Unveiling the Burden of Hepatitis A in Salerno, Italy: A Comprehensive 9-Year Retrospective Study (2015–2023) on the Seroprevalence of HAV Antibodies and Age/Sex Distribution
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Processing
2.2. Data and Statistical Analyses
2.3. Limitations
2.4. Ethical Consideration Statement
3. Results
3.1. Seroprevalence of HAV in the 2015–2023 Timeframe
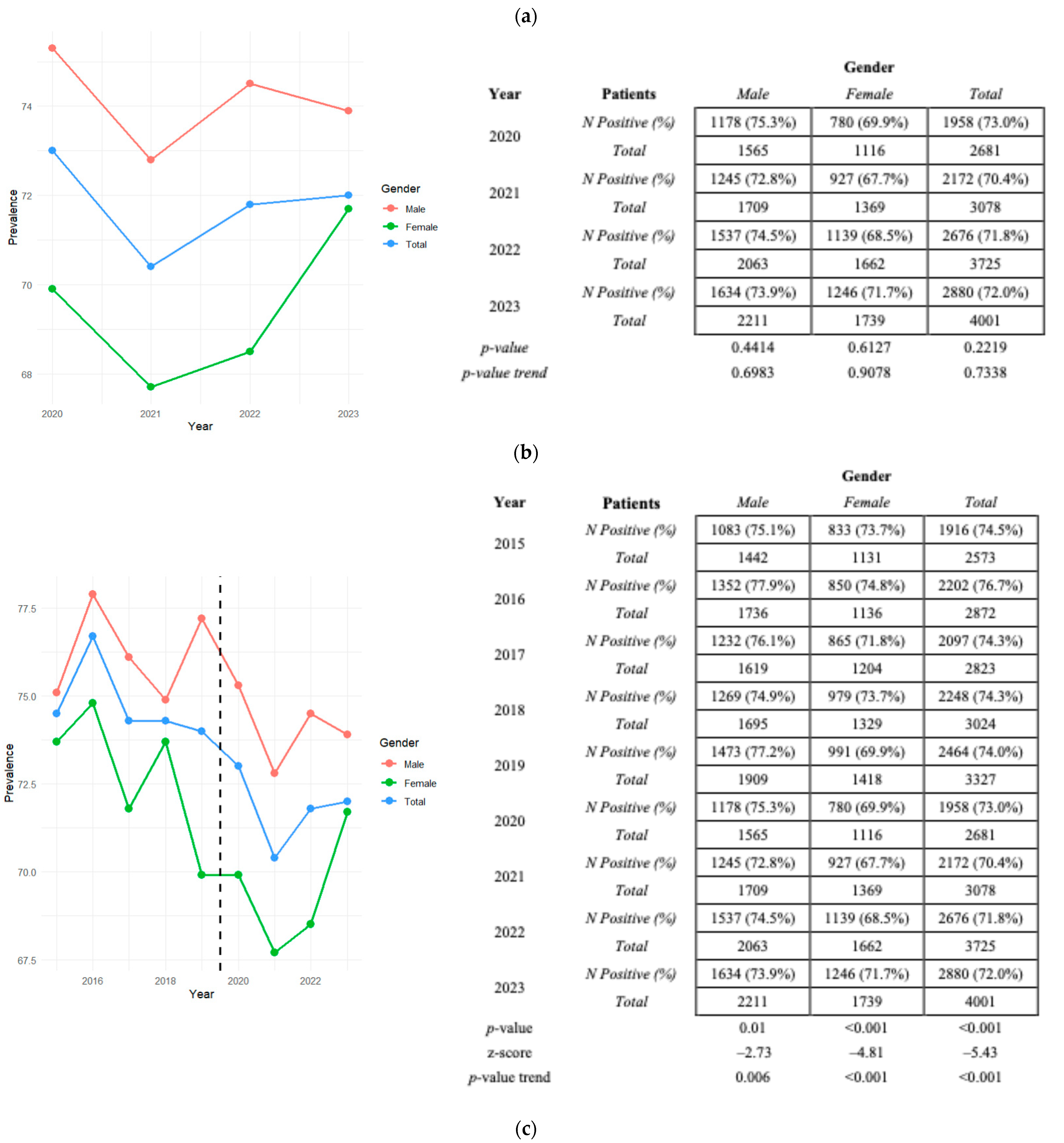
3.2. HAV Seroprevalence Positivity in Pre- and Pandemic Periods
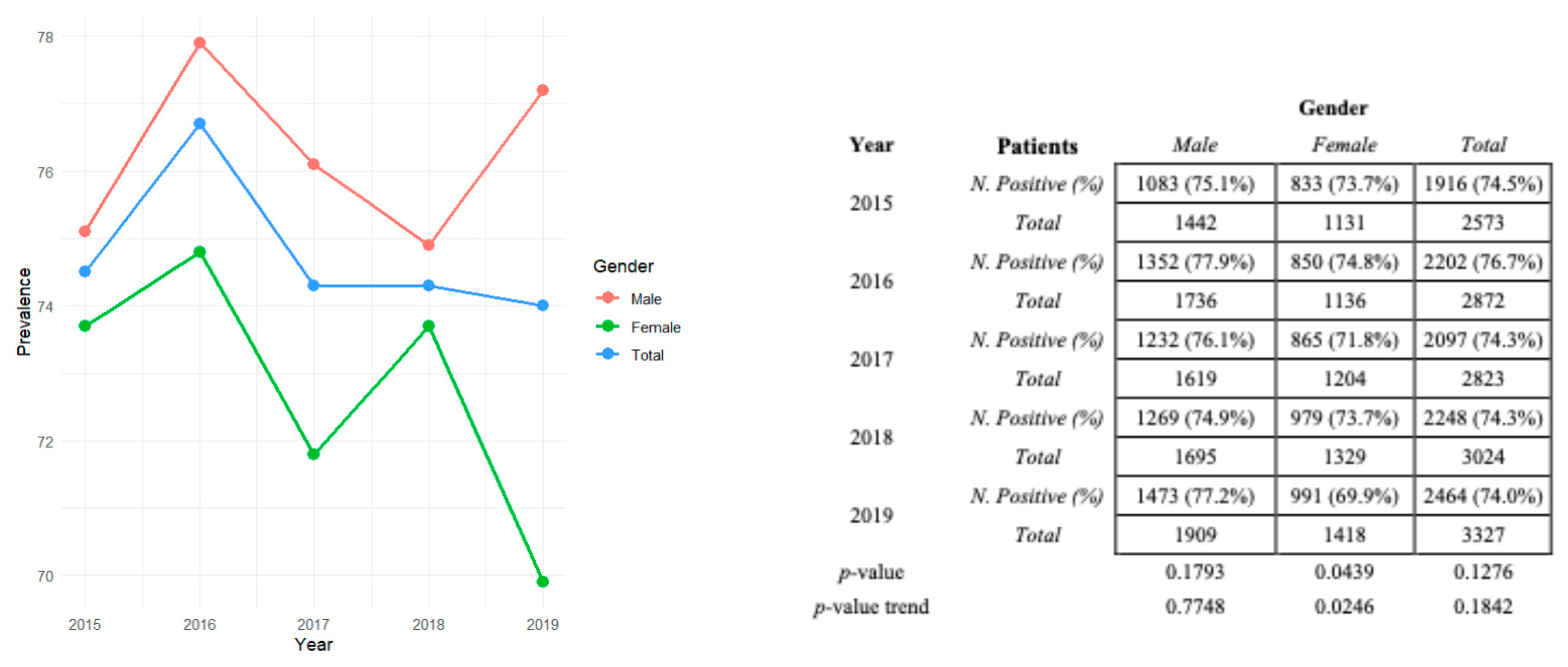
3.3. Distribution of HAV Seroprevalence by Gender and Absolute and Relative Frequencies in the Pre-Pandemic Period (2015–2019)
3.4. Distribution of HAV Seroprevalence by Gender and Absolute and Relative Frequencies in the Pandemic Period (2020–2023)
3.5. Distribution of HAV Seroprevalence Divided by Sex per Year with Relative p-Values and p-Value Trends in Pre-Pandemic and Pandemic Eras
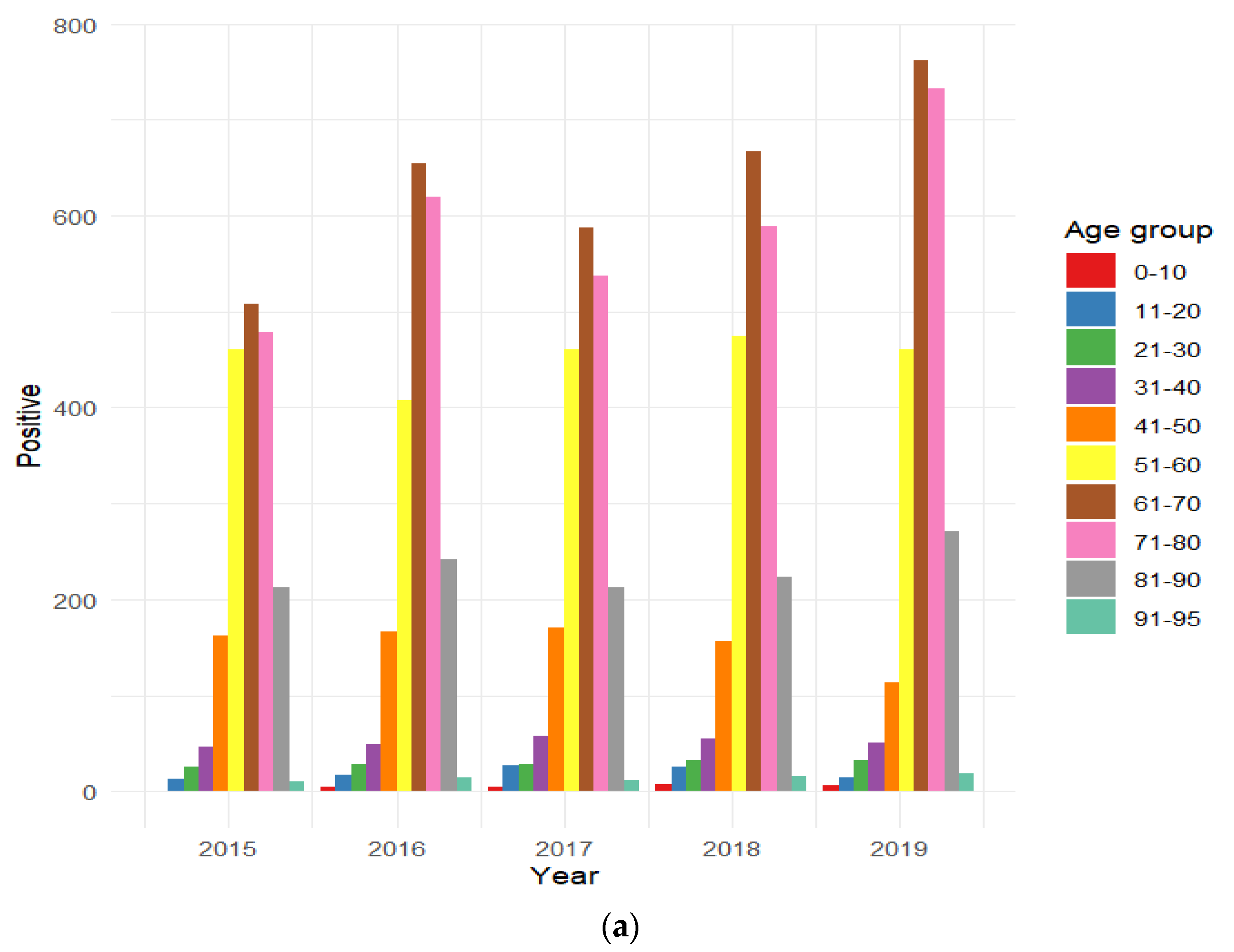
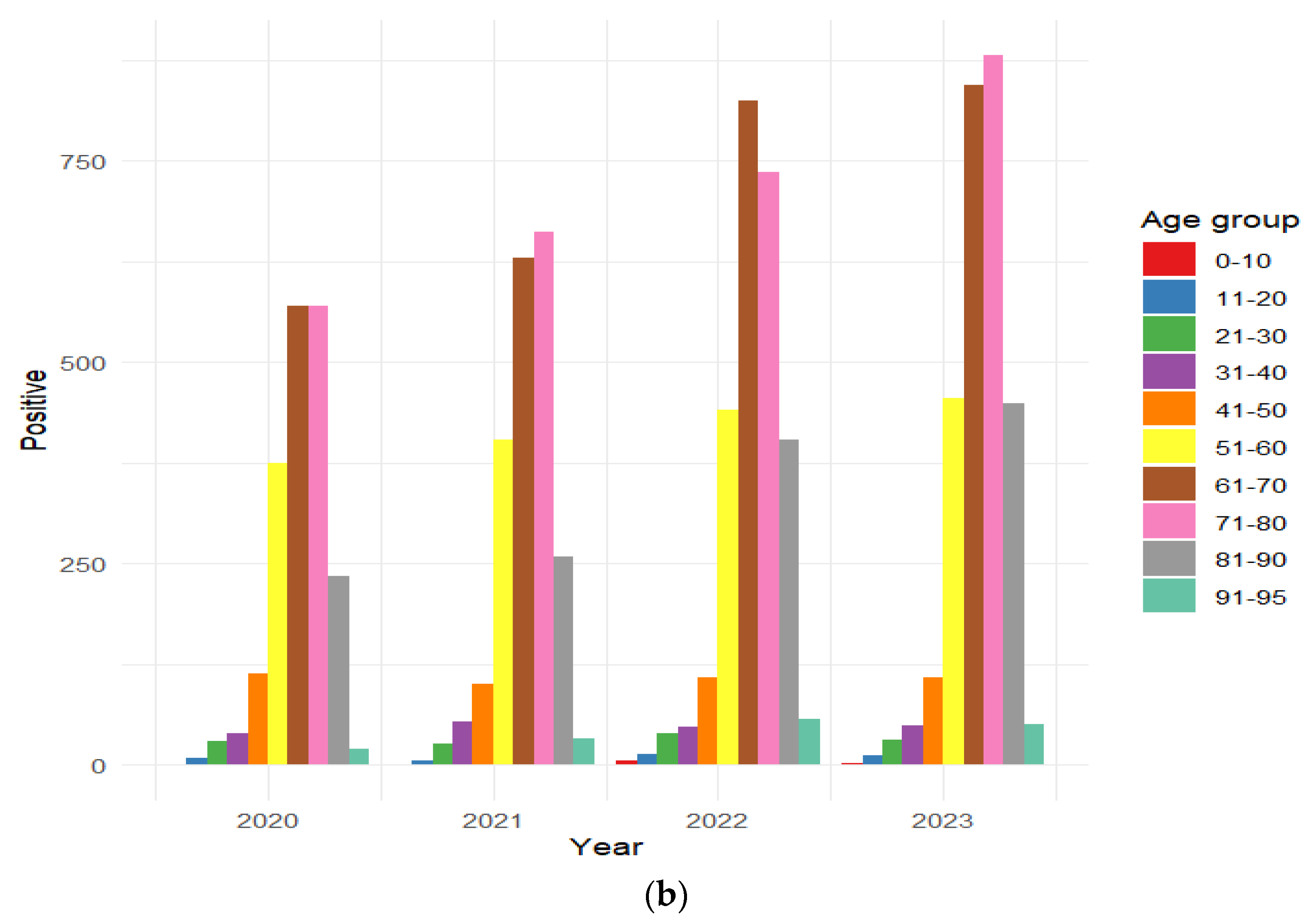
3.6. Distribution of HAV-Positive Patients Stratified by Age Group
