A Systematic Review of Ureteral Reimplantation Techniques in Endometriosis: Laparoscopic Versus Robotic-Assisted Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Studies Eligibility
2.3. Study Selection and Data Extraction
2.4. Objectives
2.5. Statistical Analysis
3. Results
4. Discussion
4.1. Outcome Analysis of Laparoscopic and Robotic-Assisted Surgery
4.1.1. Hospital Stay and Duration of Surgery
4.1.2. Recurrence of Endometriosis-Related Ureteral Obstruction
4.1.3. Complication Comparison between Laparoscopic and Robotic-Assisted Surgery
4.2. Surgical Techniques for Ureteral Reimplantation
5. Conclusions and Limitations
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Vercellini, P.; Consonni, D.; Barbara, G.; Buggio, L.; Frattaruolo, M.P.; Somigliana, E. Adenomyosis and Reproductive Performance after Surgery for Rectovaginal and Colorectal Endometriosis: A Systematic Review and Meta-Analysis. Reprod. Biomed. Online 2014, 28, 704–713. [Google Scholar] [CrossRef] [PubMed]
- Massimello, F.; Sardo, A.D.S.; Bifulco, G.; Angioni, S.; Cela, V. New Technologies in the Surgical Management of Endometriosis. AboutOpen 2023, 10, 50–54. [Google Scholar] [CrossRef]
- Di Michele, S.; Bramante, S.; Angioni, S.; Bernassola, M.; De Vita, T.; Iaccarino, D.A.; Giannoni, L.; Rosati, M. Superficial Peritoneal Endometriosis Vaporization Using a CO2 Laser: A Long-Term Single-Center Experience. J. Clin. Med. 2024, 13, 1722. [Google Scholar] [CrossRef] [PubMed]
- Abrao, M.S.; Dias, J.A.; Bellelis, P.; Podgaec, S.; Bautzer, C.R.; Gromatsky, C. Endometriosis of the Ureter and Bladder Are Not Associated Diseases. Fertil. Steril. 2009, 91, 1662–1667. [Google Scholar] [CrossRef]
- Maccagnano, C.; Pellucchi, F.; Rocchini, L.; Ghezzi, M.; Scattoni, V.; Montorsi, F.; Rigatti, P.; Colombo, R. Ureteral Endometriosis: Proposal for a Diagnostic and Therapeutic Algorithm with a Review of the Literature. Urol. Int. 2013, 91, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Vercellini, P.; Pisacreta, A.; Pesole, A.; Vicentini, S.; Stellato, G.; Crosignani, P.G. Is Ureteral Endometriosis an Asymmetric Disease? BJOG Int. J. Obstet. Gynaecol. 2000, 107, 559–561. [Google Scholar] [CrossRef]
- Seracchioli, R.; Raimondo, D.; Di Donato, N.; Leonardi, D.; Spagnolo, E.; Paradisi, R.; Montanari, G.; Caprara, G.; Zannoni, L. Histological Evaluation of Ureteral Involvement in Women with Deep Infiltrating Endometriosis: Analysis of a Large Series. Hum. Reprod. Oxf. Engl. 2015, 30, 833–839. [Google Scholar] [CrossRef]
- Daniilidis, A.; Angioni, S.; Di Michele, S.; Dinas, K.; Gkrozou, F.; D’Alterio, M.N. Deep Endometriosis and Infertility: What Is the Impact of Surgery? J. Clin. Med. 2022, 11, 6727. [Google Scholar] [CrossRef]
- Nezhat, C.; Paka, C.; Gomaa, M.; Schipper, E. Silent Loss of Kidney Seconary to Ureteral Endometriosis. J. Soc. Laparoendosc. Surg. 2012, 16, 451–455. [Google Scholar] [CrossRef]
- Yohannes, P. Ureteral Endometriosis. J. Urol. 2003, 170, 20–25. [Google Scholar] [CrossRef]
- Seracchioli, R.; Mabrouk, M.; Montanari, G.; Manuzzi, L.; Concetti, S.; Venturoli, S. Conservative Laparoscopic Management of Urinary Tract Endometriosis (UTE): Surgical Outcome and Long-Term Follow-Up. Fertil. Steril. 2010, 94, 856–861. [Google Scholar] [CrossRef] [PubMed]
- Horn, L.-C.; Do Minh, M.; Stolzenburg, J.-U. Intrinsic Form of Ureteral Endometriosis Causing Ureteral Obstruction and Partial Loss of Kidney Function. Urol. Int. 2004, 73, 181–184. [Google Scholar] [CrossRef]
- Leonardi, M.; Espada, M.; Kho, R.M.; Magrina, J.F.; Millischer, A.-E.; Savelli, L.; Condous, G. Endometriosis and the Urinary Tract: From Diagnosis to Surgical Treatment. Diagnostics 2020, 10, 771. [Google Scholar] [CrossRef] [PubMed]
