Endocrine Characteristics and Obstetric Outcomes of PCOS Patients with Successful IVF and Non-IVF Pregnancies
Abstract
:1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Azziz, R.; Carmina, E.; Chen, Z.; Dunaif, A.; Laven, J.S.E.; Legro, R.S.; Lizneva, D.; Natterson-Horowtiz, B.; Teede, H.J.; Yildiz, B.O. Polycystic ovary syndrome. Nat. Reviews. Dis. Primers 2016, 2, 16057. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Chen, C. Hormonal changes in PCOS. J. Endocrinol. 2024, 261, e230342. [Google Scholar] [CrossRef] [PubMed]
- Zehravi, M.; Maqbool, M.; Ara, I. Polycystic ovary syndrome and infertility: An update. Int. J. Adolesc. Med. Health 2021, 34, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Osibogun, O.; Ogunmoroti, O.; Michos, E.D. Polycystic ovary syndrome and cardiometabolic risk: Opportunities for cardiovascular disease prevention. Trends Cardiovasc. Med. 2020, 30, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Potdar, N.; Iyasere, C. Early pregnancy complications including recurrent pregnancy loss and obesity. Best Pract. Res. Clin. Obstet. Gynaecol. 2023, 90, 102372. [Google Scholar] [CrossRef] [PubMed]
- Balen, A.H.; Tan, S.L.; MacDougall, J.; Jacobs, H.S. Miscarriage rates following in-vitro fertilization are increased in women with polycystic ovaries and reduced by pituitary desensitization with Buserelin. Hum. Reprod. 1993, 8, 959–964. [Google Scholar] [CrossRef] [PubMed]
- Glueck, C.J.; Wang, P.; Goldenberg, N.; Sieve-Smith, L. Pregnancy outcomes among women with polycystic ovary syndrome treated with metformin. Hum. Reprod. 2002, 17, 2858–2864. [Google Scholar] [CrossRef] [PubMed]
- Homburg, R. Pregnancy complications in PCOS. Best Pract. Res. Clin. Endocrinol. Metab. 2006, 20, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, A.A.; Rajeswari, V.D. Polycystic ovary syndrome (PCOS) increases the risk of subsequent gestational diabetes mellitus (GDM): A novel therapeutic perspective. Life Sci. 2022, 310, 121069. [Google Scholar] [CrossRef] [PubMed]
- Palomba, S.; de Wilde, M.A.; Falbo, A.; Koster, M.P.H.; La Sala, G.B.; Fauser, B.C.J.M. Pregnancy complications in women with polycystic ovary syndrome. Hum. Reprod. Update 2015, 21, 575–592. [Google Scholar] [CrossRef] [PubMed]
- Joham, A.E.; Ranasinha, S.; Zoungas, S.; Moran, L.; Teede, H.J. Gestational diabetes and type 2 diabetes in reproductive-aged women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2014, 99, E447–E452. [Google Scholar] [CrossRef] [PubMed]
- Teede, H.; Deeks, A.; Moran, L. Polycystic ovary syndrome: A complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC Med. 2010, 8, 41. [Google Scholar] [CrossRef] [PubMed]
- Balen, A.H.; Morley, L.C.; Misso, M.; Franks, S.; Legro, R.S.; Wijeyaratne, C.N.; Stener-Victorin, E.; Fauser, B.C.J.M.; Norman, R.J.; Teede, H. The management of anovulatory infertility in women with polycystic ovary syndrome: An analysis of the evidence to support the development of global WHO guidance. Hum. Reprod. Update 2016, 22, 687–708. [Google Scholar] [CrossRef] [PubMed]
- Artini, P.G.; Obino, M.E.R.; Sergiampietri, C.; Pinelli, S.; Papini, F.; Casarosa, E.; Cela, V. PCOS and pregnancy: A review of available therapies to improve the outcome of pregnancy in women with polycystic ovary syndrome. Expert Rev. Endocrinol. Metab. 2018, 13, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Morley, L.C.; Tang, T.; Yasmin, E.; Norman, R.J.; Balen, A.H. Insulin-sensitising drugs (metformin, rosiglitazone, pioglitazone, D-chiro-inositol) for women with polycystic ovary syndrome, oligo amenorrhoea and subfertility. Cochrane Database Syst. Rev. 2017, 11, CD003053. [Google Scholar] [CrossRef] [PubMed]