3.7. Distribution of HAV Seroprevalence by Gender/Age Group in Both the Pre-Pandemic and Pandemic Periods
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jacobsen, K.H. Globalization and the Changing Epidemiology of Hepatitis A Virus. Cold Spring Harb. Perspect. Med. 2018, 8, a031716. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Jing, W.; Liu, J.; Liu, M. The global trends and regional differences in incidence and mortality of hepatitis A from 1990 to 2019 and implications for its prevention. Hepatol. Int. 2021, 15, 1068–1082. [Google Scholar] [CrossRef] [PubMed]
- Kunanitthaworn, N.; Mueangmo, O.; Saheng, J.; Wongjak, W.; Lertsiriladakul, T.; Chaito, T.; Nantarat, P.; Sudjaritruk, T. Seroprevalence of hepatitis A virus antibodies among children and adolescents living in Northern Thailand: An implication for hepatitis A immunization. Sci. Rep. 2023, 13, 17432. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.; Lemon, S.M. Hepatitis A virus: From discovery to vaccines. Hepatology 2006, 43, S164–S172. [Google Scholar] [CrossRef]
- Lemon, S.M.; Walker, C.M. Hepatitis A Virus and Hepatitis E Virus: Emerging and Re-Emerging Enterically Transmitted Hepatitis Viruses. Cold Spring Harb. Perspect. Med. 2018, 9, a031823. [Google Scholar] [CrossRef]
