.A.; Miller, J.; Lease, E.D. Expected effect of the lung Composite Allocation Score system on US lung transplantation. Am. J. Transplant. 2022, 22, 2971–2980, Erratum in: Am. J. Transplant. 2024, 24, 1520. [Google Scholar] [CrossRef]
- Ringshausen, F.C.; Sauer-Heilborn, A.; Büttner, T.; Dittrich, A.M.; Schwerk, N.; Ius, F.; Nährlich, L.; Welte, T.; Greer, M. Lung transplantation for end-stage cystic fibrosis before and after the availability of elexacaftor-tezacaftor-ivacaftor, Germany, 2012–2021. Eur. Respir. J. 2023, 61, 2201402. [Google Scholar] [CrossRef]
- Hoeper, M.M.; Badesch, D.B.; Ghofrani, H.A.; Gibbs, J.S.R.; Gomberg-Maitland, M.; McLaughlin, V.V.; Preston, I.R.; Souza, R.; Waxman, A.B.; STELLAR Trial Investigators; et al. Phase 3 Trial of Sotatercept for Treatment of Pulmonary Arterial Hypertension. N. Engl. J. Med. 2023, 388, 1478–1490. [Google Scholar] [CrossRef]
- Iablonskii, P.; Carlens, J.; Mueller, C.; Aburahma, K.; Niehaus, A.; Boethig, D.; Franz, M.; Floethmann, K.; Sommer, W.; Optenhoefel, J.; et al. Indications and outcome after lung transplantation in children under 12 years of age: A 16-year single center experience. J. Heart Lung Transplant. 2022, 41, 226–236. [Google Scholar] [CrossRef]
- Alessandri, F.; Di Nardo, M.; Ramanathan, K.; Brodie, D.; MacLaren, G. Extracorporeal membrane oxygenation for COVID-19-related acute respiratory distress syndrome: A narrative review. J. Intensive Care 2023, 11, 5. [Google Scholar] [CrossRef]
- Avella, D.; Neumann, H.; Bharat, A. Lung Transplantation for Coronavirus Disease-2019 Patients and Coronavirus Disease-2019 in Lung Transplant Recipients. Clin. Chest Med. 2023, 44, 191–199. [Google Scholar] [CrossRef]
- Lepper, P.M.; Barrett, N.A.; Swol, J.; Lorusso, R.; Di Nardo, M.; Belliato, M.; Belohlavek, J.; Broman, L.M. Perception of prolonged extracorporeal membrane oxygenation in Europe: An EuroELSO survey. Perfusion 2020, 35, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Bojic, A.; Schellongowski, P.; Robak, O.; Hermann, A.; Buchtele, N.; Nagler, B.; Lamm, W.; Staudinger, T. Long-term Respiratory Extracorporeal Membrane Oxygenation and Prognosis: A Retrospective Analysis. ASAIO J. 2021, 67, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Lee, S.O.; Shim, T.S.; Choi, S.H.; Kim, H.R.; Kim, Y.H.; Kim, D.K.; Do, K.-H.; Choi, I.-C.; Hong, S.-B.; et al. Lung Transplantation as a Therapeutic Option in Acute Respiratory Distress Syndrome. Transplantation 2018, 102, 829–837. [Google Scholar] [CrossRef]
- Harano, T.; Ryan, J.P.; Chan, E.G.; Noda, K.; Morrell, M.R.; Luketich, J.D.; Sanchez, P.G. Lung transplantation for the treatment of irreversible acute respiratory distress syndrome. Clin. Transplant. 2021, 35, e14182. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, J.; Lepper, P.M.; Berastegui, C.; Montull, B.; Wald, A.; Parmar, J.; Magnusson, J.M.; Schönrath, F.; Laisaar, T.; Michel, S.; et al. Lung transplantation for acute respiratory distress syndrome: A retrospective European cohort study. Eur. Respir. J. 2022, 59, 2102078. [Google Scholar] [CrossRef] [PubMed]
- Frick, A.E.; Gan, C.T.; Vos, R.; Schwarz, S.; Kraft, F.; Kifjak, D.; Neyrinck, A.P.; Van Raemdonck, D.E.; Klepetko, W.; Jaksch, P.; et al. Lung transplantation for acute respiratory distress syndrome: A multicenter experience. Am. J. Transplant. 2022, 22, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Cypel, M.; Keshavjee, S. When to consider lung transplantation for COVID-19. Lancet Respir. Med. 2020, 8, 944–946. [Google Scholar] [CrossRef]
- Bharat, A.; Machuca, T.N.; Querrey, M.; Kurihara, C.; Garza-Castillon, R., Jr.; Kim, S.; Lung, K.; Yeldandi, A.; Bharat, A. Early outcomes after lung transplantation for severe COVID-19: A series of the first consecutive cases from four countries. Lancet Respir. Med. 2021, 9, 487–497. [Google Scholar] [CrossRef]
- Roach, A.; Chikwe, J.; Catarino, P.; Rampolla, R.; Noble, P.W.; Megna, D.; Chen, Q.; Emerson, D.; Egorova, N.; Keshavjee, S.; et al. Lung Transplantation for COVID-19-Related Respiratory Failure in the United States. N. Engl. J. Med. 2022, 386, 1187–1188. [Google Scholar] [CrossRef]
- Lang, C.; Ritschl, V.; Augustin, F.; Lang, G.; Moser, B.; Taghavi, S.; Murakoezy, G.; Lambers, C.; Flick, H.; Koestenberger, M.; et al. Clinical relevance of lung transplantation for COVID-19 ARDS: A nationwide study. Eur. Respir. J. 2022, 60, 2102404. [Google Scholar] [CrossRef]
- Razia, D.; Olson, M.T.; Grief, K.; Walia, R.; Bremner, R.M.; Smith, M.A.; Tokman, S. Hospitalized patients with irreversible lung injury from COVID-19 have higher morbidity but similar 1-year survival after lung transplant compared to hospitalized patients transplanted for restrictive lung disease. J. Heart Lung Transplant. 2023, 42, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Freischlag, K.; Lynch, T.J.; Ievlev, V.; Gries, C.; Keech, J.C.; Arshava, E.V.; Pena, T.; Klesney-Tait, J.A.; Parekh, K.R. A Matched Survival Analysis of Lung Transplant Recipients with Coronavirus Disease 2019-Related Respiratory Failure. Ann. Thorac. Surg. 2023, 116, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Bermudez, C.; Bermudez, F.; Courtwright, A.; Richards, T.; Diamond, J.; Cevasco, M.; Blumberg, E.; Christie, J.; Usman, A.; Crespo, M.M. Lung transplantation for COVID-2019 respiratory failure in the United States: Outcomes 1-year posttransplant and the impact of preoperative extracorporeal membrane oxygenation support. J. Thorac. Cardiovasc. Surg. 2024, 167, 384–395. [Google Scholar] [CrossRef] [PubMed]
- Copeland, H.; Hayanga, J.A.; Neyrinck, A.; MacDonald, P.; Dellgren, G.; Bertolotti, A.; Khuu, T.; Burrows, F.; Copeland, J.G.; Gooch, D.; et al. Donor heart and lung procurement: A consensus statement. J. Heart Lung Transplant. 2020, 39, 501–517, Erratum in: J. Heart Lung Transplant. 2020, 39, 734. [Google Scholar] [CrossRef] [PubMed]
- Kirk, R.; Dipchand, A.I.; Davies, R.R.; Miera, O.; Chapman, G.; Conway, J.; Denfield, S.; Gossett, J.G.; Johnson, J.; McCulloch, M.; et al. ISHLT consensus statement on donor organ acceptability and management in pediatric heart transplantation. J. Heart Lung Transplant. 2020, 39, 331–341. [Google Scholar] [CrossRef]
- Yu, W.S.; Son, J. Donor Selection, Management, and Procurement for Lung Transplantation. J. Chest Surg. 2022, 55, 277–282. [Google Scholar] [CrossRef]
- Zhou, A.L.; Larson, E.L.; Ruck, J.M.; Ha, J.S.; Casillan, A.J.; Bush, E.L. Current status and future potential of ex vivo lung perfusion in clinical lung transplantation. Artif. Organs. 2023, 47, 1700–1709. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, N.; Zhang, Z.; Li, Y.; Gao, J.; Chen, C.; Wen, Z. Diagnostic and Therapeutic Implications of Ex Vivo Lung Perfusion in Lung Transplantation: Potential Benefits and Inherent Limitations. Transplantation 2023, 107, 105–116. [Google Scholar] [CrossRef]
- Ahmad, K.; Pluhacek, J.L.; Brown, A.W. Ex Vivo Lung Perfusion: A Review of Current and Future Application in Lung Transplantation. Pulm. Ther. 2022, 8, 149–165. [Google Scholar] [CrossRef]
- Miller, C.L.; O, J.M.; Allan, J.S.; Madsen, J.C. Novel approaches for long-term lung transplant survival. Front. Immunol. 2022, 13, 931251. [Google Scholar] [CrossRef]
- Watanabe, T.; Cypel, M.; Keshavjee, S. Ex vivo lung perfusion. J. Thorac. Dis. 2021, 13, 6602–6617. [Google Scholar] [CrossRef] [PubMed]
- Iske, J.; Hinze, C.A.; Salman, J.; Haverich, A.; Tullius, S.G.; Ius, F. The potential of ex vivo lung perfusion on improving organ quality and ameliorating ischemia reperfusion injury. Am. J. Transplant. 2021, 21, 3831–3839. [Google Scholar] [CrossRef] [PubMed]
