Shedding Light on the COVID-19 Pandemic: Placental Expression of Cell Biomarkers in Negative, Vaccinated, and Positive Pregnant Women
Abstract
:1. Introduction
2. Materials and Methods
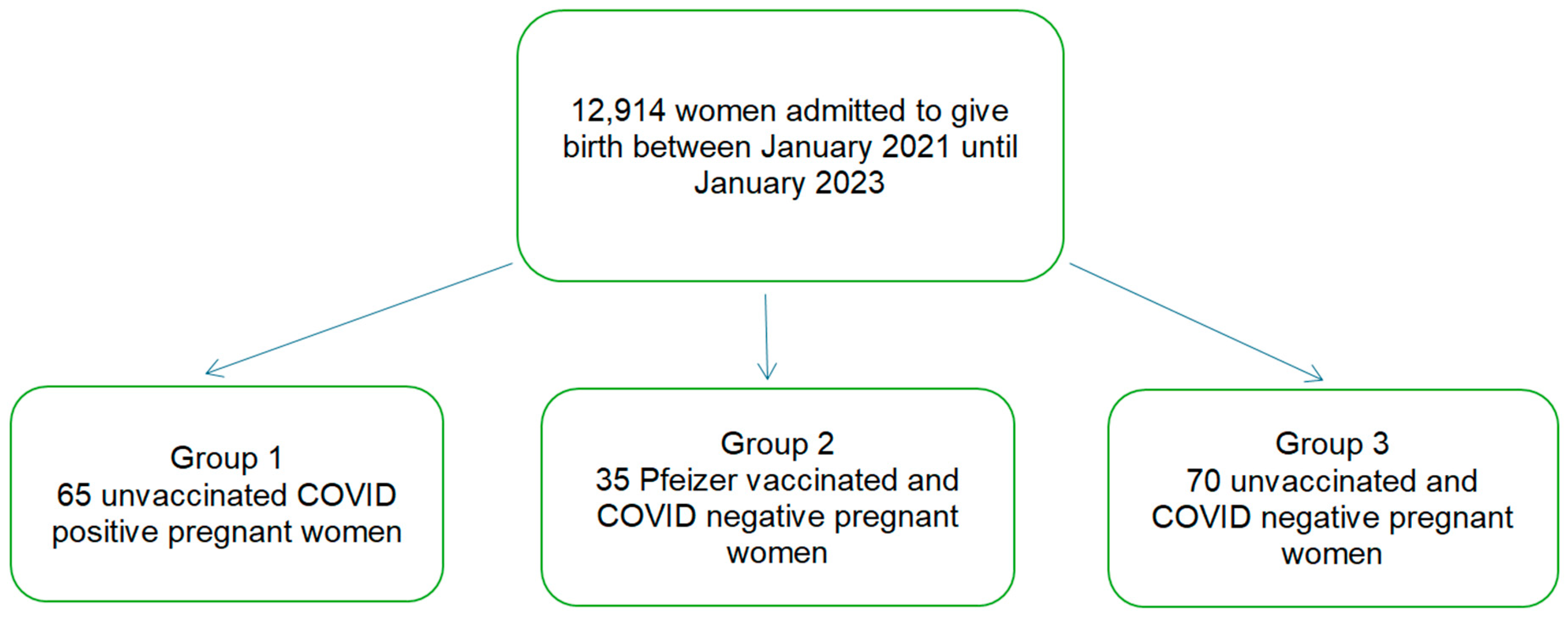
2.1. Patients and Tissue Samples
Exclusion Criteria
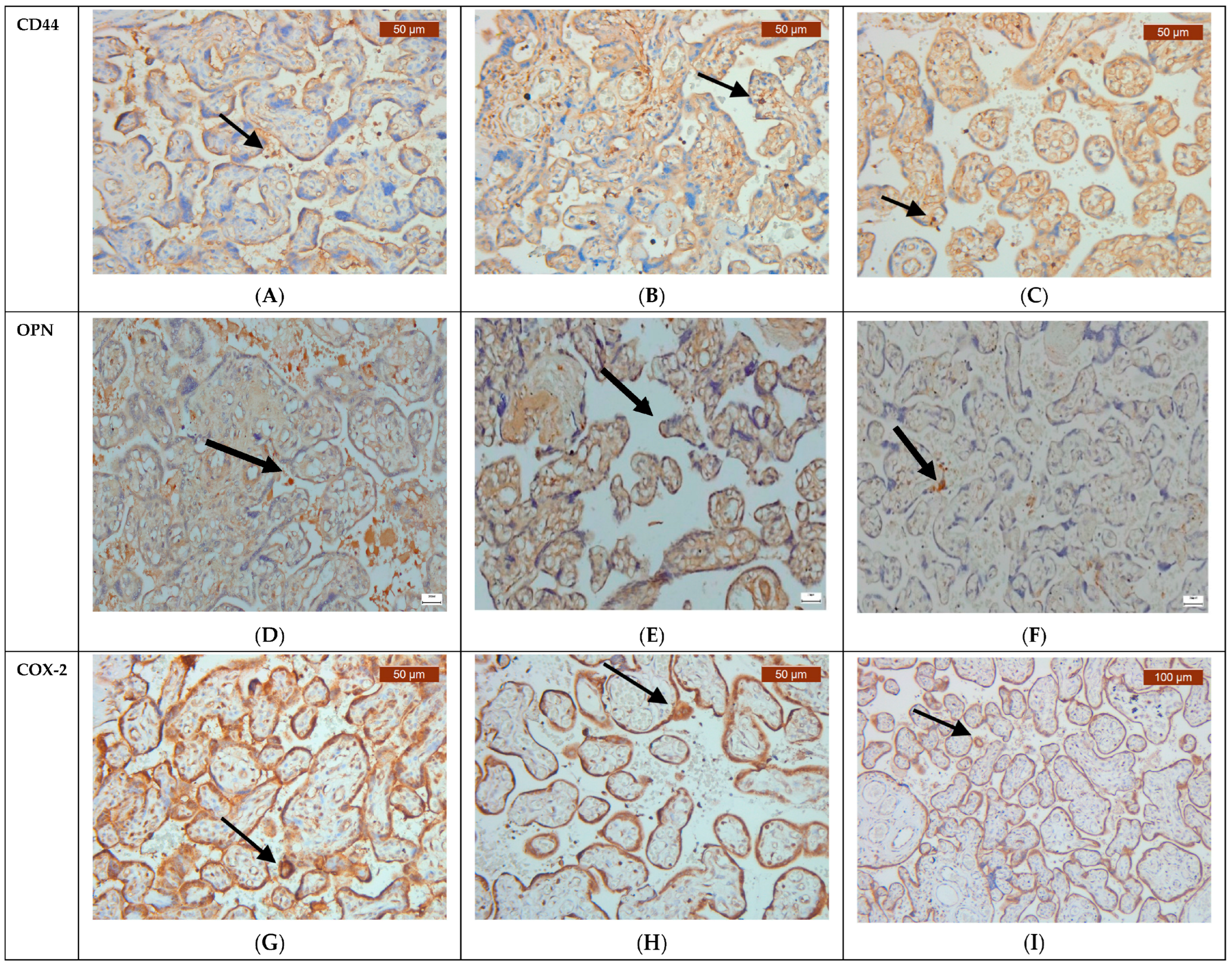
2.2. Immunohistochemistry
2.3. Statistical Analysis
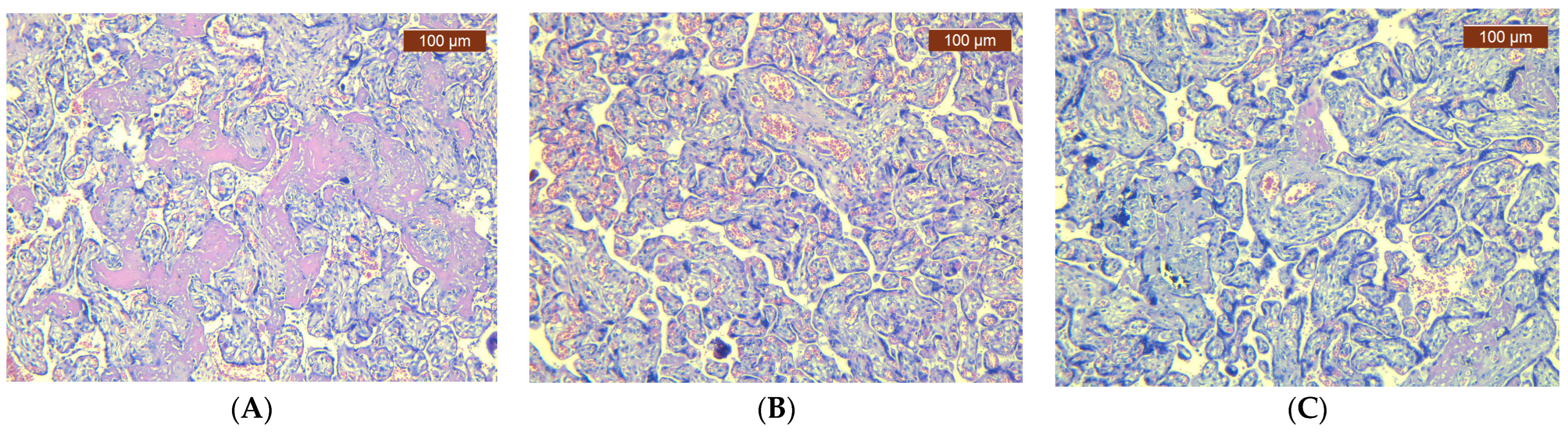
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shanes, E.D.; Mithal, L.B.; Otero, S.; Azad, H.A.; Miller, E.S.; Goldstein, J.A. Placental Pathology in COVID-19. Am. J. Clin. Pathol. 2020, 154, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, D.A.; Bugatti, M.; Santoro, A.; Facchetti, F. Molecular Pathology Demonstration of SARS-CoV-2 in Cytotrophoblast from Placental Tissue with Chronic Histiocytic Intervillositis, Trophoblast Necrosis and COVID-19. J. Dev. Biol. 2021, 9, 33. [Google Scholar] [CrossRef] [PubMed]
- Bertero, L.; Borella, F.; Botta, G.; Carosso, A.; Cosma, S.; Bovetti, M.; Carosso, M.; Abbona, G.; Collemi, G.; Papotti, M.; et al. Placenta histopathology in SARS-CoV-2 infection: Analysis of a consecutive series and comparison with control cohorts. Virchows Arch. 2021, 479, 715–728. [Google Scholar] [CrossRef] [PubMed]
- Di Girolamo, R.; Khalil, A.; Alameddine, S.; D’Angelo, E.; Galliani, C.; Matarrelli, B.; Buca, D.; Liberati, M.; Rizzo, G.; D’Antonio, F. Placental histopathology after SARS-CoV-2 infection in pregnancy: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. MFM 2021, 3, 100468. [Google Scholar] [CrossRef] [PubMed]
- Garg, R.; Agarwal, R.; Yadav, D.; Singh, S.; Kumar, H.; Bhardwaj, R. Histopathological Changes in Placenta of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection and Maternal and Perinatal Outcome in COVID-19. J. Obstet. Gynaecol. India 2023, 73, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Patberg, E.T.; Adams, T.; Rekawek, P.; Vahanian, S.A.; Akerman, M.; Hernandez, A.; Rapkiewicz, A.V.; Ragolia, L.; Sicuranza, G.; Chavez, M.R.; et al. Coronavirus disease 2019 infection and placental histopathology in women delivering at term. Am. J. Obstet. Gynecol. 