- Walker, C.M. Adaptive Immune Responses in Hepatitis A Virus and Hepatitis E Virus Infections. Cold Spring Harb. Perspect. Med. 2018, 9, a033472. [Google Scholar] [CrossRef]
- Klevens, R.M.; Miller, J.T.; Iqbal, K.; Thomas, A.; Rizzo, E.M.; Hanson, H.; Sweet, K.; Phan, Q.; Cronquist, A.; Khudyakov, Y.; et al. The evolving epidemiology of hepatitis a in the United States: Incidence and molecular epidemiology from population-based surveillance, 2005–2007. Arch. Intern. Med. 2010, 170, 1811–1818. [Google Scholar] [CrossRef]
- Schwarz, N.G.; Revillion, M.; Roque-Afonso, A.M.; Dussaix, E.; Giraud, M.; Liberpre, C.; Couturier, E.; Astagneau, E.D. A food-borne outbreak of hepatitis A virus (HAV) infection in a secondary school in Upper Normandy, France, in November 2006. Euro Surveill. 2008, 13, 18885. [Google Scholar] [CrossRef]
- Croci, L.; De Medici, D.; Scalfaro, C.; Fiore, A.; Toti, L. The survival of hepatitis A virus in fresh produce. Int. J. Food Microbiol. 2001, 73, 29–34. [Google Scholar] [CrossRef]