- Legro, R.S.; Arslanian, S.A.; Ehrmann, D.A.; Hoeger, K.M.; Murad, M.H.; Pasquali, R.; Welt, C.K. Diagnosis and treatment of polycystic ovary syndrome: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2013, 98, 4565–4592, Erratum in J. Clin. Endocrinol. Metab. 2021, 106, e2462. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Morgante, G.; Massaro, M.G.; Scolaro, V.; Cappelli, V.; Luddi, A.; Troìa, L.; De Leo, V. Metformin doses and body mass index: Clinical outcomes in insulin resistant polycystic ovary syndrome women. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 8136–8142. [Google Scholar] [CrossRef] [PubMed]
- Kluge, L.; Källén, K.; Thurin-Kjellberg, A.; Wennerholm, U.B.; Bergh, C. The association between body mass index and live birth and maternal and perinatal outcomes after in-vitro fertilization: A national cohort study. Front. Endocrinol. 2023, 14, 1239702. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Balen, A.H.; Dresner, M.; Scott, E.M.; Drife, J.O. Should obese women with polycystic ovary syndrome receive treatment for infertility? BMJ 2006, 332, 434–435. [Google Scholar] [CrossRef] [PubMed]
- Teede, H.J.; Tay, C.T.; Laven, J.J.E.; Dokras, A.; Moran, L.J.; Piltonen, T.T.; Costello, M.F.; Boivin, J.; Redman, L.M.; Boyle, J.A.; et al. Recommendations from the 2023 international evidence-based guideline for the assessment and management of polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2023, 108, 2447–2469. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huang, S.; Du, X.; Wang, R.; Li, R.; Wang, H.; Luo, L.; O’Leary, S.; Qiao, J.; Mol, B.W.J. Ovulation induction and intrauterine insemination in infertile women with polycystic ovary syndrome: A comparison of drugs. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 231, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Ghosh, S.; Singh, S.; Chakravarty, A.; Ganesh, A.; Rajani, S.; Chakravarty, B.N. Congenital malformations among babies born following letrozole or clomiphene for infertility treatment. PLoS ONE 2014, 9, e108219. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Weiss, N.S.; Kostova, E.; Nahuis, M.; Mol, B.W.J.; van der Veen, F.; van Wely, M. Gonadotrophins for ovulation induction in women with polycystic ovary syndrome. Cochrane Database Syst. Rev. 2019, 1, CD010290. [Google Scholar] [CrossRef] [PubMed]
- Bourdon, M.; Maignien, C.; Pocate-Cheriet, K.; Plu Bureau, G.; Marcellin, L.; Patrat, C.; Chapron, C.; Santulli, P. The freeze-all strategy after IVF: Which indications? Reprod. Biomed. Online 2021, 42, 529–545. [Google Scholar] [CrossRef] [PubMed]
- Salem, W. Assisted reproductive technology: Pergnancy and maternal outcomes. In UptoDate; Connor, R.F., Ed.; Wolters Kluwer: Groningen, The Netherland, 2024; Available online: https://www.uptodate.com/contents/assisted-reproductive-technology-pregnancy-and-maternal-outcomes?search=25.%09Salem%2C%20W.%20Assisted%20reproductive%20technology%3A%20Pergnancy%20and%20maternal%20outcomes&source=search_result&selectedTitle=1%7E150&usage_type=default&display_rank=1 (accessed on 7 September 2024).
- Berceanu, C.; Mehedinţu, C.; Berceanu, S.; Voicu, N.L.; Brătilă, E.; Istrate-Ofiţeru, A.M.; Navolan, D.B.; Niculescu, M.; Szasz, F.A.; Căpitănescu, R.G.; et al. Morphological and ultrasound findings in multiply pregnancy placentation. Rom. J. Morphol. Embryol. 2018, 59, 435–453. [Google Scholar] [PubMed]
- Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum. Reprod. 2004, 19, 41–47. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen, 5th ed.; World Health Organization: Geneva, Switzerland, 2010; Available online: https://iris.who.int/handle/10665/44261 (accessed on 16 August 2024).