2021, 224, 382.e1–382.e18, Erratum in: Am. J. Obstet. Gynecol. 2023, 228, 128. https://doi.org/10.1016/j.ajog.2021.04.255. [Google Scholar] [CrossRef]
- Vikesaa, J.; Hansen, T.V.; Jønson, L.; Borup, R.; Wewer, U.M.; Christiansen, J.; Nielsen, F.C. RNA-binding IMPs promote cell adhesion and invadopodia formation. EMBO J. 2006, 25, 1456–1468. [Google Scholar] [CrossRef]
- Obut, M.; Oğlak, S.C. Expression of CD44 and IL-10 in normotensive and preeclamptic placental tissue. Ginekol. Pol. 2020, 91, 334–341. [Google Scholar] [CrossRef]
- Thapa, R.; Wilson, G.D. The Importance of CD44 as a Stem Cell Biomarker and Therapeutic Target in Cancer. Stem Cells Int. 2016, 2016, 2087204. [Google Scholar] [CrossRef]
- Higashibata, Y.; Sakuma, T.; Kawahata, H.; Fujihara, S.; Moriyama, K.; Okada, A.; Yasui, T.; Kohri, K.; Kitamura, Y.; Nomura, S. Identification of promoter regions involved in cell- and developmental stage-specific osteopontin expression in bone, kidney, placenta, and mammary gland: An analysis of transgenic mice. J. Bone Miner. Res. 2004, 19, 78–88. [Google Scholar] [CrossRef]
- Thomas, J.R.; Appios, A.; Zhao, X.; Dutkiewicz, R.; Donde, M.; Lee, C.Y.C.; Naidu, P.; Lee, C.; Cerveira, J.; Liu, B.; et al. Phenotypic and functional characterization of first-trimester human placental macrophages, Hofbauer cells. J. Exp. Med. 2021, 218, e20200891. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Li, X.; Seo, H.; McLendon, B.A.; Kramer, A.C.; Bazer, F.W.; Johnson, G.A. Osteopontin (OPN)/Secreted Phosphoprotein 1 (SPP1) Binds Integrins to Activate Transport of Ions across the Porcine Placenta. Front. Biosci. 2022, 27, 117. [Google Scholar] [CrossRef] [PubMed]