- Ahmad, T.; Adnan, F.; Nadeem, M.; Kakar, S.; Anjum, S.; Saad, A.; Waheed, A.; Arshad, N. Assessment of the risk for human health of Enterovirus and Hepatitis A virus in clinical and water sources from three metropolitan cities of Pakistan. Ann. Agric. Environ. Med. 2018, 25, 708–713. [Google Scholar] [CrossRef]
- Shin, E.-C.; Jeong, S.-H. Natural History, Clinical Manifestations, and Pathogenesis of Hepatitis A. Cold Spring Harb. Perspect. Med. 2018, 8, a031708. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, K.H.; Wiersma, S.T. Hepatitis A virus seroprevalence by age and world region, 1990 and 2005. Vaccine 2010, 28, 6653–6657. [Google Scholar] [CrossRef] [PubMed]
- Cheek, J.E.; Hennessy, T.W.; Redd, J.T.; Cobb, N.; Bryan, R.T. Epidemic assistance from the Centers for Disease Control and Prevention involving American Indians and Alaska Natives, 1946–2005. Am. J. Epidemiol. 2011, 174, S89–S96. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.cdc.gov/hepatitis/hcp/populations-settings/msm.html#:~:text=Hepatitis%20A%20virus%20(HAV)%2C,the%20virus%20or%20become%20infected (accessed on 8 August 2024).