- Moreno Garijo, J.; Roscoe, A. Ex-vivo lung perfusion. Curr. Opin. Anaesthesiol. 2020, 33, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Whitson, B.A.; Prekker, M.E.; Herrington, C.S.; Whelan, T.P.; Radosevich, D.M.; Hertz, M.I.; Dahlberg, P.S. Primary graft dysfunction and long-term pulmonary function after lung transplantation. J. Heart Lung Transplant. 2007, 26, 1004–1011. [Google Scholar] [CrossRef]
- Warnecke, G.; Moradiellos, J.; Tudorache, I.; Kühn, C.; Avsar, M.; Wiegmann, B.; Sommer, W.; Ius, F.; Kunze, C.; Gottlieb, J.; et al. Normothermic perfusion of donor lungs for preservation and assessment with the Organ Care System Lung before bilateral transplantation: A pilot study of 12 patients. Lancet 2012, 380, 1851–1858. [Google Scholar] [CrossRef]
- Tikkanen, J.M.; Cypel, M.; Machuca, T.N.; Azad, S.; Binnie, M.; Chow, C.-W.; Chaparro, C.; Hutcheon, M.; Yasufuku, K.; de Perrot, M.; et al. Functional outcomes and quality of life after normothermic ex vivo lung perfusion lung transplantation. J. Heart Lung Transplant. 2015, 34, 547–556. [Google Scholar] [CrossRef]
- Slama, A.; Schillab, L.; Barta, M.; Benedek, A.; Mitterbauer, A.; Hoetzenecker, K.; Taghavi, S.; Lang, G.; Matilla, J.; Ankersmit, H.; et al. Standard donor lung procurement with normothermic ex vivo lung perfusion: A prospective randomized clinical trial. J. Heart Lung Transplant. 2017, 36, 744–753. [Google Scholar] [CrossRef]
- Warnecke, G.; Van Raemdonck, D.; Smith, M.A.; Massard, G.; Kukreja, J.; Rea, F.; Loor, G.; De Robertis, F.; Nagendran, J.; Dhital, K.K.; et al. Normothermic ex-vivo preservation with the portable Organ Care System Lung device for bilateral lung transplantation (INSPIRE): A randomised, open-label, non-inferiority, phase 3 study. Lancet Respir. Med. 2018, 6, 357–367. [Google Scholar] [CrossRef]
- Cypel, M.; Yeung, J.C.; Liu, M.; Anraku, M.; Chen, F.; Karolak, W.; Sato, M.; Laratta, J.; Azad, S.; Madonik, M.; et al. Normothermic ex vivo lung perfusion in clinical lung transplantation. N. Engl. J. Med. 2011, 364, 1431–1440. [Google Scholar] [CrossRef]
- Fisher, A.; Andreasson, A.; Chrysos, A.; Lally, J.; Mamasoula, C.; Exley, C.; Wilkinson, J.; Qian, J.; Watson, G.; Lewington, O.; et al. An observational study of Donor Ex Vivo Lung Perfusion in UK lung transplantation: DEVELOP-UK. Health Technol. Assess. 2016, 20, 1–276. [Google Scholar] [CrossRef]
- Loor, G.; Warnecke, G.; Villavicencio, M.A.; Smith, M.A.; Kukreja, J.; Ardehali, A.; Hartwig, M.; Daneshmand, M.A.; Hertz, M.I.; Huddleston, S.; et al. Portable normothermic ex-vivo lung perfusion, ventilation, and functional assessment with the Organ Care System on donor lung use for transplantation from extended-criteria donors (EXPAND): A single-arm, pivotal trial. Lancet Respir. Med. 2019, 7, 975–984. [Google Scholar] [CrossRef] [PubMed]
- Abdoul, N.; Legeai, C.; Cantrelle, C.; Mercier, O.; Olland, A.; Mordant, P.; Thomas, P.A.; Jougon, J.; Tissot, A.; Maury, J.-M.; et al. Impact of ex vivo lung perfusion on brain-dead donor lung utilization: The French experience. Am. J. Transplant. 2022, 22, 1409–1417. [Google Scholar] [CrossRef] [PubMed]
- Noda, K.; Chan, E.G.; Furukawa, M.; Ryan, J.P.; Clifford, S.; Luketich, J.D.; Sanchez, P.G.; UPMC EVLP Group. Single-center experience of ex vivo lung perfusion and subsequent lung transplantation. Clin. Transplant. 2023, 37, e14901. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Malas, J.; Krishnan, A.; Thomas, J.; Megna, D.; Egorova, N.; Chikwe, J.; Bowdish, M.E.; Catarino, P. Limited cumulative experience with ex vivo lung perfusion is associated with inferior outcomes after lung transplantation. J. Thorac. Cardiovasc. Surg. 2024, 167, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Halpern, S.E.; Kesseli, S.J.; Au, S.; Krischak, M.K.; Olaso, D.G.; Smith, H.; Tipton, G.; Jamieson, I.R.; Barbas, A.S.; Haney, J.C.; et al. Lung transplantation after ex vivo lung perfusion versus static cold storage: An institutional cost analysis. Am. J. Transplant. 2022, 22, 552–564. [Google Scholar] [CrossRef]
- Peel, J.K.; Keshavjee, S.; Naimark, D.; Liu, M.; Del Sorbo, L.; Cypel, M.; Barrett, K.; Pullenayegum, E.M.; Sander, B. Determining the impact of ex-vivo lung perfusion on hospital costs for lung transplantation: A retrospective cohort study. J. Heart Lung Transplant. 2023, 42, 356–367. [Google Scholar] [CrossRef]
- Ali, A.; Wang, A.; Ribeiro, R.V.P.; Beroncal, E.L.; Baciu, C.; Galasso, M.; Gomes, B.; Mariscal, A.; Hough, O.; Brambate, E.; et al. Static lung storage at 10 °C maintains mitochondrial health and preserves donor organ function. Sci. Transl. Med. 2021, 13, eabf7601. [Google Scholar] [CrossRef]
- Ali, A.; Hoetzenecker, K.; de la Cruz, J.L.C.-C.; Schwarz, S.; Gil Barturen, M.; Tomlinson, G.; Yeung, J.; Donahoe, L.; Yasufuku, K.; Pierre, A.; et al. Extension of Cold Static Donor Lung Preservation at 10 °C. NEJM Evid. 2023, 2, EVIDoa2300008. [Google Scholar] [CrossRef]
- Provoost, A.-L.; Novysedlak, R.; Van Raemdonck, D.; Van Slambrouck, J.; Prisciandaro, E.; Vandervelde, C.M.; Barbarossa, A.; Jin, X.; Denaux, K.; De Leyn, P.; et al. Lung transplantation following controlled hypothermic storage with a portable lung preservation device: First multicenter European experience. Front. Cardiovasc. Med. 2024, 11, 1370543. [Google Scholar] [CrossRef]
- Ius, F.; Tudorache, I.; Warnecke, G. Extracorporeal support, during and after lung transplantation: The history of an idea. J. Thorac. Dis. 2018, 10, 5131–5148. [Google Scholar] [CrossRef]
- Rajagopal, K.; Hoeper, M.M. State of the Art: Bridging to lung transplantation using artificial organ support technologies. J. Heart Lung Transplant. 2016, 35, 1385–1398. [Google Scholar] [CrossRef] [PubMed]
- Reeb, J.; Olland, A.; Renaud, S.; Lejay, A.; Santelmo, N.; Massard, G.; Falcoz, P.E. Vascular access for extracorporeal life support: Tips and tricks. J. Thorac. Dis. 2016, 8 (Suppl 4), S353–S363. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, R.; DeCamp, M.; Bharat, A. A novel strategy for cardiopulmonary support during lung transplantation. J. Thorac. Dis. 2018, 10, E142–E144. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Guzman, E.; Sharma, N.S.; Wille, K.; Hoopes, C.W. Use of a novel pulmonary artery cannula to provide extracorporeal membrane oxygenation as a bridge to lung transplantation. J. Heart Lung Transplant. 2016, 35, 1051–1053. [Google Scholar] [CrossRef]
- Bermudez, C.A.; Zaldonis, D.; Fan, M.H.; Pilewski, J.M.; Crespo, M.M. Prolonged Use of the Hemolung Respiratory Assist System as a Bridge to Redo Lung Transplantation. Ann. Thorac. Surg. 2015, 100, 2330–2333. [Google Scholar] [CrossRef]
- Fischer, S.; Simon, A.R.; Welte, T.; Hoeper, M.M.; Meyer, A.; Tessmann, R.; Gohrbandt, B.; Gottlieb, J.; Haverich, A.; Strueber, M. Bridge to lung transplantation with the novel pumpless interventional lung assist device NovaLung. J. Thorac. Cardiovasc. Surg. 2006, 131, 719–723. [Google Scholar] [CrossRef][Green Version]
- Strueber, M.; Hoeper, M.M.; Fischer, S.; Cypel, M.; Warnecke, G.; Gottlieb, J.; Pierre, A.; Welte, T.; Haverich, A.; Simon, A.R.; et al. Bridge to thoracic organ transplantation in patients with pulmonary artery hypertension using a pumpless lung assist device. Am. J. Transplant. 2009, 9, 853–857. [Google Scholar] [CrossRef]
- Todd, E.M.; Roy, S.B.; Hashimi, A.S.; Serrone, R.; Panchanathan, R.; Kang, P.; Varsch, K.E.; Steinbock, B.E.; Huang, J.; Omar, A.; et al. Extracorporeal membrane oxygenation as a bridge to lung transplantation: A single-center experience in the present era. J. Thorac. Cardiovasc. Surg. 2017, 154, 1798–1809. [Google Scholar] [CrossRef]
- Biscotti, M.; Gannon, W.D.; Agerstrand, C.; Abrams, D.; Sonett, J.; Brodie, D.; Bacchetta, M. Awake Extracorporeal Membrane Oxygenation as Bridge to Lung Transplantation: A 9-Year Experience. Ann. Thorac. Surg. 2017, 104, 412–419. [Google Scholar] [CrossRef]