- Szczuko, M.; Kikut, J.; Komorniak, N.; Bilicki, J.; Celewicz, Z.; Ziętek, M. The Role of Arachidonic and Linoleic Acid Derivatives in Pathological Pregnancies and the Human Reproduction Process. Int. J. Mol. Sci. 2020, 21, 9628. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.J.; Sung, G.J.; Marquardt, R.; Young, S.L.; Lessey, B.A.; Kim, T.H.; Cheon, Y.P.; Jeong, J.W. SIRT1 plays an important role in implantation and decidualization during mouse early pregnancy. Biol. Reprod. 2022, 106, 1072–1082. [Google Scholar] [CrossRef] [PubMed]
- Helmi, Z.; Al-Badri, H. Association of placental histopathological findings with COVID-19 and its predictive factors. Rev. Bras. Ginecol. Obstet. 2024, 46, e-rbgo3. [Google Scholar] [CrossRef]
- Debelenko, L.; Katsyv, I.; Chong, A.M.; Peruyero, L.; Szabolcs, M.; Uhlemann, A.C. Trophoblast damage with acute and chronic intervillositis: Disruption of the placental barrier by severe acute respiratory syndrome coronavirus 2. Hum. Pathol. 2021, 109, 69–79. [Google Scholar] [CrossRef]
- Chen, J.; Du, L.; Wang, F.; Shao, X.; Wang, X.; Yu, W.; Bi, S.; Chen, D.; Pan, X.; Zeng, S.; et al. Cellular and molecular atlas of the placenta from a COVID-19 pregnant woman infected at midgestation highlights the defective impacts on foetal health. Cell Prolif. 2022, 55, e13204. [Google Scholar] [CrossRef]
- Surekha, M.V.; Suneetha, N.; Balakrishna, N.; Putcha, U.K.; Satyanarayana, K.; Geddam, J.J.B.; Sreenu, P.; Tulja, B.; Mamidi, R.S.; Rutter, G.A.; et al. Impact of COVID-19 during pregnancy on placental pathology, maternal and neonatal outcome—A cross-sectional study on anemic term pregnant women from a tertiary care hospital in southern India. Front. Endocrinol. 2023, 14, 1092104. [Google Scholar] [CrossRef]
- Tartaglia, S.; Di Ilio, C.; Romanzi, F.; Moresi, S.; Nardi, E.; Bevilacqua, E.; Arena, V.; Lanzone, A. Effects of SARS-CoV-2 mRNA vaccine on placental histopathology: Comparison of a population of uncomplicated COVID-19 positive pregnant women. Placenta 2024, 149, 64–71. [Google Scholar] [CrossRef]