- Wooten, D.A. Forgotten but Not Gone: Learning from the Hepatitis A Outbreak and Public Health Response in San Diego. Top. Antivir. Med. 2019, 26, 117–121. [Google Scholar] [PubMed]
- Havelaar, A.H.; Kirk, M.D.; Torgerson, P.R.; Gibb, H.J.; Hald, T.; Lake, R.J.; Praet, N.; Bellinger, D.C.; De Silva, N.R.; Gargouri, N.; et al. World Health Organization Global Estimates and Regional Comparisons of the Burden of Foodborne Disease in 2010. PLoS Med. 2015, 12, e1001923. [Google Scholar] [CrossRef] [PubMed]
- Erkan, T.; Kutlu, T.; Cullu, F.; Tümay, G.T. A case of vertical transmission of hepatitis A virus infection. Acta Paediatr. 2007, 87, 1008–1009. [Google Scholar] [CrossRef]
- Renge, R.L.; Dani, V.S.; Chitambar, S.D.; Arankalle, V.A. Vertical transmission of Hepatitis A. Indian J. Pediatr. 2002, 69, 535–536. [Google Scholar] [CrossRef]
- Elinav, E.; Ben–Dov, I.Z.; Shapira, Y.; Daudi, N.; Adler, R.; Shouval, D.; Ackerman, Z. Acute Hepatitis a Infection in Pregnancy Is Associated with High Rates of Gestational Complications and Preterm Labor. Gastroenterology 2006, 130, 1129–1134. [Google Scholar] [CrossRef]
- McDuffie, R.S.; Bader, T. Fetal meconium peritonitis after maternal hepatitis A. Am. J. Obstet. Gynecol. 1999, 180, 1031–1032. [Google Scholar] [CrossRef]
- Leikin, E.; Lysikiewicz, A.; Garry, D.; Tejani, N. Intrauterine transmission of hepatitis A virus. Obstet. Gynecol. 1996, 88, 690–691. [Google Scholar] [CrossRef]
- Daudi, N.; Shouval, D.; Stein-Zamir, C.; Ackerman, Z. Breastmilk hepatitis A virus RNA in nursing mothers with acute hepatitis A virus infection. Breastfeed. Med. 2012, 7, 313–315. [Google Scholar] [CrossRef] [PubMed]
- Moro, P.L.; Museru, O.I.; Niu, M.; Lewis, P.; Broder, K. Reports to the Vaccine Adverse Event Reporting System after hepatitis A and hepatitis AB vaccines in pregnant women. Am. J. Obstet. Gynecol. 2013, 210, 561.e1–561.e6. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, B.; Jindal, H.; Malik, J.S.; Choudhry, S. Vaccination for pregnant women: Need to address. Hum. Vaccines Immunother. 2014, 10, 3627–3628. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Kim, E.J.; Lee, J.H.; Choi, S.S.; Kim, M.S.; Jung, S.S.; Han, G.Y.; Yun, H.S.; Chun, D.S.; Oh, S.S.; et al. Molecular characterization of hepatitis A virus isolated from acute gastroenteritis patients in the Seoul region of Korea. Eur. J. Clin. Microbiol. Infect. Dis. 2009, 28, 1177–1182. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.A.; Fontaine, M.J.; Gonzalez, C.L.; Layon, A.G.; Goodnough, L.T.; Galel, S.A. Case report of a transfusion-associated hepatitis A infection. Transfusion 2014, 54, 2202–2206. [Google Scholar] [CrossRef] [PubMed]
- Galimberti, F.; Bonomi Boseggia, S.; Tragni, E. Consequences of COVID-19 pandemic on healthcare services. G. Ital. Farm. Farm. 2021, 13, 5–16. [Google Scholar]
- Almasio, P.L.; Amoroso, P. HAV infection in chronic liver disease: A rationale for vaccination. Vaccine 2003, 21, 2238–2241. [Google Scholar] [CrossRef]
- Available online: https://www.epicentro.iss.it/epatite/dati-seieva (accessed on 8 August 2024).
- Available online: https://www.ecdc.europa.eu/en/publications-data/hepatitis-annual-epidemiological-report-2021 (accessed on 8 August 2024).