- Ius, F.; Natanov, R.; Salman, J.; Kuehn, C.; Sommer, W.; Avsar, M.; Siemeni, T.; Bobylev, D.; Poyanmehr, R.; Boethig, D.; et al. Extracorporeal membrane oxygenation as bridge to lung transplantation may not impact overall mortality risk after transplantation: Results from a 7-year single-centre experience. Eur. J. Cardiothorac. Surg. 2018, 54, 334–340. [Google Scholar] [CrossRef]
- Hoetzenecker, K.; Donahoe, L.; Yeung, J.C.; Azad, S.; Fan, E.; Ferguson, N.D.; Del Sorbo, L.; de Perrot, M.; Pierre, A.; Yasufuku, K.; et al. Extracorporeal life support as a bridge to lung transplantation-experience of a high-volume transplant center. J. Thorac. Cardiovasc. Surg. 2018, 155, 1316–1328. [Google Scholar] [CrossRef] [PubMed]
- Hakim, A.H.; Ahmad, U.; McCurry, K.R.; Johnston, D.R.; Pettersson, G.B.; Budev, M.; Murthy, S.; Blackstone, E.H.; Tong, M.Z. Contemporary Outcomes of Extracorporeal Membrane Oxygenation Used as Bridge to Lung Transplantation. Ann. Thorac. Surg. 2018, 106, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Fuehner, T.; Kuehn, C.; Hadem, J.; Wiesner, O.; Gottlieb, J.; Tudorache, I.; Olsson, K.M.; Greer, M.; Sommer, W.; Welte, T.; et al. Extracorporeal membrane oxygenation in awake patients as bridge to lung transplantation. Am. J. Respir. Crit. Care Med. 2012, 185, 763–768. [Google Scholar] [CrossRef] [PubMed]
- Bermudez, C.A.; Rocha, R.V.; Zaldonis, D.; Bhama, J.K.; Crespo, M.M.; Shigemura, N.; Pilewski, J.M.; Sappington, P.L.; Boujoukos, A.J.; Toyoda, Y. Extracorporeal membrane oxygenation as a bridge to lung transplant: Midterm outcomes. Ann. Thorac. Surg. 2011, 92, 1226–1231. [Google Scholar] [CrossRef]
- Lang, G.; Taghavi, S.; Aigner, C.; Renyi-Vamos, F.; Jaksch, P.; Augustin, V.; Nagayama, K.; Ghanim, B.; Klepetko, W. Primary lung transplantation after bridge with extracorporeal membrane oxygenation: A plea for a shift in our paradigms for indications. Transplantation 2012, 93, 729–736. [Google Scholar] [CrossRef]
- Bittner, H.B.; Lehmann, S.; Rastan, A.; Garbade, J.; Binner, C.; Mohr, F.W.; Barten, M.J. Outcome of extracorporeal membrane oxygenation as bridge to lung transplantation and graft recovery. Ann. Thorac. Surg. 2012, 94, 942–949. [Google Scholar] [CrossRef]
- Javidfar, J.; Brodie, D.; Iribarne, A.; Jurado, J.; Lavelle, M.; Brenner, K.; Arcasoy, S.; Sonett, J.; Bacchetta, M. Extracorporeal membrane oxygenation as a bridge to lung transplantation and recovery. J. Thorac. Cardiovasc. Surg. 2012, 144, 716–721. [Google Scholar] [CrossRef]
- Schmidt, F.; Sasse, M.; Boehne, M.; Mueller, C.; Bertram, H.; Kuehn, C.; Warnecke, G.; Ono, M.; Seidemann, K.; Jack, T.; et al. Concept of “awake venovenous extracorporeal membrane oxygenation” in pediatric patients awaiting lung transplantation. Pediatr. Transplant. 2013, 17, 224–230. [Google Scholar] [CrossRef]
- Toyoda, Y.; Bhama, J.K.; Shigemura, N.; Zaldonis, D.; Pilewski, J.; Crespo, M.; Bermudez, C. Efficacy of extracorporeal membrane oxygenation as a bridge to lung transplantation. J. Thorac. Cardiovasc. Surg. 2013, 145, 1065–1070. [Google Scholar] [CrossRef]
- Lafarge, M.; Mordant, P.; Thabut, G.; Brouchet, L.; Falcoz, P.-E.; Haloun, A.; Le Pimpec-Barthes, F.; Maury, J.-M.; Reynaud-Gaubert, M.; Saint-Raymond, C.; et al. Experience of extracorporeal membrane oxygenation as a bridge to lung transplantation in France. J. Heart Lung Transplant. 2013, 32, 905–913. [Google Scholar] [CrossRef]
- Lang, G.; Kim, D.; Aigner, C.; Matila, J.; Taghavi, S.; Jaksch, P.; Murakoezi, G.; Klepetko, W. Awake extracorporeal membrane oxygenation bridging for pulmonary retransplantation provides comparable results to elective retransplantation. J. Heart Lung Transplant. 2014, 14, 1224–1228. [Google Scholar] [CrossRef] [PubMed]
- Dellgren, G.; Riise, G.C.; Swärd, K.; Gilljam, M.; Rexius, H.; Liden, H.; Silverborn, M. Extracorporeal membrane oxygenation as a bridge to lung transplantation: A long-term study. Eur. J. Cardiothorac. Surg. 2015, 47, 95–100. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hayanga, A.J.; Aboagye, J.; Esper, S.; Shigemura, N.; Bermudez, C.A.; Cunha, J.D.; Bhama, J. Extracorporeal membrane oxygenation as a bridge to lung transplantation in the United States: An evolving strategy in the management of rapidly advancing pulmonary disease. J. Thorac. Cardiovasc. Surg. 2015, 149, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Hayes, D., Jr.; Tobias, J.D.; Tumin, D. Center Volume and Extracorporeal Membrane Oxygenation Support at Lung Transplantation in the Lung Allocation Score Era. Am. J. Respir. Crit. Care Med. 2016, 194, 317–326. [Google Scholar] [CrossRef]
- Schechter, M.A.; Ganapathi, A.M.; Englum, B.R.; Speicher, P.J.; Daneshmand, M.A.; Davis, R.D.; Hartwig, M.G. Spontaneously Breathing Extracorporeal Membrane Oxygenation Support Provides the Optimal Bridge to Lung Transplantation. Transplantation 2016, 100, 2699–2704. [Google Scholar] [CrossRef]
- Yeo, H.J.; Lee, S.; Yoon, S.H.; Lee, S.E.; Cho, W.H.; Jeon, D.; Kim, Y.S.; Kim, D. Extracorporeal Life Support as a Bridge to Lung Transplantation in Patients with Acute Respiratory Failure. Transplant. Proc. 2017, 49, 1430–1435. [Google Scholar] [CrossRef]
- Tsiouris, A.; Budev, M.M.; Yun, J.J. Extracorporeal Membrane Oxygenation as a Bridge to Lung Transplantation in the United States: A Multicenter Survey. ASAIO 2018, 64, 689–693. [Google Scholar] [CrossRef]
- Tudorache, I.; Sommer, W.; Kühn, C.; Wiesner, O.; Hadem, J.; Fühner, T.; Ius, F.; Avsar, M.; Schwerk, N.; Böthig, D.; et al. Lung Transplantation for Severe Pulmonary Hypertension–Awake Extracorporeal Membrane Oxygenation for Postoperative Left Ventricular Remodeling. Transplantation 2015, 99, 451–458. [Google Scholar] [CrossRef]
- Salman, J.; Ius, F.; Sommer, W.; Siemeni, T.; Kuehn, C.; Avsar, M.; Boethig, D.; Molitoris, U.; Bara, C.; Gottlieb, J.; et al. Mid-term results of bilateral lung transplant with postoperatively extended intraoperative extracorporeal membrane oxygenation for severe pulmonary hypertension. Eur. J. Cardiothorac. Surg. 2017, 52, 163–170. [Google Scholar] [CrossRef]
- Turner, D.A.; Cheifetz, I.M.; Rehder, K.J.; Williford, W.L.; Bonadonna, D.; Banuelos, S.J.; Peterson-Carmichael, S.; Lin, S.S.; Davis, R.D.; Zaas, D. Active rehabilitation and physical therapy during extracorporeal membrane oxygenation while awaiting lung transplantation: A practical approach. Crit. Care Med. 2011, 39, 2593–2598. [Google Scholar] [CrossRef]
- Rahimi, R.A.; Skrzat, J.; Reddy, D.R.S.; Zanni, J.M.; Fan, E.; Stephens, R.S.; Needham, D.M. Physical Rehabilitation of Patients in the Intensive Care Unit Requiring Extracorporeal Membrane Oxygenation: A Small Case Series. Phys. Ther. 2013, 93, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Hayes, K.; Hodgson, C.L.; Pellegrino, V.A.; Snell, G.; Tarrant, B.; Fuller, L.M.; Holland, A.E. Physical Function in Subjects Requiring Extracorporeal Membrane Oxygenation Before or After Lung Transplantation. Respir. Care 2018, 63, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Kolaitis, N.A.; Soong, A.; Shrestha, P.; Zhuo, H.; Neuhaus, J.; Katz, P.P.; Greenland, J.R.; Golden, J.; Leard, L.E.; Shah, R.J.; et al. Improvement in patient-reported outcomes after lung transplantation is not impacted by the use of extracorporeal membrane oxygenation as a bridge to transplantation. J. Thorac. Cardiovasc. Surg. 2018, 156, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Capoccia, M.; Brewer, J.M.; Rackauskas, M.; Becker, T.K.; Maybauer, D.M.; Stukov, Y.; Lorusso, R.; Maybauer, M.O. Outcome of Veno-Pulmonary Extracorporeal Life Support in Lung Transplantation Using ProtekDuo Cannula: A Systematic Review and Description of Configurations. J. Clin. Med. 2024, 13, 4111. [Google Scholar] [CrossRef]