- Rainey, W.E.; Carr, B.R.; Sasano, H.; Suzuki, T.; Mason, J.I. Dissecting human adrenal androgen production. Trends Endocrinol. Metab. 2002, 13, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.H.; Rizvi, S.A.; Shahid, R.; Manzoor, R. Dehydroepiandrosterone sulfate (DHEAS) levels in polycystic ovarian syndrome (PCOS). J. Coll. Physicians Surg. Pak. 2021, 31, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.L. The molecular basis of premature adrenarche: An hypothesis. Acta Paediatr. 1999, 88, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.C.; Fan, L.; Qin, A.P. The effect of dehydroepiandrosterone (DHEA) supplementation on women with diminished ovarian reserve (DOR) in IVF cycle: Evidence from a meta-analysis. J. Gynecol. Obstet. Hum. Reprod. 2017, 46, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Montoya-Botero, P.; Rodriguez-Purata, J.; Polyzos, N.P. Androgen supplementation in assisted reproduction: Where are we in 2019? Curr. Opin. Obstet. Gynecol. 2019, 31, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Hossein Rashidi, B.; Hormoz, B.; Shahrokh Tehraninejad, E.; Shariat, M.; Mahdavi, A. Testosterone and dehydroepiandrosterone sulphate levels and IVF/ICSI results. Gynecol. Endocrinol. 2009, 25, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Peeva, M.; Badeghiesh, A.; Baghlaf, H.; Dahan, M.H. Adverse obstetric outcomes in women with PCOS and multiple gestations. Reprod. Biomed. Online 2023, 46, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Sha, T.; Wang, X.; Cheng, W.; Yan, Y. A meta-analysis of pregnancy-related outcomes and complications in women with polycystic ovary syndrome undergoing IVF. Reprod. Biomed. Online 2019, 39, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Kjerulff, L.E.; Sanchez-Ramos, L.; Duffy, D. Pregnancy outcomes in women with polycystic ovary syndrome: A metaanalysis. Am. J. Obstet. Gynecol. 2011, 204, 558.e1–558.e6. [Google Scholar] [CrossRef] [PubMed]
- Boomsma, C.M.; Eijkemans, M.J.; Hughes, E.G.; Visser, G.H.; Fauser, B.C.; Macklon, N.S. A meta-analysis of pregnancy outcomes in women with polycystic ovary syndrome. Hum. Reprod. Update 2006, 12, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Guo, H.; Wang, B.; Chen, Q.; Zhu, Q. Neonatal outcomes in women with polycystic ovary syndrome after frozen–thawed embryo transfer. Fertil. Steril. 2021, 115, 447–454. [Google Scholar] [CrossRef] [PubMed]