- Todorova, T.; Stoykova, Z.; Kostadinova, T. An Increased Occurrence of Viral Hepatitis A during the COVID-19 Pandemic. Infect. Dis. 2021, 53, 963–964. [Google Scholar] [CrossRef]
- Marrone, A.; Nevola, R.; Sellitto, A.; Cozzolino, D.; Romano, C.; Cuomo, G.; Aprea, C.; Schwartzbaum, M.X.P.; Ricozzi, C.; Imbriani, S.; et al. Remdesivir Plus Dexamethasone Versus Dexamethasone Alone for the Treatment of Coronavirus Disease 2019 (COVID-19) Patients Requiring Supplemental O2 Therapy: A Prospective Controlled Nonrandomized Study. Clin. Infect. Dis. 2022, 75, e403–e409. [Google Scholar] [CrossRef]
- Sarialioğlu, F.; Belen, F.B.; Hayran, K.M. Hepatitis A Susceptibility Parallels High COVID-19 Mortality. Turk. J. Med. Sci. 2021, 51, 382–384. [Google Scholar] [CrossRef]
- Gutierrez-Reyes, G.; Gutierrez-Ruiz, M.C.; Kershenobich, D. Liver fibrosis and chronic viral hepatitis. Arch. Med. Res. 2007, 38, 644–651. [Google Scholar] [CrossRef] [PubMed]
- Galiero, R.; Loffredo, G.; Simeon, V.; Caturano, A.; Vetrano, E.; Medicamento, G.; Alfano, M.; Beccia, D.; Brin, C.; Colantuoni, S.; et al. Impact of liver fibrosis on COVID-19 in-hospital mortality in Southern Italy. PLoS ONE 2024, 19, e0296495. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rzymski, P.; Zarębska-Michaluk, D.; Genowska, A.; Tyszko, P.; Strukcinskiene, B.; Flisiak, R. Trends of Hepatitis A Virus Infection in Poland: Assessing the Potential Impact of the COVID-19 Pandemic and War in Ukraine. Viruses 2024, 16, 469. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Petakh, P.; Tymchyk, V.; Kamyshnyi, O. Communicable diseases in Ukraine during the period of 2018–2023: Impact of the COVID-19 pandemic and war. Travel Med. Infect. Dis. 2024, 60, 102733. [Google Scholar] [CrossRef] [PubMed]
- Levican, J.; Ampuero, M.; Rabello, C.; Venegas, I.; Quarleri, J.; Gaggero, A. Changing molecular epidemiology of Hepatitis A virus in Santiago, Chile from 2010 to 2021. Infect. Genet. Evol. 2023, 111, 105428. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). WHO Immunological Basis for Immunization Series: Module 18: Hepatitis A; Update 2019; WHO: Geneva, Switzerland, 2019; Available online: https://apps.who.int/iris/handle/10665/326501 (accessed on 8 August 2024).
- Zanella, B.; Boccalini, S.; Biamonte, M.A.; Giorgetti, D.; Menicacci, M.; Bonito, B.; Ninci, A.; Tiscione, E.; Puggelli, F.; Mereu, G.; et al. A Study of Hepatitis A Seroprevalence in a Paediatric and Adolescent Population of the Province of Florence (Italy) in the Period 2017–2018 Confirms Tuscany a Low Endemic Area. Vaccines 2021, 9, 1194. [Google Scholar] [CrossRef] [PubMed]
- Sagnelli, E.; Stroffolini, T.; Almasio, P.; Mele, A.; Coppola, N.; Ferrigno, L.; Scolastico, C.; Onofrio, M.; Imparato, M.; Filippini, P. Exposure to HAV infection in patients with chronic liver disease in Italy, a multicentre study. J. Viral Hepat. 2005, 13, 67–71. [Google Scholar] [CrossRef]
- Ansaldi, F.; Bruzzone, B.; Rota, M.C.; Bella, A.; Ciofi degli Atti, M.; Durando, P.; Gasparini, R.; Icardi, G.; Serologic Study Group. Hepatitis A incidence and hospital-based seroprevalence in Italy: A nation-wide study. Eur. J. Epidemiol. 2007, 23, 45–53. [Google Scholar] [CrossRef]
- Armstrong, G.L.; Bell, B.P. Hepatitis A virus infections in the United States: Model-based estimates and implications for childhood immunization. Pediatrics 2002, 109, 839–845. [Google Scholar] [CrossRef]
- World Health Organization (WHO). WHO Position Paper on Hepatitis A Vaccines; Weekly Epidemiological Record, 87(28–29); WHO: Geneva, Switzerland, 6 June 2012; pp. 261–276. Available online: https://www.who.int/publications/i/item/who-wer8728-29-261-276 (accessed on 8 August 2024).