- Saeed, O.; Stein, L.H.; Cavarocchi, N.; Tatooles, A.J.; Mustafa, A.; Jorde, U.P.; Alvarez, C.; Gluck, J.; Saunders, P.; Abrol, S.; et al. Outcomes by cannulation methods for venovenous extracorporeal membrane oxygenation during COVID-19: A multicenter retrospective study. Artif. Organs. 2022, 46, 1659–1668. [Google Scholar] [CrossRef]
- Bermudez, C.A.; Shiose, A.; Esper, S.A.; Shigemura, N.; D’cunha, J.; Bhama, J.K.; Richards, T.J.; Arlia, P.; Crespo, M.M.; Pilewski, J.M. Outcomes of Intraoperative Venoarterial Extracorporeal Membrane Oxygenation Versus Cardiopulmonary Bypass During Lung Transplantation. Ann. Thorac. Surg. 2014, 98, 1936–1943. [Google Scholar] [CrossRef]
- Biscotti, M.; Yang, J.; Sonett, J.; Bacchetta, M. Comparison of extracorporeal membrane versus cardiopulmonary bypass for lung transplantation. J. Thorac. Cardiovasc. Surg. 2014, 148, 2410–2416. [Google Scholar] [CrossRef]
- Machuca, T.N.; Collaud, S.; Mercier, O.; Cheung, M.; Cunningham, V.; Kim, S.J.; Azad, S.; Singer, L.; Yasufuku, K.; de Perrot, M.; et al. Outcomes of intraoperative extracorporeal membrane oxygenation versus cardiopulmonary bypass for lung transplantation. J. Thorac. Cardiovasc. Surg. 2015, 149, 1152–1157. [Google Scholar] [CrossRef]
- Winter, H.; Müller, H.-H.; Meiser, B.; Neurohr, C.; Behr, J.; Guenther, S.; Hagl, C.; Hoechter, D.J.; von Dossow, V.; Schramm, R. The Munich Lung Transplant Group: Intraoperative Extracorporeal Circulation in Lung Transplantation. Thorac. Cardiovasc. Surg. 2015, 63, 706–714. [Google Scholar] [CrossRef]
- Pettenuzzo, T.; Faggi, G.; Di Gregorio, G.; Schiavon, M.; Marulli, G.; Gregori, D.; Rea, F.; Ori, C.; Feltracco, P. Blood Products Transfusion and Mid-Term Outcomes of Lung Transplanted Patients Under Extracorporeal Membrane Oxygenation Support. Prog. Transplant. 2018, 28, 314–321. [Google Scholar] [CrossRef]
- Hoetzenecker, K.; Schwarz, S.; Muckenhuber, M.; Benazzo, A.; Frommlet, F.; Schweiger, T.; Bata, O.; Jaksch, P.; Ahmadi, N.; Muraközy, G.; et al. Intraoperative extracorporeal membrane oxygenation and the possibility of postoperative prolongation improve survival in bilateral lung transplantation. J. Thorac. Cardiovasc. Surg. 2018, 155, 2193–2206. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.S.; Paik, H.C.; Haam, S.J.; Lee, C.Y.; Nam, K.S.; Jung, H.S.; Do, Y.W.; Shu, J.W.; Lee, J.G. Transition to routine use of venoarterial extracorporeal oxygenation during lung transplantation could improve early outcomes. J. Thorac. Dis. 2016, 8, 1712–1720. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cosgun, T.; Tomaszek, S.; Opitz, I.; Wilhelm, M.; Schuurmans, M.M.; Weder, W.; Inci, I. Single-center experience with intraoperative extracorporeal membrane oxygenation use in lung transplantation. Int. J. Artif. Organs, 2017; epub ahead of print. [Google Scholar] [CrossRef]
- Magouliotis, D.E.; Tasiopoulou, V.S.; Svokos, A.A.; Svokos, A.A.; Zacharoulis, D. Extracorporeal membrane oxygenation versus cardiopulmonary bypass during lung transplantation: A meta-analysis. Gen. Thorac. Cardiovasc. Surg. 2018, 66, 38–47. [Google Scholar] [CrossRef]
- Hoechter, D.J.; Shen, Y.-M.; Kammerer, T.; Günther, S.; Weig, T.; Schramm, R.; Hagl, C.; Born, F.; Meiser, B.; Preissler, G.; et al. Extracorporeal Circulation During Lung Transplantation Procedures: A meta-Analysis. ASAIO J. 2017, 63, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Ius, F.; Sommer, W.; Tudorache, I.; Avsar, M.; Siemeni, T.; Salman, J.; Molitoris, U.; Gras, C.; Juettner, B.; Puntigam, J.; et al. Five-year experience with intraoperative extracorporeal membrane oxygenation in lung transplantation: Indications and midterm results. J. Heart Lung Transplant. 2016, 35, 39–58. [Google Scholar] [CrossRef] [PubMed]
- Ius, F.; Aburahma, K.; Boethig, D.; Salman, J.; Sommer, W.; Draeger, H.; Poyanmehr, R.; Avsar, M.; Siemeni, T.; Bobylev, D.; et al. Long-term outcomes after intraoperative extracorporeal membrane oxygenation during lung transplantation. J. Heart Lung Transplant. 2020, 39, 915–925. [Google Scholar] [CrossRef] [PubMed]
- Franz, M.; Siemeni, T.; Aburahma, K.; Yablonski, P.; Poyanmehr, R.; Avsar, M.; Bobylev, D.; Sommer, W.; Boethig, D.; Greer, M.; et al. Lung transplant and severe coronary artery disease: Results from a single-centre experience. Eur. J. Cardiothorac. Surg. 2022, 62, ezac348. [Google Scholar] [CrossRef]
- Hartwig, M.G.; Walczak, R.; Lin, S.S.; Davis, R.D. Improved Survival but Marginal Allograft Function in Patients Treated with Extracorporeal Membrane Oxygenation after Lung Transplantation. Ann. Thorac. Surg. 2012, 93, 366–371. [Google Scholar] [CrossRef]
- Vlasselaers, D.; Verleden, G.M.; Meyns, B.; Van Raemdonck, D.; Demedts, M.; Lerut, A.; Lauwers, P. Femoral Venoarterial Extracorporeal Membrane Oxygenation for Severe Reimplantation Response After Lung Transplantation. Chest 2000, 118, 559–561. [Google Scholar] [CrossRef]
- Dahlberg, P.S.; Prekker, M.E.; Herrington, C.S.; Hertz, M.I.; Park, S.J. Medium-Term Results of Extracorporeal Membrane Oxygenation for Severe Acute Lung Injury After Lung Transplantation. J. Heart Lung Transplant. 2004, 23, 979–984. [Google Scholar] [CrossRef] [PubMed]
- Oto, T.; Rosenfeldt, F.; Rowland, M.; Pick, A.; Rabinov, M.; Preovolos, A.; Snell, G.; Williams, T.; Esmore, D. Extracorporeal Membrane Oxygenation After Lung Transplantation: Evolving Technique Improves Outcomes. Ann. Thorac. Surg. 2004, 78, 1230–1235. [Google Scholar] [CrossRef]
- Mason, D.P.; Boffa, D.J.; Murthy, S.C.; Gildea, T.R.; Budev, M.M.; Mehta, A.C.; McNeill, A.M.; Smedira, N.G.; Feng, J.; Rice, T.W.; et al. Extended use of extracorporeal membrane oxygenation after lung transplantation. J. Thorac. Cardiovasc. Surg. 2006, 132, 954–960. [Google Scholar] [CrossRef] [PubMed]
- Fischer, S.; Bohn, D.; Rycus, P.; Pierre, A.F.; de Perrot, M.; Waddell, T.K.; Keshavjee, S. Extracorporeal membrane Oxygenation for Primary Graft Dysfunction After Lung Transplantation: Analysis of the Extracorporeal Life Support Organization (ELSO) Registry. J. Heart Lung Transplant. 2007, 26, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Bermudez, C.A.; Adusumilli, P.S.; McCurry, K.R.; Zaldonis, D.; Crespo, M.M.; Pilewski, J.M.; Toyoda, Y. Extracorporeal Membrane Oxygenation for Primary Graft Dysfunction After Lung Transplantation: Long-Term Survival. Ann. Thorac. Surg. 2009, 87, 854–860. [Google Scholar] [CrossRef] [PubMed]
- Marasco, S.F.; Vale, M.; Preovolos, A.; Pellegrino, V.; Lee, G.; Snell, G.; Williams, T. Institution of extracorporeal membrane oxygenation late after lung transplantation—A futile exercise? Clin. Transplant. 2012, 26, E71–E77. [Google Scholar] [CrossRef]
- Mulvihill, M.S.; Yerokun, B.A.; Davis, R.P.; Renney, D.N.; Daneshmand, M.A.; Hartwig, M. Extracorporeal membrane oxygenation following lung transplantation: Indications and survival. J. Heart Lung Transplant. 2018, 37, 259–267. [Google Scholar] [CrossRef]
- Moser, B.; Jaksch, P.; Taghavi, S.; Muraközy, G.; Lang, G.; Hager, H.; Krenn, C.; Roth, G.; Faybik, P.; Bacher, A.; et al. Lung transplantation for idiopathic pulmonary arterial hypertension on intraoperative and postoperatively prolonged extracorporeal membrane oxygenation provides optimally controlled reperfusion and excellent outcome. Eur. J. Cardiothorac. Surg. 2018, 53, 178–185. [Google Scholar] [CrossRef]
- Terasaki, P.I. Humoral theory of transplantation. Am. J. Transplant. 2003, 3, 665–673. [Google Scholar] [CrossRef]
- Campbell, P. Clinical relevance of human leukocyte antigen antibodies in liver, heart, lung and intestine transplantation. Curr. Opin. Organ. Transplant. 2013, 18, 463–469. [Google Scholar] [CrossRef]