- Valdespino-Vázquez, M.Y.; Helguera-Repetto, C.A.; León-Juárez, M.; Villavicencio-Carrisoza, O.; Flores-Pliego, A.; Moreno-Verduzco, E.R.; Díaz-Pérez, D.L.; Villegas-Mota, I.; Carrasco-Ramírez, E.; López-Martínez, I.E.; et al. Fetal and placental infection with SARS-CoV-2 in early pregnancy. J. Med. Virol. 2021, 93, 4480–4487. [Google Scholar] [CrossRef]
- Maranto, M.; Zaami, S.; Restivo, V.; Termini, D.; Gangemi, A.; Tumminello, M.; Culmone, S.; Billone, V.; Cucinella, G.; Gullo, G. Symptomatic COVID-19 in Pregnancy: Hospital Cohort Data between May 2020 and April 2021, Risk Factors and Medicolegal Implications. Diagnostics 2023, 13, 1009. [Google Scholar] [CrossRef] [PubMed]
- Incognito, G.G.; Distefano, R.E.C.; Campo, G.; Gulino, F.A.; Gulisano, C.; Gullotta, C.; Gullo, G.; Cucinella, G.; Tuscano, A.; Bruno, M.T.; et al. Comparison of Maternal and Neonatal Outcomes between SARS-CoV-2 Variants: A Retrospective, Monocentric Study. J. Clin. Med. 2023, 12, 6329. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Maranto, M.; Gullo, G.; Bruno, A.; Minutolo, G.; Cucinella, G.; Maiorana, A.; Casuccio, A.; Restivo, V. Factors Associated with Anti-SARS-CoV-2 Vaccine Acceptance among Pregnant Women: Data from Outpatient Women Experiencing High-Risk Pregnancy. Vaccines 2023, 11, 454. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kreis, N.N.; Ritter, A.; Louwen, F.; Yuan, J. A Message from the Human Placenta: Structural and Immunomodulatory Defense against SARS-CoV-2. Cells 2020, 9, 1777. [Google Scholar] [CrossRef]
- Sarno, L.; Locci, M.; Fulgione, C.; Perillo, F.; Dell’Isola, A.; Mantelli, D.; Sibillo, C.; Saccone, G.; Maruotti, G.M.; Terracciano, D.; et al. Characteristics of Placental Histopathology in Women with Uncomplicated Pregnancies Affected by SARS-CoV-2 Infection at the Time of Delivery: A Single-Center Experience. Biomedicines 2022, 10, 3003. [Google Scholar] [CrossRef]
- Balsak, D.; Togrul, C.; Ekinci, C.; Cavus, Y.; Tahaoglu, A.E.; Deveci, E.; Gül, T.; Karaman, E.; Ekinci, A.; Sakar, N. Severe pre-eclampsia complicated by HELLP syndrome alterations in the structure of the umbilical cord (morphometric and immunohistochemical study). Biotechnol. Biotechnol. Equip. 2015, 29, 345–350. [Google Scholar] [CrossRef]