- Gentile, C.; Alberini, I.; Manini, I.; Rossi, S.; Montomoli, E.; Pozzi, T.; Rizzo, C.; Alfonsi, V. Hepatitis A seroprevalence in Tuscany, Italy. Euro Surveill. 2009, 14, 19146. [Google Scholar] [CrossRef]
- Chironna, M.; Prato, R.; Sallustio, A.; Martinelli, D.; Tafuri, S.; Quarto, M.; Germinario, C. Hepatitis A in Puglia (South Italy) after 10 years of universal vaccination: Need for strict monitoring and catch-up vaccination. BMC Infect. Dis. 2012, 12, 271. [Google Scholar] [CrossRef] [PubMed]
- Valdenassi, L.; Franzini, M.; Ricevuti, G.; Rinaldi, L.; Galoforo, A.C.; Tirelli, U. Potential mechanisms by which the oxygen-ozone (O2-O3) therapy could contribute to the treatment against the coronavirus COVID-19. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 4059–4061. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elshafy, D.N.; Nadeem, R.; Bahgat, M.M. Prevalence of enteric viruses in wastewater in Egypt after the COVID-19 pandemic. J. Water Health 2022, 20, 1668–1672. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, S.; Xia, G.L.; Dimitrova, Z.; Lin, Y.; Montgomery, M.; Augustine, R.; Kamili, S.; Khudyakov, Y. Changing Molecular Epidemiology of Hepatitis A Virus Infection, United States, 1996–2019. Emerg. Infect. Dis. 2021, 27, 1742–1745. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Years | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 | 2023 | 2015–2023 |
|---|---|---|---|---|---|---|---|---|---|---|
| Samples | ||||||||||
| Positive n. (%) | 1916 (74.5) | 2202 (76.7) | 2097 (74.3) | 2248 (74.3) | 2464 (74.1) | 1958 (73) | 2172 (70.4) | 2676 (71.8) | 2880 (72) | 20,613 (73.3) |
| Negative n. (%) | 657 (25.5) | 670 (23.3) | 726 (25.7) | 776 (25.7) | 863 (25.9) | 723 (27) | 906 (29.6) | 1049 (28.2) | 1121 (28) | 7491 (26.7) |
| Total samples n. | 2573 | 2872 | 2823 | 3024 | 3327 | 2681 | 3078 | 3725 | 4001 | 28,104 |
| Positive Absolute and Relative Frequences | Negative Absolute and Relative Frequences | Total | p-Value | |
|---|---|---|---|---|
| HAV (2015–2019) N. (%) | 10,927 (74.7%) | 3692 (25.3%) | 14,619 | 0.009 |
| HAV (2020–2023) N. (%) | 9686 (71.8%) | 3799 (28.2%) | 13,485 |
| Years Gender | 2015 | 2016 | 2017 | 2018 | 2019 | 2015–2019 | Positive and Negative | Positive N. (%) |
|---|---|---|---|---|---|---|---|---|
| Female N. (%) | 833 (43.5) | 850 (38.6) | 865 (41.2) | 979 (43.5) | 991 (40.2) | 4518 (41.3) | n = 6218 | 4518 (72.7) |
| Male N. (%) | 1083 (56.5) | 1352 (61.4) | 1232 (58.5) | 1269 (56.5) | 1473 (59.8) | 6409 (58.7) | n = 8401 | 6409 (76.3) |
| Total positive N | 1916 | 2202 | 2097 | 2248 | 2464 | 10,927 | p-value | <0.001 |
| Years Gender | 2020 | 2021 | 2022 | 2023 | 2020–2023 | Positive and Negative | Positive N. (%) |
|---|---|---|---|---|---|---|---|
| Female N. (%) | 780 (39.8) | 927 (42.7) | 1139 (42.6) | 1246 (43.3) | 4092 (42.2) | n = 5940 | 4092 (68.9) |
| Male N. (%) | 1178 (60.2) | 1245 (57.3) | 1537 (57.4) | 1634 (56.7) | 5594 (57.8) | n = 7545 | 5594 (74.1) |
| Total positive N. | 1958 | 2172 | 2676 | 2880 | 9686 | p-value | <0.001 |
| (a) | 2015 | 2016 | 2017 | 2018 | 2019 | 2015–2019 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age/Gender | M | F | M | F | M | F | M | F | M | F | M | F |
| 0–10 | 0% | 0.1% | 0.1% | 0.4% | 0.2% | 0.3% | 0.5% | 0.1% | 0.2% | 0.3% | 0.2% | 0.2% |
| 11–20 | 0.7% | 0.6% | 1.0% | 0.4% | 1.8% | 0.6% | 1.4% | 0.8% | 0.8% | 0.3% | 1.2% | 0.5% |
| 21–30 | 1.8% | 0.6% | 1.0% | 1.6% | 1.0% | 1.8% | 1.6% | 1.3% | 1.7% | 0.8% | 1.4% | 1.2% |
| 31–40 | 2.7% | 2.0% | 2.4% | 2.0% | 3.2% | 2.1% | 2.2% | 2.8% | 2.6% | 1.2% | 2.6% | 2.0% |
| 41–50 | 8.4% | 8.5% | 7.6% | 7.5% | 8.1% | 8.1% | 6.9% | 7.0% | 4.4% | 5.0% | 7.0% | 7.1% |