- Jordan, S.C.; Vo, A.A. Donor-specific antibodies in allograft recipients: Etiology, impact and therapeutic approaches. Curr. Opin. Organ. Transplant. 2014, 19, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Loupy, A.; Lefaucheur, C. Antibody-mediated rejection of solid-organ allografts. N. Engl. J. Med. 2018, 379, 1150–1160. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.P.; Kobashigawa, J.A. Antibody-medicated rejection after heart transplantation: Diagnosis and clinical implications. Curr. Opin. Organ. Transplant. 2020, 25, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Stewart, S.; Fishbein, M.C.; Snell, G.I.; Berry, G.J.; Boehler, A.; Burke, M.M.; Glanville, A.; Gould, F.K.; Magro, C.; Marboe, C.C.; et al. Revision of the 1996 working formulation for the standardization of nomenclature in the diagnosis of lung rejection. J. Heart Lung Transplant. 2007, 26, 1229–1242. [Google Scholar] [CrossRef] [PubMed]
- Roden, A.C.; Aisner, D.L.; Allen, T.C.; Aubry, M.C.; Barrios, R.J.; Beasley, M.B.; Cagle, P.T.; Capelozzi, V.L.; Dacic, S.; Ge, Y.; et al. Diagnosis of Acute Cellular Rejection and Antibody-Mediated Rejection on Lung Transplant Biopsies: A Perspective From Members of the Pulmonary Pathology Society. Arch. Pathol. Lab. Med. 2017, 141, 437–444. [Google Scholar] [CrossRef]
- Levine, D.J.; Glanville, A.R.; Aboyoun, C.; Belperio, J.; Benden, C.; Berry, G.J.; Hachem, R.; Hayes, D.; Neil, D.; Reinsmoen, N.L.; et al. Antibody-mediated rejection of the lung: A consensus report of the International Society for Heart and Lung Transplantation. J. Heart Lung Transplant. 2016, 35, 397–406. [Google Scholar] [CrossRef]
- Tambur, A.R.; Campbell, P.; Chong, A.S.; Feng, S.; Ford, M.L.; Gebel, H.; Gill, R.G.; Kelsoe, G.; Kosmoliaptsis, V.; Mannon, R.B.; et al. Sensitization in Transplantation: Assessment of Risk (STAR) 2017 Working Group Meeting Report. Am. J. Transplant. 2018, 18, 1604–1614. [Google Scholar] [CrossRef]
- Roux, A.; Levine, D.; Zeevi, A.; Hachem, R.; Halloran, K.; Halloran, P.; Gibault, L.; Taupin, J.; Neil, D.; Loupy, A.; et al. Banff lung report: Current knowledge and future research perspectives for diagnosis and treatment of pulmonary antibody-mediated rejection (AMR). Am. J. Transplant. 2019, 19, 21–31. [Google Scholar] [CrossRef]
- Pavlisko, E.N.; Adam, B.A.; Berry, G.J.; Calabrese, F.; Cortes-Santiago, N.; Glass, C.H.; Goddard, M.; Greenland, J.R.; Kreisel, D.; Levine, D.J.; et al. The 2022 Banff Meeting Lung Report. Am. J. Transplant. 2024, 24, 542–548. [Google Scholar] [CrossRef]
- Chong, A.S. B cells as antigen-presenting cells in transplantation rejection and tolerance. Cell Immunol. 2020, 349, 104061. [Google Scholar] [CrossRef]
- Reinsmoen, N.L.; Mirocha, J.; Ensor, C.R.; Marrari, M.; Chaux, G.; Levine, D.J.; Zhang, X.; Zeevi, A. A 3-center study reveals new insights into the impact of non-HLA antibodies on lung transplantation outcome. Transplantation 2017, 101, 1215–1221. [Google Scholar] [CrossRef] [PubMed]
- Rao, U.; Mohanakumar, T.; Ahn, C.; Gao, A.; Kaza, V. Prevalence of antibodies to lung self-antigens (Kalpha1 tubulin and collagen V) and donor specific antibodies to HLA in lung transplant recipients and implications for lung transplant outcomes: Single center experience. Transpl. Immunol. 2019, 54, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Elrefaei, M.; Taupin, J.-L.; Hitchman, K.M.; Hiho, S.; Gareau, A.J.; Iasella, C.J.; Marrari, M.; Belousova, N.; Bettinotti, M.; et al. Chronic lung allograft dysfunction is associated with an increased number of non-HLA antibodies. J. Heart Lung Transplant. 2024, 43, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Hachem, R.R. The impact of non-HLA antibodies on outcomes after lung transplantation and implications for therapeutic approaches. Hum. Immunol. 2019, 80, 583–587. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Reinsmoen, N.L. Impact and production of non-HLA-specific antibodies in solid organ transplantation. Int. J. Immunogenet. 2020, 47, 235–242. [Google Scholar] [CrossRef]
- Kardol-Hoefnagel, T.; Otten, H.G. A comprehensive overview of the clinical relevance and treatment options for antibody-mediated rejection with non-HLA antibodies. Transplantation 2021, 105, 1459–1470. [Google Scholar] [CrossRef]
- Scornik, J.C.; Zander, D.S.; Baz, M.A.; Donnelly, W.H.; Staples, E.D. Susceptibility of lung transplants to preformed donor-specific HLA antibodies as detected by flow cytometry. Transplantation 1999, 68, 1542–1546. [Google Scholar] [CrossRef]
- Hadjiliadis, D.; Chaparro, C.; Reinsmoen, N.L.; Gutierrez, C.; Singer, L.G.; Steele, M.P.; Waddell, T.K.; Davis, R.D.; Hutcheon, M.A.; Palmer, S.M.; et al. Pre-transplant panel reactive antibody in lung transplant recipients is associated with significantly worse post-transplant survival in a multicenter study. J. Heart Lung Transplant. 2005, 24, S249–S254. [Google Scholar] [CrossRef]
- Masson, E.; Chabod, M.S.J.; Thevenin, C.; Gonin, F.; Rebibou, J.M.; Tiberghien, P. Hyperacute rejection after lung transplantation caused by undetected low-titer anti-HLA antibodies. J. Heart Lung Transplant. 2007, 26, 642–645. [Google Scholar] [CrossRef]
- Shah, A.S.; Nwakanma, L.; Simpkins, C.; Williams, J.; Chang, D.C.; Conte, J.V. Pretransplant panel reactive antibodies in human lung transplantation: An analysis of over 10,000 patients. Ann. Thorac. Surg. 2008, 85, 1919–1924. [Google Scholar] [CrossRef]
- Lachmann, N.; Todorova, K.; Schulze, H.; Schönemann, C. Luminex® and its applications for solid organ transplantation, hematopoietic stem cell transplantation, and transfusion. Transfus. Med. Hemother. 2013, 40, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Brugière, O.; Roux, A.; Le Pavec, J.; Sroussi, D.; Parquin, F.; Pradère, P.; Dupin, C.; Bunel, V.; Mourin, G.; Jebrak, G.; et al. Role of C1q-binding anti-HLA antibodies as a predictor of lung allograft outcome. Eur. Respir. J. 2018, 52, 1701898. [Google Scholar] [CrossRef] [PubMed]
- Iasella, C.J.; Ensor, C.R.; Marrari, M.; Mangiola, M.; Xu, Q.; Nolley, E.; Moore, C.A.; Morrell, M.R.; Pilewski, J.M.; Sanchez, P.G.; et al. Donor-specific antibody characteristics, including persistence and complement-binding capacity, increase risk for chronic lung allograft dysfunction. J. Heart Lung Transplant. 2020, 39, 1417–1425. [Google Scholar] [CrossRef] [PubMed]
- Brugière, O.; Suberbielle, C.; Thabut, G.; Lhuillier, E.; Dauriat, G.; Metivier, A.-C.; Gautreau, C.; Charron, D.; Mal, H.; Parquin, F.; et al. Lung transplantation in patients with pretransplantation donor-specific antibodies detected by Luminex assay. Transplantation 2013, 95, 761–765. [Google Scholar] [CrossRef]
- Girnita, A.L.; McCurry, K.R.; Iacono, A.T.; Duquesnoy, R.; Corcoran, T.E.; Awad, M.; Spichty, K.J.; Yousem, S.A.; Burckart, G.; Dauber, J.H.; et al. HLA-specific antibodies are associated with high-grade and persistent-recurrent lung allograft acute rejection. J. Heart Lung Transplant. 2004, 23, 1135–1141. [Google Scholar] [CrossRef]
- Hachem, R.R.; Yusen, R.D.; Meyers, B.F.; Aloush, A.A.; Mohanakumar, T.; Patterson, G.A.; Trulock, E.P. Anti-HLA antibodies and pre-emptive antibody-directed therapy after lung transplantation. J. Heart Lung Transplant. 2010, 29, 973–980. [Google Scholar] [CrossRef]
- Snyder, L.D.; Wang, Z.; Chen, D.-F.; Reinsmoen, N.L.; Finlen-Copeland, C.A.; Davis, W.A.; Zaas, D.W.; Palmer, S.M. Implications for human leukocyte antigen antibodies after lung transplantation. A 10-year experience in 441 patients. Chest 2013, 144, 226–233. [Google Scholar] [CrossRef]
- Lobo, L.J.; Aris, R.M.; Schmitz, J.; Neuringer, I.P. Donor-specific antibodies are associated with antibody-mediated rejection, acute cellular rejection, bronchiolitis obliterans syndrome, and cystic fibrosis after lung transplantation. J. Heart Lung Transplant. 2013, 32, 70–77. [Google Scholar] [CrossRef]
- Witt, C.A.; Gaut, J.P.; Yusen, R.D.; Byers, D.E.; Iuppa, J.A.; Bain, K.B.; Patterson, G.A.; Mohanakumar, T.; Trulock, E.P.; Hachem, R.R. Acute Antibody-Mediated Rejection After Lung Transplantation: A Retrospective, Single Center, Case Series. J. Heart Lung Transplant. 2013, 32, 1034–1040. [Google Scholar] [CrossRef]