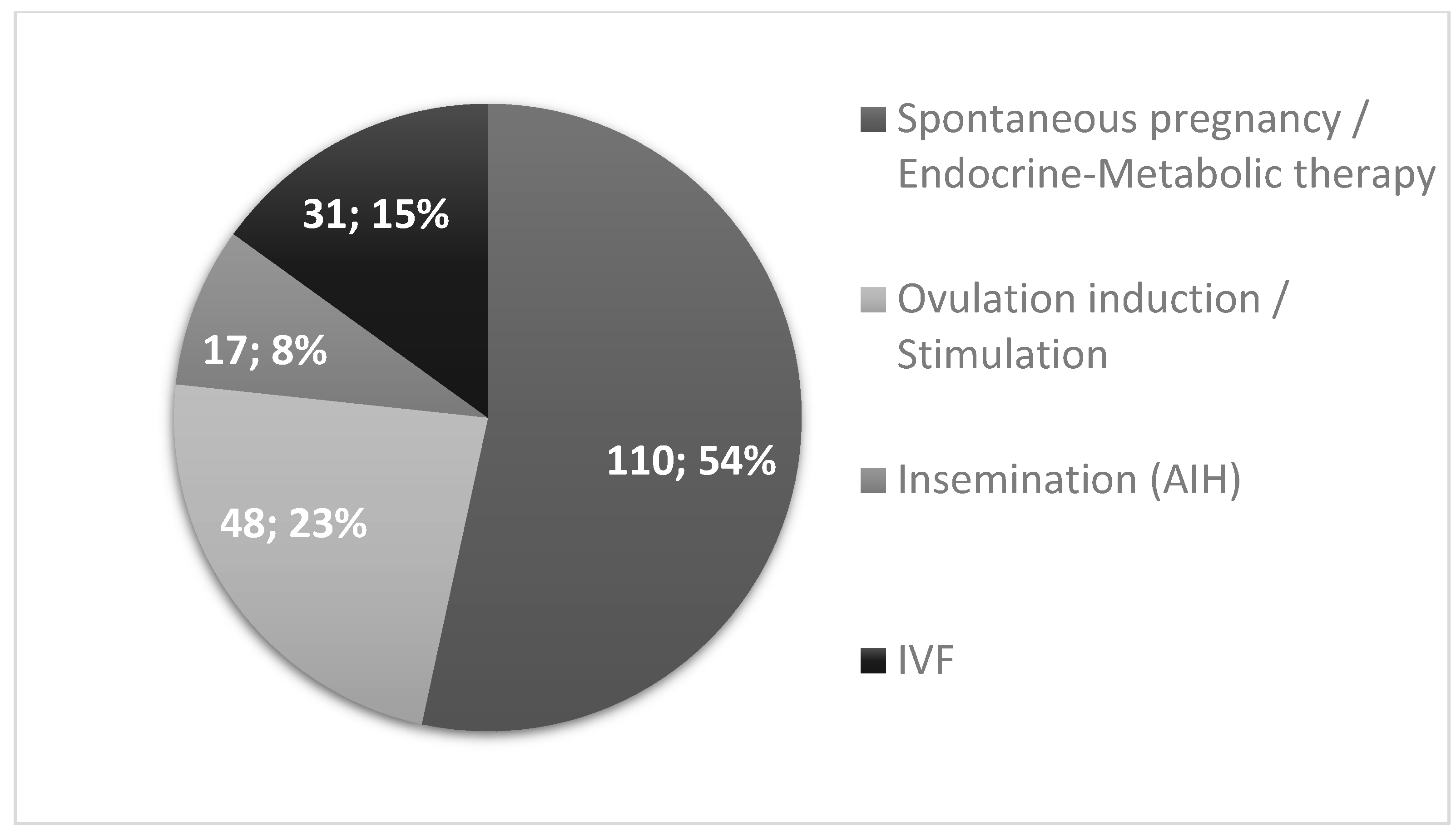

| IVF | Non-IVF | ||||
|---|---|---|---|---|---|
| Mean ± S.D. | (n) | Mean ± S.D. | (n) | p | |
| Maternal age (y) | 33.58 ± 4.36 | (31) | 29.83 ± 4.38 | (174) | <0.001 |
| Gestational age (wk) | 37.23 ± 2.55 | (31) | 38.54 ± 2.28 | (169) | 0.004 |
| Birth weight (g) | 3069 ± 683 | (31) | 3362 ± 638 | (168) | 0.02 |
| Birth weight % | 51.37 ± 33.67 | (31) | 54.85 ± 29.82 | (168) | 0.56 |
| TSH (mU/L) | 1.91 ± 0.96 | (21) | 2.14 ± 1.28 | (88) | 0.44 |
| E2 (CD3) (ng/L) | 49.63 ± 37.55 | (21) | 53.86 ± 43.17 | (75) | 0.68 |
| FSH (CD3) (IU/L) | 6.43 ± 2.35 | (21) | 6.11 ± 2.16 | (73) | 0.56 |
| LH (CD3) (IU/L) | 7.74 ± 6.79 | (21) | 9.82 ± 8.23 | (73) | 0.29 |
| LH/FSH (CD3) | 1.24 ± 1.19 | (21) | 1.57 ± 0.86 | (72) | 0.16 |
| PROG (CD21) (μg/L) | 9.83 ± 10.00 | (15) | 5.63 ± 7.39 | (60) | 0.07 |
| PRL (μg/L) | 19.82 ± 15.53 | (23) | 16.30 ± 9.60 | (99) | 0.17 |
| TEST (nmol/L) | 1.44 ± 1.31 | (12) | 1.61 ± 0.76 | (56) | 0.55 |
| SHBG (nmol/L) | 72.01 ± 54.40 | (12) | 54.29 ± 37.60 | (51) | 0.19 |
| AD (μg/L) | 2.20 ± 1.11 | (13) | 3.16 ± 1.66 | (58) | 0.05 |
| DHEAS (μmol/L) | 6.11 ± 3.13 | (13) | 8.09 ± 3.37 | (58) | 0.05 |
| BMI (kg/m2) | 29.52 ± 4.37 | (9) | 28.04 ± 6.26 | (50) | 0.50 |
| 25-OH-Vitamin-D (nmol/L) | 61.00 ± 6.88 | (5) | 64.04 ± 25.54 | (24) | 0.80 |
| IVF | Non-IVF | ||||
|---|---|---|---|---|---|
| Mean ± S.D. | (n) | Mean ± S.D. | (n) | p | |
| Maternal age (y) | 33.57 ± 4.26 | (23) | 29.94 ± 4.26 | (163) | <0.001 |
| Gestational age (wk) | 38.09 ± 1.47 | (23) | 38.62 ± 2.26 | (158) | 0.08 |
| Birth weight (g) | 3289.13 ± 496.08 | (23) | 3376.62 ± 628.71 | (157) | 0.52 |
| Birth weight % | 54.09 ± 32.63 | (23) | 55.11 ± 29.83 | (154) | 0.88 |
| IVF | Non-IVF | ||||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | OR for IVF | 95% C.I. | p | |
| Metformin | |||||||
| Yes | 7 | 24.1 | 35 | 26.9 | 0.86 | 0.34–2.20 | 0.76 |
| No | 22 | 75.9 | 95 | 73.1 | |||
| Thyroxine substitution | |||||||
| Yes | 7 | 24.1 | 22 | 17.2 | 1.53 | 0.58–4.03 | 0.39 |
| No | 22 | 75.9 | 106 | 82.8 | |||
| Hyperandrogenism | |||||||
| Yes | 6 | 20.7 | 42 | 33.1 | 0.53 | 0.20–1.40 | 0.20 |