- Zhu, R.; Wang, S.C.; Sun, C.; Tao, Y.; Piao, H.L.; Wang, X.Q.; Du, M.R.; Li, D.-J. Hyaluronan-CD44 interaction promotes growth of decidual stromal cells in human first-trimester pregnancy. PLoS ONE 2013, 8, e74812. [Google Scholar] [CrossRef]
- Li, M.C.; Fang, Q.; He, Z.M.; Gao, Y.; Zhou, Y. Placental expression of osteopontin(OPN) in monochorionic twins with discordant growth. Placenta 2013, 34, 288–290. [Google Scholar] [CrossRef]
- Song, Y.B.; Sun, M.Y. Correlation analysis between COX-2 gene polymorphism and eclampsia. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 6404–6410. [Google Scholar] [CrossRef]
- Pannunzio, A.; Coluccia, M. Cyclooxygenase-1 (COX-1) and COX-1 Inhibitors in Cancer: A Review of Oncology and Medicinal Chemistry Literature. Pharmaceuticals 2018, 11, 101. [Google Scholar] [CrossRef]
- Gately, S. The Contributions of Cyclooxygenase-2 to Tumor Angiogenesis. Cancer Metastasis 2000, 19, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Mancini, A.D.; Di Battista, J.A. The cardinal role of the phospholipase A2/cyclooxygenase-2/prostaglandin E synthase/prostaglandin E2 (PCPP) axis in inflammostasis. Inflamm. Res. 2011, 60, 1083–1092. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Pan, L.; Tang, L.; Liu, J. Alpha-enolase 1 knockdown facilitates the proliferation and invasion of villous trophoblasts by upregulating COX-2. Mol. Genet. Genom. Med. 2023, 11, e2220. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Chang, X.; Bai, J.; Chen, Z.J.; Li, W.P.; Zhang, C. The Study of Cyclooxygenase 2 in Human Decidua of Preeclampsia. Biol. Reprod. 2016, 95, 56. [Google Scholar] [CrossRef] [PubMed]
- Shah, B.H.; Catt, K.J. Roles of LPA3 and COX-2 in implantation. Trends Endocrinol. Metab. 2005, 16, 397–399. [Google Scholar] [CrossRef]