- Smith, J.D.; Ibrahim, M.W.; Newell, H.; Danskine, A.J.; Soresi, S.; Burke, M.M.; Rose, M.L.; Carby, M. Pre-transplant donor HLA-specific antibodies: Characteristics causing detrimental effects on survival after lung transplantation. J. Heart Lung Transplant. 2014, 33, 1074–1082. [Google Scholar] [CrossRef]
- Kim, M.; Townsend, K.R.; Wood, I.G.; Boukedes, S.; Guleria, I.; Gabardi, S.; El-Chemaly, S.; Camp, P.C.; Chandraker, A.K.; Milford, E.L.; et al. Impact of pretransplant anti-HLA antibodies on outcomes in lung transplant candidates. Am. J. Respir. Crit. Care Med. 2014, 189, 1234–1239. [Google Scholar] [CrossRef] [PubMed]
- Morrell, M.R.; Pilewski, J.M.; Gries, C.J.; Pipeling, M.R.; Crespo, M.M.; Ensor, C.R.; Yousem, S.A.; D’cunha, J.; Shigemura, N.; Bermudez, C.A.; et al. De novo donor-specific HLA antibodies are associated with early and high-grade bronchiolitis obliterans syndrome and death after lung transplantation. J. Heart Lung Transplant. 2014, 33, 1288–1294. [Google Scholar] [CrossRef] [PubMed]
- Safavi, S.; Robinson, D.R.; Soresi, S.; Carby, M.; Smith, J.D. De novo donor HLA-specific antibodies predict development of bronchiolitis obliterans syndrome after lung transplantation. J. Heart Lung Transplant. 2014, 33, 1273–1281. [Google Scholar] [CrossRef] [PubMed]
- Bosanquet, J.P.; Witt, C.A.; Bemiss, B.C.; Byers, D.E.; Yusen, R.D.; Patterson, A.G.; Kreisel, D.; Mohanakumar, T.; Trulock, E.P.; Hachem, R.R. The impact of pre-transplant allosensitization on outcomes after lung transplantation. J. Heart Lung Transplant. 2015, 34, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Roux, A.; Le Lan, I.B.; Holifanjaniaina, S.; Thomas, K.A.; Hamid, A.M.; Picard, C.; Grenet, D.; De Miranda, S.; Douvry, B.; Foch Lung Transplantation Group; et al. Antibody-Mediated Rejection in Lung Transplantation: Clinical Outcomes and Donor-Specific Antibody Characteristics. Am. J. Transplant. 2016, 16, 1216–1228. [Google Scholar] [CrossRef]
- Visentin, J.; Chartier, A.; Massara, L.; Linares, G.; Guidicelli, G.; Blanchard, E.; Parrens, M.; Begueret, H.; Dromer, C.; Taupin, J.-L. Lung intragraft donor-specific antibodies as a risk factor for graft loss. J. Heart Lung Transplant. 2016, 35, 1418–1426. [Google Scholar] [CrossRef]
- Ius, F.; Sommer, W.; Tudorache, I.; Kühn, C.; Avsar, M.; Siemeni, T.; Salman, J.; Hallensleben, M.; Kieneke, D.; Greer, M.; et al. Early-donor-specific antibodies in lung transplantation: Risk factors and impact on survival. J. Heart Lung Transplant. 2014, 33, 1255–1263. [Google Scholar] [CrossRef]
- Le Pavec, J.; Suberbielle, C.; Lamrani, L.; Feuillet, S.; Savale, L.; Dorfmüller, P.; Stephan, F.; Mussot, S.; Mercier, O.; Fadel, E. De-novo donor-specific anti-HLA antibodies 30 days after lung transplantation are associated with worse outcomes. J. Heart Lung Transplant. 2016, 35, 1067–1077. [Google Scholar] [CrossRef]
- Tikkanen, J.M.; Singer, L.G.; Kim, S.J.; Li, Y.; Binnie, M.; Chaparro, C.; Chow, C.-W.; Martinu, T.; Azad, S.; Keshavjee, S.; et al. De Novo DQ Donor-Specific Antibodies Are Associated with Chronic Lung Allograft Dysfunction after Lung Transplantation. Am. J. Respir. Crit. Care Med. 2016, 194, 596–606. [Google Scholar] [CrossRef]
- Zazueta, O.E.; Preston, S.E.; Moniodis, A.; Fried, S.; Kim, M.; Townsend, K.; Wood, I.; Boukedes, S.; Guleria, I.; Camp, P.; et al. The presence of pretransplant HLA antibodies does not impact the development of chronic lung allograft dysfunction or CLAD-related death. Transplantation 2017, 101, 2207–2212. [Google Scholar] [CrossRef]
- Verleden, S.E.; Vanaudenaerde, B.M.; Emonds, M.-P.; Van Raemdonck, D.E.; Neyrinck, A.P.; Verleden, G.M.; Vos, R. Donor-specific and –nonspecific HLA antibodies and outcome post lung transplantation. Eur. Respir. J. 2017, 50, 1701248. [Google Scholar] [CrossRef] [PubMed]
- Schmitzer, M.; Winter, H.; Kneidinger, N.; Meimarakis, G.; Dick, A.; Schramm, R.; Klotz, L.V.; Preissler, G.; Strobl, N.; von Dossow, V.; et al. Persistence of de novo donor-specific HLA-antibodies after lung transplantation: A potential marker of decreased patient survival. HLA 2018, 92, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Courtwright, A.M.; Cao, S.; Wood, I.; Mallidi, H.R.; Kawasawa, J.; Moniodis, A.; Ng, J.; El-Chemaly, S.; Goldberg, H.J. Clinical outcomes of lung transplantation in the presence of donor-specific antibodies. Ann. Am. Thorac. Soc. 2019, 16, 1131–1137. [Google Scholar] [CrossRef] [PubMed]
- Keller, M.; Yang, S.; Ponor, L.; Bon, A.; Cochrane, A.; Philogene, M.; Bush, E.; Shah, P.; Matthews, J.; Brown, A.W.; et al. Preemptive treatment of de novo donor-specific antibodies in lung transplant patients reduces subsequent risk of chronic lung allograft dysfunction or death. Am. J. Transplant. 2023, 23, 559–564. [Google Scholar] [CrossRef]
- Hachem, R.R.; Kamoun, M.; Budev, M.M.; Askar, M.; Ahya, V.N.; Lee, J.C.; Levine, D.J.; Pollack, M.S.; Dhillon, G.S.; Weill, D.; et al. Human Leukocyte antigens antibodies after lung transplantation: Primary results of the HALT study. Am. J. Transplant. 2018, 37, 1119–1130. [Google Scholar] [CrossRef]
- Bouquegneau, A.; Loheac, C.; Aubert, O.; Bouatou, Y.; Viglietti, D.; Empana, J.P.; Ulloa, C.; Hassan Murad, M.; Legendre, C.; Glotz, D.; et al. Complement-activating donor-specific anti-HLA antibodies and solid organ transplant survival: A systematic review and meta-analysis. PLoS Med. 2018, 15, e1002572. [Google Scholar]
- Roux, A.; Thomas, K.A.; Sage, E.; Suberbielle-Boissel, C.; Beaumont-Azuar, L.; Parquin, F.; Le Guen, M.; Harre, N.; Hamid, A.M.; Reed, E.F. Donor-specific HLA antibody-mediated complement activation is a significant indicator of antibody-mediated rejection and poor long-term graft outcome during lung transplantation: A single center cohort study. Transpl. Int. 2018, 31, 761–772. [Google Scholar] [CrossRef]
- Miyamoto, E.; Motoyama, H.; Sato, M.; Aoyama, A.; Menju, T.; Shikuma, K.; Sowa, T.; Yoshizawa, A.; Saito, M.; Takahagi, A.; et al. Association of local intrapulmonary production of antibodies specific to donor major histocompatibility complex class I with the progression of chronic rejection of lung allografts. Transplantation 2017, 101, e156–e165. [Google Scholar] [CrossRef]
- Sacreas, A.; Taupin, J.L.; Emonds, M.P.; Daniëls, L.; Van Raemdonck, D.E.; Vos, R.; Verleden, G.M.; Vanaudenaerde, B.M.; Roux, A.; Verleden, S.E. Intragraft donor-specific anti-HLA antibodies in phenotypes of chronic lung allograft dysfunction. Eur. Respir. J. 2019, 54, 1900847. [Google Scholar] [CrossRef]
- Berry, G.; Burke, M.; Andersen, C.; Angelini, A.; Bruneval, P.; Calabrese, F.; Fishbein, M.C.; Goddard, M.; Leone, O.; Maleszewski, J.; et al. Pathology of pulmonary antibody-mediated rejection: 2012 update from the Pathology Council of the ISHLT. J. Heart Lung Transplant. 2013, 32, 14–21. [Google Scholar] [CrossRef]
- Kataria, A.; Kumar, D.; Gupta, G. Donor-derived Cell-free DNA in Solid Organ Transplant Diagnostics: Indications, Limitations, and Future Directions. Transplantation 2021, 105, 1203–1211. [Google Scholar] [CrossRef] [PubMed]
- Keller, M.; Agbor-Enoh, S. Cell-free DNA in lung transplantation: Research tool or clinical workhorse? Curr. Opin. Organ. Transplant. 2022, 27, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Kauke, T.; Kneidinger, N.; Martin, B.; Dick, A.; Schneider, C.; Schramm, R.; Meimarakis, G.; Preissler, G.; Eickelberg, O.; Von Dossow, V.; et al. Bronchiolitis obliterans syndrome due to donor-specific HLA antibodies. Tissue Antigens 2015, 86, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Westall, G.P.; Snell, G.I. Antibody-mediated rejection in lung transplantation: Fable, spin, or fact? Transplantation 2014, 98, 927–930. [Google Scholar] [CrossRef]