| No | 23 | 79.3 | 85 | 66.9 | |||
| Irregular cycle | |||||||
| Yes | 12 | 41.4 | 75 | 58.6 | 0.50 | 0.22–1.13 | 0.10 |
| No | 17 | 58.6 | 53 | 41.4 | |||
| Andrologic abnormality | |||||||
| Yes | 6 | 66.7 | 7 | 36.8 | 3.43 | 0.65–18.22 | 0.15 |
| No | 3 | 33.3 | 12 | 63.2 | |||
| Miscarriage in history | |||||||
| Yes | 7 | 30.4 | 29 | 23.8 | 1.40 | 0.53–3.74 | 0.50 |
| No | 16 | 69.6 | 93 | 76.2 | |||
| Insulin resistance | |||||||
| Yes | 13 | 43.3 | 52 | 30.6 | 1.74 | 0.79–3.83 | 0.17 |
| No | 17 | 56.7 | 118 | 69.4 | |||
| Age > 31 y | |||||||
| Yes | 21 | 67.7 | 61 | 35.1 | 3.89 | 1.72–8.79 | 0.001 |
| No | 10 | 32.3 | 113 | 34.9 | |||
| LH/FSH < 1.3 | |||||||
| Yes | 16 | 76.2 | 34 | 47.2 | 3.58 | 1.18–10.81 | 0.03 |
| No | 5 | 33.8 | 38 | 52.8 | |||
| AD < 2.5 (μg/L) | |||||||
| Yes | 7 | 53.8 | 24 | 41.4 | 1.65 | 0.49–5.54 | 0.42 |
| No | 6 | 46.2 | 34 | 58.6 | |||
| DHEAS < 6.5 (μmol/L) | |||||||
| Yes | 8 | 61.5 | 17 | 29.3 | 3.86 | 1.10–13.50 | 0.04 |
| No | 5 | 38.5 | 41 | 70.7 | |||
| OR for IVF per 1 Unit Change | 95% C.I. | p | |
|---|---|---|---|
| Age (y) | 1.222 | 1.11–1.35 | <0.001 |
| DHEAS (μmol/L) | 0.82 | 0.66–1.01 | 0.06 |
| AD (μg/L) | 0.58 | 0.33–1.02 | 0.056 |
| Test. (nmol/L) | 0.78 | 0.35–1.74 | 0.55 |
| LH/FSH | 0.62 | 0.31–1.22 | 0.16 |
| IVF | Non-IVF | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | (%) | n | (%) | OR | 95% C.I. | p | aOR | 95% C.I. | p | |
| Sex of newborn | ||||||||||
| Female | 16 | (51.6) | 78 | (46.2) | 1.24 | 0.58–2.68 | 0.58 | 1.63 | 0.72–3.68 | 0.24 |
| Male | 15 | (48.4) | 91 | (53.8) | ||||||
| Preterm delivery | ||||||||||
| Yes | 9 | (29.0) | 14 | (8.3) | 4.53 | 1.75–11.70 | 0.002 | 5.06 | 1.81–14.18 | 0.002 |
| No | 22 | (71.0) | 155 | (91.7) | ||||||
| Cesarean delivery | ||||||||||
| Yes | 22 | (71.0) | 73 | (43.2) | 3.22 | 1.40–7.40 | 0.006 | 2.82 | 1.19–6.71 | 0.019 |
| No | 9 | (29.0) | 96 | (56.8) | ||||||
| Gestational diabetes | ||||||||||
| Yes | 14 | (48.3) | 60 | (46.9) | 1.06 | 0.47–2.37 | 0.89 | 0.93 | 0.38–2.24 | 0.87 |
| No | 15 | (51.7) | 68 | (53.1) | ||||||
| Preeclampsia | ||||||||||
| Yes | 3 | (10.0) | 17 | (8.6) | 1.18 | 0.32–4.39 | 0.80 | 1.22 | 0.31–4.85 | 0.77 |
| No | 27 | (90.0) | 149 | (91.4) | ||||||