- Gullo, G.; Scaglione, M.; Cucinella, G.; Riva, A.; Coldebella, D.; Cavaliere, A.F.; Signore, F.; Buzzaccarini, G.; Spagnol, G.; Laganà, A.S.; et al. Congenital Zika Syndrome: Genetic Avenues for Diagnosis and Therapy, Possible Management and Long-Term Outcomes. J. Clin. Med. 2022, 11, 1351. [Google Scholar] [CrossRef]
| Clinical and Demographic Characteristics of the Women | No. | Mean | St. Dev. | Percentile 25 | Median | Percentile 75 | Min | Max | Kruskal–Wallis p-Value | |
|---|---|---|---|---|---|---|---|---|---|---|
| Maternal age (years) | Negative and unvaccinated | 70 | 28 | 4 | 27 | 29 | 31 | 19 | 34 | <0.001 |
| Positive | 65 | 31 | 5 | 28 | 33 | 34 | 18 | 39 | ||
| Pfizer vaccinated | 35 | 30 | 5 | 27 | 30 | 33 | 18 | 39 | ||
| Gestational age at delivery (weeks) | Negative and unvaccinated | 70 | 40 | 1 | 38 | 40 | 41 | 37 | 41 | <0.001 |
| Positive | 65 | 38 | 3 | 37 | 38 | 39 | 28 | 40 | ||
| Pfizer vaccinated | 35 | 38 | 3 | 37 | 39 | 40 | 29 | 41 | ||
| Fetal weight (grams) | Negative and unvaccinated | 70 | 3507 | 354 | 3260 | 3495 | 3670 | 2800 | 4490 | 0.01 |
| Positive | 65 | 3237 | 536 | 2920 | 3300 | 3600 | 1980 | 4540 | ||
| Pfizer vaccinated | 35 | 3291 | 522 | 2920 | 3390 | 3650 | 1990 | 4570 | ||
| Apgar score at 1 min (point) | Negative and unvaccinated | 70 | 9 | 1 | 8 | 9 | 9 | 8 | 9 | 0.67 |
| Positive | 65 | 8 | 1 | 8 | 9 | 9 | 4 | 9 | ||
| Pfizer vaccinated | 35 | 8 | 1 | 8 | 9 | 9 | 4 | 10 | ||
| Hemoglobin antepartum (milligrams/deciliter) | Negative and unvaccinated | 70 | 12.3 | 1.1 | 11.8 | 12.1 | 13.6 | 10.0 | 13.7 | 0.76 |
| Positive | 65 | 12.2 | 0.8 | 11.9 | 12.3 | 12.5 | 9.7 | 13.4 | ||
| Pfizer vaccinated | 35 | 12.3 | 0.9 | 11.9 | 12.3 | 13.0 | 9.8 | 14.3 | ||
| Hematocrit antepartum (%) | Negative and unvaccinated | 70 | 36.3 | 3.4 | 35.0 | 37.0 | 38.9 | 28.1 | 40.0 | 0.578 |
| Positive | 65 | 36.4 | 2.6 | 35.3 | 36.0 | 38.0 | 29.3 | 41.0 | ||
| Pfizer vaccinated | 35 | 36.8 | 2.9 | 35.3 | 36.9 | 38.8 | 28.1 | 42.1 | ||