| 51–60 | 22.3% | 26.3% | 18.4% | 18.6% | 21.1% | 23.1% | 20.4% | 22.0% | 17.7% | 28.3% | 19.8% | 22.0% |
| 61–70 | 26.8% | 26.2% | 30.2% | 29.0% | 29.5% | 26.0% | 31.0% | 28.0% | 32.4% | 28.8% | 30.1% | 27.6% |
| 71–80 | 27.0% | 22.3% | 29.2% | 26.4% | 25% | 26.5% | 26.5% | 25.8% | 29.6% | 30.0% | 27.6% | 26.3% |
| 81–90 | 9.8% | 12.7% | 9.7% | 13.0% | 9.6% | 10.9% | 9.3% | 10.8% | 10.2% | 12.2% | 9.7% | 11.9% |
| 90–95 | 0.5% | 0.6% | 0.4% | 1.0% | 0.6% | 0.6% | 0.3% | 1.2% | 0.4% | 1.2% | 0.4% | 1.0% |
| Total | 56.5% | 43.5% | 61.4% | 38.6% | 58.8% | 41.2% | 56.5% | 43.5% | 59.8% | 40.2% | 58.7% | 41.3% |
| Total positive | 1916 | 2202 | 2097 | 2248 | 2464 | 10,927 | ||||||
| p-value | 0.022 | 0.045 | 0.059 | 0.087 | 0.002 | <0.001 | ||||||
| (b) | 2020 | 2021 | 2022 | 2023 | 2020–2023 | |||||||
| Age/Gender | M | F | M | F | M | F | M | F | M | F | ||
| 0–10 | 0% | 0% | 0% | 0% | 0.2% | 0.3% | 0.1% | 0% | 0.1% | 0.1% | ||
| 11–20 | 0.2% | 0.8% | 0.1% | 0.4% | 0.2% | 1.0% | 0.3% | 0.5% | 0.2% | 0.7% | ||
| 21–30 | 1.5% | 1.5% | 1.5% | 0.9% | 1.5% | 1.4% | 1.5% | 0.5% | 1.5% | 1.0% | ||
| 31–40 | 2.3% | 1.5% | 2.8% | 1.9% | 1.4% | 2.4% | 1.7% | 1.7% | 2.0% | 1.9% | ||
| 41–50 | 6.3% | 5.1% | 4.7% | 4.6% | 3.4% | 4.8% | 3.2% | 4.4% | 4.3% | 4.7% | ||
| 51–60 | 20.0% | 17.8% | 18.6% | 18.7% | 16.7% | 16.0% | 16.3% | 15.2% | 17.7% | 16.7% | ||
| 61–70 | 29.6% | 28.2% | 31.3% | 25.9% | 33.7% | 27.0% | 30.7% | 27.4% | 31.4% | 27.1% | ||
| 71–80 | 28.4% | 30.3% | 30.5% | 30.4% | 28.6% | 26.0% | 32.3% | 28.3% | 30.1% | 28.5% | ||
| 81–90 | 11.4% | 12.8% | 9.8% | 14.7% | 12.8% | 18.1% | 12.9% | 19.1% | 11.9% | 16.6% | ||
| 90–95 | 0.3% | 1.9% | 0.7% | 2.5% | 1.5% | 3.0% | 0.9% | 2.9% | 0.9% | 2.6% | ||
| Total | 60.2% | 39.8% | 57.3% | 42.7% | 57.4% | 42.6% | 56.7% | 43.3% | 57.8% | 42.2% | ||
| Total positive | 1958 | 2172 | 2676 | 2880 | 9686 | |||||||
| p-value | 0.006 | <0.001 | <0.001 | <0.001 | <0.001 | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serretiello, E.; Iervolino, D.; Di Siervi, G.; Gallo, L.; Bernardi, F.F.; Pagliano, P.; Boccia, G.; Folliero, V.; Franci, G.; Rinaldi, L. Unveiling the Burden of Hepatitis A in Salerno, Italy: A Comprehensive 9-Year Retrospective Study (2015–2023) on the Seroprevalence of HAV Antibodies and Age/Sex Distribution. J. Clin. Med. 2024, 13, 5534. https://doi.org/10.3390/jcm13185534
Serretiello E, Iervolino D, Di Siervi G, Gallo L, Bernardi FF, Pagliano P, Boccia G, Folliero V, Franci G, Rinaldi L. Unveiling the Burden of Hepatitis A in Salerno, Italy: A Comprehensive 9-Year Retrospective Study (2015–2023) on the Seroprevalence of HAV Antibodies and Age/Sex Distribution. Journal of Clinical Medicine. 2024; 13(18):5534. https://doi.org/10.3390/jcm13185534
Chicago/Turabian StyleSerretiello, Enrica, Domenico Iervolino, Giuseppe Di Siervi, Luigi Gallo, Francesca F. Bernardi, Pasquale Pagliano, Giovanni Boccia, Veronica Folliero, Gianluigi Franci, and Luca Rinaldi. 2024. "Unveiling the Burden of Hepatitis A in Salerno, Italy: A Comprehensive 9-Year Retrospective Study (2015–2023) on the Seroprevalence of HAV Antibodies and Age/Sex Distribution" Journal of Clinical Medicine 13, no. 18: 5534. https://doi.org/10.3390/jcm13185534
APA StyleSerretiello, E., Iervolino, D., Di Siervi, G., Gallo, L., Bernardi, F. F., Pagliano, P., Boccia, G., Folliero, V., Franci, G., & Rinaldi, L. (2024). Unveiling the Burden of Hepatitis A in Salerno, Italy: A Comprehensive 9-Year Retrospective Study (2015–2023) on the Seroprevalence of HAV Antibodies and Age/Sex Distribution. Journal of Clinical Medicine, 13(18), 5534. https://doi.org/10.3390/jcm13185534