| IVF | Non-IVF | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | (%) | n | (%) | OR | 95% C.I. | p | aOR | 95% C.I. | p | |
| Sex of newborn | ||||||||||
| Female | 13 | (56.5) | 74 | (46.8) | 1.48 | 0.61–3.56 | 0.39 | 1.96 | 0.77–4.99 | 1.16 |
| Male | 10 | (43.5) | 84 | (53.2) | ||||||
| Preterm delivery | ||||||||||
| Yes | 4 | (17.4) | 10 | (6.3) | 3.12 | 0.89–10.92 | 0.07 | 3.38 | 0.88–12.88 | 0.075 |
| No | 19 | (82.6) | 148 | (93.7) | ||||||
| Cesarean delivery | ||||||||||
| Yes | 15 | (65.2) | 67 | (42.4) | 2.55 | 1.02–6.35 | 0.04 | 2.25 | 0.87–5.81 | 0.09 |
| No | 8 | (34.8) | 91 | (57.6) | ||||||
| Gestational diabetes | ||||||||||
| Yes | 11 | (52.4) | 56 | (45.9) | 1.30 | 0.51–3.28 | 0.58 | 1.13 | 0.41–3.07 | 0.81 |
| No | 10 | (47.6) | 66 | (54.1) | ||||||
| Preeclampsia | ||||||||||
| Yes | 3 | (13.6) | 13 | (8.1) | 2.38 | 0.70–8.05 | 0.16 | 2.38 | 0.65–8.66 | 0.18 |
| No | 19 | (86.4) | 147 | (91.9) | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orosz, M.; Borics, F.; Rátonyi, D.; Tibor Krasznai, Z.; Vida, B.; Herman, T.; Csehely, S.; Jakab, A.; Lukács, L.; Lampé, R.; et al. Endocrine Characteristics and Obstetric Outcomes of PCOS Patients with Successful IVF and Non-IVF Pregnancies. J. Clin. Med. 2024, 13, 5602. https://doi.org/10.3390/jcm13185602
Orosz M, Borics F, Rátonyi D, Tibor Krasznai Z, Vida B, Herman T, Csehely S, Jakab A, Lukács L, Lampé R, et al. Endocrine Characteristics and Obstetric Outcomes of PCOS Patients with Successful IVF and Non-IVF Pregnancies. Journal of Clinical Medicine. 2024; 13(18):5602. https://doi.org/10.3390/jcm13185602
Chicago/Turabian StyleOrosz, Mónika, Fanni Borics, Dávid Rátonyi, Zoárd Tibor Krasznai, Beáta Vida, Tünde Herman, Szilvia Csehely, Attila Jakab, Luca Lukács, Rudolf Lampé, and et al. 2024. "Endocrine Characteristics and Obstetric Outcomes of PCOS Patients with Successful IVF and Non-IVF Pregnancies" Journal of Clinical Medicine 13, no. 18: 5602. https://doi.org/10.3390/jcm13185602
APA StyleOrosz, M., Borics, F., Rátonyi, D., Tibor Krasznai, Z., Vida, B., Herman, T., Csehely, S., Jakab, A., Lukács, L., Lampé, R., & Deli, T. (2024). Endocrine Characteristics and Obstetric Outcomes of PCOS Patients with Successful IVF and Non-IVF Pregnancies. Journal of Clinical Medicine, 13(18), 5602. https://doi.org/10.3390/jcm13185602