| Hemoglobin postpartum (milligrams/deciliter) | Negative and unvaccinated | 70 | 10.9 | 0.8 | 10.4 | 10.9 | 11.4 | 9.0 | 13.0 | 0.61 |
| Positive | 65 | 11.0 | 0.9 | 10.4 | 11.0 | 11.6 | 8.9 | 12.8 | ||
| Pfizer vaccinated | 35 | 11.1 | 0.8 | 10.5 | 11.0 | 11.6 | 9.7 | 12.9 | ||
| Hematocrite postpartum (%) | Negative and unvaccinated | 70 | 31.8 | 2.9 | 29.0 | 31.1 | 34.3 | 27.8 | 37.0 | 0.52 |
| Positive | 65 | 32.3 | 2.8 | 30.4 | 32.3 | 33.6 | 27.7 | 38.5 | ||
| Pfizer vaccinated | 35 | 32.4 | 2.8 | 29.9 | 32.5 | 34.3 | 27.5 | 37.5 | ||
| Leucocyte value (103/L) | Negative and unvaccinated | 70 | 12,293 | 2272 | 10,350 | 11,485 | 14,600 | 9900 | 16,500 | <0.001 |
| Positive | 65 | 10,186 | 2590 | 8280 | 10,120 | 12,100 | 5390 | 16,400 | ||
| Pfizer vaccinated | 35 | 10,936 | 2922 | 8320 | 10,350 | 12,280 | 5390 | 16,500 | ||
| Platelet value (106/L) | Negative and unvaccinated | 70 | 220,500 | 78,240 | 149,000 | 217,500 | 278,000 | 122,000 | 355,000 | 0.76 |
| Positive | 65 | 218,523 | 63,791 | 156,000 | 211,000 | 260,000 | 132,000 | 355,000 | ||
| Pfizer vaccinated | 35 | 227,543 | 71,222 | 156,000 | 218,000 | 278,000 | 135,000 | 360,000 | ||
| CRP (C-reactive protein) value (milligrams/deciliter) | Negative and unvaccinated | 70 | 3.12 | 1.52 | 2.00 | 2.75 | 4.10 | 0.90 | 10.00 | 0.89 |
| Positive | 65 | 3.09 | 1.30 | 1.90 | 3.00 | 4.80 | 1.07 | 4.90 | ||
| Pfizer vaccinated | 35 | 3.18 | 1.20 | 2.30 | 2.80 | 4.10 | 1.00 | 4.90 | ||
| CD44 (Cluster of Differentiation 44) | p-Value | OPN (Osteopontin) | p-Value | COX-2 (Cyclooxygenase-2) | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Negative | Positive | Negative | Positive | Negative | Positive | Total | ||||||
| Group | COVID-positive | Count | 23 | 42 | <0.001 * Positive vs. vaccinated | 53 | 12 | <0.001 * Positive vs. vaccinated | 4 | 61 | 0.295 F Positive vs. vaccinated | 65 |
| % within lot | 35.4% | 64.6% | 81.5% | 18.5% | 6.2% | 93.8% | 100.0% | |||||