- Chong, A.S. Mechanisms of organ transplant injury mediated by B cells and antibodies: Implications for antibody-mediated rejection. Am. J. Transplant. 2020, 20, 23–32. [Google Scholar] [CrossRef]
- Hulbert, A.L.; Pavlisko, E.N.; Palmer, S.M. Current challenges and opportunities in the management of antibody-mediated rejection in lung transplantation. Curr. Opin. Organ. Transplant. 2018, 23, 308–315. [Google Scholar] [CrossRef]
- Habal, M.V.; Farr, M.; Restaino, S.; Chong, A. Desensitization in the era of precision medicine: Moving from the bench to bedside. Transplantation 2019, 103, 1574–1581. [Google Scholar] [CrossRef]
- Wan, S.S.; Ying, T.D.; Wyburn, K.; Roberts, D.M.; Wyld, M.; Chadban, S.J. The treatment of antibody-mediated rejection in kidney transplantation: An updated systematic review and meta-analysis. Transplantation 2018, 102, 557–568. [Google Scholar] [CrossRef]
- Jordan, S.C.; Ammerman, N.; Choi, J.; Kumar, S.; Huang, E.; Toyoda, M.; Kim, I.; Wu, G.; Vo, A. Interleukin-6: An important mediator of allograft injury. Transplantation 2020, 104, 2497–2506. [Google Scholar] [CrossRef]
- Suchanek, O.; Clatworthy, M.R. Novel strategies to target the humoral alloimmune response. HLA 2020, 96, 667–680. [Google Scholar] [CrossRef]
- Schinstock, C.A.; Mannon, R.B.; Budde, K.; Chong, A.S.; Haas, M.; Knechtle, S.; Lefaucheur, C.; Montgomery, R.A.; Nickerson, P.; Tullius, S.G.; et al. Recommended treatment for antibody-mediated rejection after kidney transplantation: The 2019 expert consensus from the Transplantation Society Working Group. Transplantation 2020, 104, 911–922. [Google Scholar] [CrossRef] [PubMed]
- Nickerson, P.W. What have we learned about how to prevent and treat antibody-mediated rejection in kidney transplantation? Am. J. Transplant. 2020, 20, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Mayer, K.A.; Doberer, K.; Eskandary, F.; Halloran, P.F.; Böhmig, G.A. New concepts in chronic antibody-mediated rejection: Prevention and treatment. Curr. Opin. Organ. Transplant. 2021, 26, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Vo, A.A.; Choi, J.; Kim, I.; Louie, S.; Cisneros, K.; Kahwaji, J.; Toyoda, M.; Ge, S.; Haas, M.; Puliyanda, D.; et al. A phase I/II trial of the interleukin-6 receptor specific humanized monoclonal (Tocilizumab) + intravenous immunoglobulin in difficult to desensitize patients. Transplantation 2015, 99, 2356–2363. [Google Scholar] [CrossRef]
- Choi, J.; Aubert, O.; Vo, A.; Loupy, A.; Haas, M.; Puliyanda, D.; Kim, I.; Louie, S.; Kang, A.; Peng, A.; et al. Assessment of Tocilizumab (anti-interleukin-6 receptor monoclonal) as a potential treatment for chronic antibody-mediated rejection and transplant glomerulopathy in HLA-sensitized renal allograft recipients. Am. J. Transplant. 2017, 17, 2381–2389. [Google Scholar] [CrossRef]
- Pottebaum, A.A.; Venkatachalam, K.; Liu, C.; Brennan, D.C.; Murad, H.; Malone, A.F.; Alhamad, T. Efficacy and safety of Tocilizumab in the treatment of acute active antibody-mediated rejection in kidney transplant recipients. Transplant. Direct 2020, 6, e543. [Google Scholar] [CrossRef]
- Lavacca, A.; Presta, R.; Gai, C.; Mella, A.; Gallo, E.; Camussi, G.; Abbasciano, I.; Barreca, A.; Caorsi, C.; Fop, F.; et al. Early effects of first-line treatment with anti-interleukin-6 receptor antibody tocilizumab for chronic active antibody-mediated rejection in kidney transplantation. Clin. Transplant. 2020, 34, e13908. [Google Scholar] [CrossRef]
- Shin, B.H.; Everly, M.J.; Zhang, H.; Choi, J.; Vo, A.; Zhang, X.; Huang, E.; Jordan, S.C.; Toyoda, M. Impact of Tocilizumab (anti-IL-6R) treatment on immunoglobulins and anti-HLA antibodies in kidney transplant patients with chronic antibody-mediated rejection. Transplantation 2020, 104, 856–863. [Google Scholar] [CrossRef]
- Sethi, S.; Peng, A.; Najjar, R.; Vo, A.; Jordan, S.C.; Huang, E. Infectious Complications in Tocilizumab-Treated Kidney Transplant Recipients. Transplantation 2021, 105, 1818–1824. [Google Scholar] [CrossRef]
- Ius, F.; Sommer, W.; Tudorache, I.; Kühn, C.; Avsar, M.; Siemeni, T.; Salman, J.; Hallensleben, M.; Kieneke, D.; Greer, M.; et al. Preemptive treatment with therapeutic plasma exchange and rituximab for early donor-specific antibodies after lung transplantation. J. Heart Lung Transplant. 2015, 34, 50–58. [Google Scholar] [CrossRef]
- Stuckey, L.J.; Kamoun, M.; Chan, K.M. Lung transplantation across donor-specific anti-human leukocyte antigen antibodies: Utility of bortezomib therapy in early graft dysfunction. Ann. Pharmacother. 2012, 46, e2. [Google Scholar] [CrossRef] [PubMed]
- Ensor, C.R.; Yousem, S.A.; Marrari, M.; Morrell, M.R.; Mangiola, M.; Pilewski, J.M.; D’cunha, J.; Wisniewski, S.R.; Venkataramanan, R.; Zeevi, A.; et al. Proteasome inhibitor carfilzomib-based therapy for antibody-mediated rejection of the pulmonary allograft: Use and short-term findings. Am. J. Transplant. 2017, 17, 1380–1388. [Google Scholar] [CrossRef] [PubMed]
- Pham, C.; Pierce, B.J.; Nguyen, D.T.; Graviss, E.A.; Huang, H.J. Assessment of Carfilzomib Treatment Response in Lung Transplant Recipients with Antibody-mediated Rejection. Transplant. Direct 2021, 7, e68. [Google Scholar] [CrossRef] [PubMed]
- Muller, Y.D.; Aubert, J.D.; Vionnet, J.; Rotman, S.; Sadallah, S.; Aubert, V.; Pascual, M. Acute antibody-mediated rejection 1 week after lung transplantation successfully treated with Eculizumab, Intravenous Immunoglobulins, and Rituximab. Transplantation 2018, 102, e301–e303. [Google Scholar] [CrossRef]
- Dawson, K.L.; Parulekar, A.; Seethamraju, H. Treatment of hyperacute antibody-mediated lung allograft rejection with eculizumab. J. Heart Lung Transplant. 2012, 31, 1325–1326. [Google Scholar] [CrossRef]
- Schinstock, C.A.; Bentall, A.J.; Smith, B.H.; Cornell, L.D.; Everly, M.; Gandhi, M.J.; Stegall, M.D. Long-term outcomes of eculizumab-treated positive crossmatch recipients: Allograft survival, histologic findings, and natural history of the donor-specific antibodies. Am. J. Transplant. 2019, 19, 1671–1683. [Google Scholar] [CrossRef]
- Baskaran, G.; Tiriveedhi, V.; Ramachandran, S.; Aloush, A.; Grossman, B.; Hachem, R.; Mohanakumar, T. Efficacy of extracorporeal photopheresis in clearance of antibodies to donor-specific and lung-specific antigens in lung transplant recipients. J. Heart Lung Transplant. 2014, 33, 950–956. [Google Scholar] [CrossRef]
- Benazzo, A.; Worel, N.; Schwarz, S.; Just, U.; Nechay, A.; Lambers, C.; Böhmig, G.; Fischer, G.; Koren, D.; Muraközy, G.; et al. Outcome of extracorporeal photopheresis as an add-on therapy for antibody-mediated rejection in lung transplant recipients. Transfus. Med. Hemother. 2020, 47, 205–213. [Google Scholar] [CrossRef]
- Jackups, R., Jr.; Canter, C.; Sweet, S.C.; Mohanakumar, T.; Morris, G.P. Measurement of donor-specific HLA antibodies following Plasma Exchange Therapy Predicts Clinical Outcome in Pediatric Heart and Lung Transplant Recipients with Antibody-mediated Rejection. J. Clin. Apher. 2013, 28, 301–308. [Google Scholar] [CrossRef]
- Timofeeva, O.A.; Choe, J.; Alsammak, M.; Yoon, E.J.; Geier, S.S.; Mathew, L.; McCollick, A.; Carney, K.; Au, J.; Diamond, A.; et al. Guiding therapeutic plasma exchange for antibody-mediated rejection treatment in lung Transplant recipients—A retrospective study. Transpl. Int. 2021, 34, 700–708. [Google Scholar] [CrossRef]
- Roux, A.; Bunel, V.; Belousova, N.; Messika, J.; Tanaka, S.; Salpin, M.; Roussel, A.; Beaumont-Azuar, L.; Picard, C.; Brugiere, O.; et al. First use of imlifidase desensitization in a highly sensitized lung transplant candidate: A case report. Am. J. Transplant. 2023, 23, 294–297. [Google Scholar] [CrossRef] [PubMed]