| Pfeizer vaccinated | Count | 0 | 35 | 0.004 F Vaccinated vs. negative | 11 | 24 | <0.001 * Vaccinated vs. negative | 0 | 35 | All positive Vaccinated vs. negative | 35 | |
| % within lot | 0.0% | 100.0% | 31.4% | 68.6% | 0.0% | 100.0% | 100.0% | |||||
| Negative and unvaccinated | Count | 14 | 56 | 0.045 * Positive vs. negative | 56 | 14 | 0.82 * Positive vs. negative | 0 | 70 | 0.051 F Positive vs. negative | 70 | |
| % within lot | 20.0% | 80.0% | 80.0% | 20.0% | 0.0% | 100.0% | 100.0% | |||||
| Total | Count | 37 | 133 | 120 | 50 | 4 | 166 | 170 | ||||
| % within lot | 21.8% | 78.2% | 70.6% | 29.4% | 2.4% | 97.6% | 100.0% | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Condac, C.; Lozneanu, L.; Matasariu, D.R.; Ursache, A.; Bujor, I.E.; Niță, M.E.; Boiculese, V.L.; Bîrluțiu, V. Shedding Light on the COVID-19 Pandemic: Placental Expression of Cell Biomarkers in Negative, Vaccinated, and Positive Pregnant Women. J. Clin. Med. 2024, 13, 5546. https://doi.org/10.3390/jcm13185546
Condac C, Lozneanu L, Matasariu DR, Ursache A, Bujor IE, Niță ME, Boiculese VL, Bîrluțiu V. Shedding Light on the COVID-19 Pandemic: Placental Expression of Cell Biomarkers in Negative, Vaccinated, and Positive Pregnant Women. Journal of Clinical Medicine. 2024; 13(18):5546. https://doi.org/10.3390/jcm13185546
Chicago/Turabian StyleCondac, Constantin, Ludmila Lozneanu, Daniela Roxana Matasariu, Alexandra Ursache, Iuliana Elena Bujor, Maria Elena Niță, Vasile Lucian Boiculese, and Victoria Bîrluțiu. 2024. "Shedding Light on the COVID-19 Pandemic: Placental Expression of Cell Biomarkers in Negative, Vaccinated, and Positive Pregnant Women" Journal of Clinical Medicine 13, no. 18: 5546. https://doi.org/10.3390/jcm13185546
APA StyleCondac, C., Lozneanu, L., Matasariu, D. R., Ursache, A., Bujor, I. E., Niță, M. E., Boiculese, V. L., & Bîrluțiu, V. (2024). Shedding Light on the COVID-19 Pandemic: Placental Expression of Cell Biomarkers in Negative, Vaccinated, and Positive Pregnant Women. Journal of Clinical Medicine, 13(18), 5546. https://doi.org/10.3390/jcm13185546