- Vo, A.A.; Jordan, S.C. Benefits, efficacy, cost-effectiveness and infectious complications in transplant patients desensitized with intravenous immunoglobulin and anti-CD20 therapy. Clin. Exp. Immunol. 2014, 178 (Suppl. S1), 48–51. [Google Scholar] [CrossRef] [PubMed]
- Shevach, E.M. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity 2009, 30, 636–645. [Google Scholar] [CrossRef] [PubMed]
- Tedla, F.M.; Roche-Recinos, A.; Brar, A. Intravenous immunoglobulin in kidney transplantation. Curr. Opin. Organ. Transplant. 2015, 20, 630–637. [Google Scholar] [CrossRef]
- Panda, S.; Ding, J.L. Natural antibodies bridge innate and adaptive immunity. J. Immunol. 2015, 149, 13–20. [Google Scholar] [CrossRef]
- Nguyen, T.T.T.; Baumgarth, N. Natural IgM and the Development of B cell-mediated autoimmune diseases. Crit. Rev. Immunol. 2016, 36, 163–177. [Google Scholar] [CrossRef]
- Lobo, P.I. Role of Natural Autoantibodies and Natural IgM Anti-Leucocyte Autoantibodies in Health and Disease. Front. Immunol. 2016, 7, 198. [Google Scholar] [CrossRef]
- Perez, E.E.; Orange, J.S.; Bonilla, F.; Chinen, J.; Chinn, I.K.; Dorsey, M.; El-Gamal, Y.; Harville, T.O.; Hossny, E.; Mazer, B.; et al. Update on the use of immunoglobulin in human disease: A review of evidence. J. Allergy Clin. Immunol. 2017, 139, S1–S46. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.; Xiong, E.; Hong, R.; Lu, Q.; Ohno, H.; Wang, J.Y. Role of the IgM Fc receptor in immunity and tolerance. Front. Immunol. 2019, 10, 529. [Google Scholar] [CrossRef]
- Matsuda, Y.; Hiramitsu, T.; Li, X.K.; Watanabe, T. Characteristics of Immunoglobulin M Type Antibodies of Different Origins from the Immunologic and Clinical Viewpoints and Their Application in Controlling Antibody-Mediated Allograft Rejection. Pathogens 2020, 10, 4. [Google Scholar] [CrossRef]
- Maddur, M.S.; Lacroix-Desmazes, S.; Dimitrov, J.D.; Kazatchkine, M.D.; Bayry, J.; Kaveri, S.V. Natural antibodies: From first-line defence against pathogens to perpetual immune homeostasis. Clin. Rev. Allergy Immunol. 2020, 58, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Ius, F.; Sommer, W.; Verboom, M.; Erdfelder, C.; Tudorache, I.; Kühn, C.; Avsar, M.; Siemeni, T.; Salman, J.; Greer, M.; et al. IgM-Enriched Human Intravenous Immunoglobulin-Based Treatment of Patients with Early Donor Specific Anti-HLA Antibodies After Lung Transplantation. Transplantation 2016, 100, 2682–2692. [Google Scholar] [CrossRef] [PubMed]
- Ius, F.; Verboom, M.; Sommer, W.; Poyanmehr, R.; Knoefel, A.K.; Salman, J.; Kuehn, C.; Avsar, M.; Siemeni, T.; Erdfelder, C.; et al. Preemptive treatment of early donor-specific antibodies with IgA- and IgM-enriched intravenous human immunoglobulins in lung transplantation. Am. J. Transplant. 2018, 18, 2295–2304. [Google Scholar] [CrossRef] [PubMed]
- Ius, F.; Müller, C.; Sommer, W.; Verboom, M.; Hallensleben, M.; Salman, J.; Siemeni, T.; Kühn, C.; Avsar, M.; Bobylev, D.; et al. Six-year experience with treatment of early donor-specific anti-HLA antibodies in pediatric lung transplantation using a human immunoglobulin-based protocol. Pediatr. Pulmonol. 2020, 55, 754–764. [Google Scholar] [CrossRef]
- Appel, J.Z., III; Hartwig, M.G.; Davis, R.D.; Reinsmoen, N.L. Utility of peritransplant and rescue intravenous immunoglobulin and extracorporeal immunoadsorption in lung transplant recipients sensitized to HLA antigens. Hum. Immunol. 2005, 66, 378–386. [Google Scholar] [CrossRef]
- Snyder, L.D.; Gray, A.L.; Reynolds, J.M.; Arepally, G.M.; Bedoya, A.; Hartwig, M.G.; Davis, R.D.; Lopes, K.E.; Wegner, W.E.; Chen, D.F.; et al. Antibody desensitization therapy in highly sensitized lung transplant candidates. Am. J. Transplant. 2014, 14, 849–856. [Google Scholar] [CrossRef]
- Tinckam, K.J.; Keshavjee, S.; Chaparro, C.; Barth, D.; Azad, S.; Binnie, M.; Chow, C.W.; de Perrot, M.; Pierre, A.F.; Waddell, T.K.; et al. Survival in sensitized lung transplant recipients with perioperative desensitization. Am. J. Transplant. 2015, 15, 417–426. [Google Scholar] [CrossRef]
- Aversa, M.; Martinu, T.; Patriquin, C.; Cypel, M.; Barth, D.; Ghany, R.; Ma, J.; Keshavjee, S.; Singer, L.G.; Tinckam, K. Long-term outcomes of sensitized lung transplant recipients after peri-operative desensitization. Am. J. Transplant. 2021, 21, 3444–3448. [Google Scholar] [CrossRef]
- Parquin, F.; Zuber, B.; Vallée, A.; Taupin, J.-L.; Cuquemelle, E.; Malard, S.; Neuville, M.; Devaquet, J.; Le Guen, M.; Fessler, J.; et al. A virtual crossmatch-based strategy for perioperative desensitisation in lung transplant recipients with pre-formed donor-specific antibodies: 3-year outcome. Eur. Respir. J. 2021, 58, 2004090. [Google Scholar] [CrossRef]
- Heise, E.L.; Chichelnitskiy, E.; Greer, M.; Franz, M.; Aburahma, K.; Iablonskii, P.; de Manna, N.D.; Christoph, S.; Verboom, M.; Hallensleben, M.; et al. Lung transplantation despite preformed donor-specific antihuman leukocyte antigen antibodies: A 9-year single-center experience. Am. J. Transplant. 2023, 23, 1740–1756. [Google Scholar] [CrossRef]
- Butler, C.L.; Valenzuela, N.M.; Thomas, K.A.; Reed, E.F. Not all antibodies are created equal: Factors that influence antibody mediated rejection. J. Immunol. Res. 2017, 2017, 7903471. [Google Scholar] [CrossRef] [PubMed]
- Daoud, A.H.S.; Betensley, A.D. Diagnosis and treatment of antibody mediated rejection in lung transplantation: A retrospective case series. Transpl. Immunol. 2013, 28, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Otani, S.; Davis, A.K.; Cantwell, L.; Ivulich, S.; Pham, A.; Paraskeva, M.A.; Snell, G.I.; Westall, G.P. Evolving experience of treating antibody-mediated rejection following lung transplantation. Transpl. Immunol. 2014, 31, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Islam, A.K.; Sinha, N.; DeVos, J.M.; Kaleekal, T.S.; Jyothula, S.S.; Teeter, L.D.; Nguyen, D.T.M.; Eagar, T.N.; Moore, L.W.; Puppala, M.; et al. Early clearance vs persistence of de novo donor-specific antibodies following lung transplantation. Clin. Transplant. 2017, 31, e13028. [Google Scholar] [CrossRef] [PubMed]
- Vacha, M.; Chery, G.; Hulbert, A.; Byrns, J.; Benedetti, C.; Finlen Copeland, C.A.; Gray, A.; Onwuemene, O.; Palmer, S.M.; Snyder, L.D. Antibody depletion strategy for the treatment of suspected antibody-mediated rejection in lung transplant recipients: Does it work? Clin Transplant. 2017, 31, e12886. [Google Scholar] [CrossRef]
- Khush, K.K.; Cherikh, W.S.; Chambers, D.C.; Harhay, M.O.; Hayes, D.; Hsich, E.; Meiser, B.; Potena, L.; Robinson, A.; International Society for Heart and Lung Transplant; et al. The international thoracic organ transplant registry of the international society for heart and lung transplantation: Thirty-sixth adult heart transplantation report–2019; focus theme: Donor and recipient size match. J. Heart Lung Transplant. 2019, 38, 1056–1066. [Google Scholar] [CrossRef]
- Noda, K.; Furukawa, M.; Chan, E.G.; Sanchez, P.G. Expanding Donor Options for Lung Transplant: Extended Criteria, Donation After Circulatory Death, ABO Incompatibility, and Evolution of Ex Vivo Lung Perfusion. Transplantation 2023, 107, 1440–1451. [Google Scholar] [CrossRef]
- Levvey, B.J.; Snell, G.I. How do we expand the lung donor pool? Curr. Opin. Pulm. Med. 2024, 30, 398–404. [Google Scholar] [CrossRef]
- Strüber, M.; Warnecke, G.; Hafer, C.; Goudeva, L.; Fegbeutel, C.; Fischer, S.; Gottlieb, J.; Avsar, M.; Simon, A.R.; Haverich, A. Intentional ABO-incompatible lung transplantation. Am. J. Transplant. 2008, 8, 2476–2478. [Google Scholar] [CrossRef]
- Chen-Yoshikawa, T.F. ABO blood type incompatible lung transplantation. J. Thorac. Dis. 2023, 15, 3437–3442. [Google Scholar] [CrossRef]
- Wood, K.J.; Bushell, A.; Hester, J. Regulatory immune cells in transplantation. Nat. Rev. Immunol. 2012, 12, 417–430. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).