- Rivlin, M.E.; Krueger, R.P.; Wiser, W.L. Danazol in the Management of Ureteral Obstruction Secondary to Endometriosis. Fertil. Steril. 1985, 44, 274–276. [Google Scholar] [CrossRef] [PubMed]
- Maccagnano, C.; Pellucchi, F.; Rocchini, L.; Ghezzi, M.; Scattoni, V.; Montorsi, F.; Rigatti, P.; Colombo, R. Diagnosis and Treatment of Bladder Endometriosis: State of the Art. Urol. Int. 2012, 89, 249–258. [Google Scholar] [CrossRef]
- Nezhat, C.; Falik, R.; McKinney, S.; King, L.P. Pathophysiology and Management of Urinary Tract Endometriosis. Nat. Rev. Urol. 2017, 14, 359–372. [Google Scholar] [CrossRef]
- Berlanda, N.; Vercellini, P.; Carmignani, L.; Aimi, G.; Amicarelli, F.; Fedele, L. Ureteral and Vesical Endometriosis. Two Different Clinical Entities Sharing the Same Pathogenesis. Obstet. Gynecol. Surv. 2009, 64, 830–842. [Google Scholar] [CrossRef]
- Cavaco-Gomes, J.; Martinho, M.; Gilabert-Aguilar, J.; Gilabert-Estélles, J. Laparoscopic Management of Ureteral Endometriosis: A Systematic Review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 210, 94–101. [Google Scholar] [CrossRef]
- Barra, F.; Scala, C.; Biscaldi, E.; Vellone, V.G.; Ceccaroni, M.; Terrone, C.; Ferrero, S. Ureteral Endometriosis: A Systematic Review of Epidemiology, Pathogenesis, Diagnosis, Treatment, Risk of Malignant Transformation and Fertility. Hum. Reprod. Update 2018, 24, 710–730. [Google Scholar] [CrossRef]
- Working group of ESGE, ESHRE, and WES; Keckstein, J.; Becker, C.M.; Canis, M.; Feki, A.; Grimbizis, G.F.; Hummelshoj, L.; Nisolle, M.; Roman, H.; Saridogan, E.; et al. Recommendations for the Surgical Treatment of Endometriosis. Part 2: Deep Endometriosis. Hum. Reprod. Open 2020, 2020, hoaa002. [Google Scholar] [CrossRef]
- Nezhat, C.; Nezhat, F.; Green, B. Laparoscopic Treatment of Obstructed Ureter Due to Endometriosis by Resection and Ureteroureterostomy: A Case Report. J. Urol. 1992, 148, 865–868. [Google Scholar] [CrossRef] [PubMed]
- Nezhat, C.H.; Malik, S.; Nezhat, F.; Nezhat, C. Laparoscopic Ureteroneocystostomy and Vesicopsoas Hitch for Infiltrative Endometriosis. J. Soc. Laparoendosc. Surg. 2004, 8, 3–7. [Google Scholar]
- Rassweiler, J.J.; Gözen, A.S.; Erdogru, T.; Sugiono, M.; Teber, D. Ureteral Reimplantation for Management of Ureteral Strictures: A Retrospective Comparison of Laparoscopic and Open Techniques. Eur. Urol. 2007, 51, 512–522; discussion 522–523. [Google Scholar] [CrossRef]
- Nezhat, C.; Modest, A.M.; King, L.P. The Role of the Robot in Treating Urinary Tract Endometriosis. Curr. Opin. Obstet. Gynecol. 2013, 25, 308–311. [Google Scholar] [CrossRef]
- Nezhat, C.R.; Stevens, A.; Balassiano, E.; Soliemannjad, R. Robotic-Assisted Laparoscopy vs. Conventional Laparoscopy for the Treatment of Advanced Stage Endometriosis. J. Minim. Invasive Gynecol. 2015, 22, 40–44. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Dindo, D.; Demartines, N.; Clavien, P.-A. Classification of Surgical Complications: A New Proposal with Evaluation in a Cohort of 6336 Patients and Results of a Survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Ceccaroni, M.; Ceccarello, M.; Caleffi, G.; Clarizia, R.; Scarperi, S.; Pastorello, M.; Molinari, A.; Ruffo, G.; Cavalleri, S. Total Laparoscopic Ureteroneocystostomy for Ureteral Endometriosis: A Single-Center Experience of 160 Consecutive Patients. J. Minim. Invasive Gynecol. 2019, 26, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Schonman, R.; Dotan, Z.; Weintraub, A.Y.; Goldenberg, M.; Seidman, D.S.; Schiff, E.; Soriano, D. Long-Term Follow-up after Ureteral Reimplantation in Patients with Severe Deep Infiltrating Endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 171, 146–149. [Google Scholar] [CrossRef]
- Bourdel, N.; Cognet, S.; Canis, M.; Berdugo, O.; Botchorishvili, R.; Rabischong, B.; Jardon, K. Laparoscopic Ureteroneocystostomy: Be Prepared! J. Minim. Invasive Gynecol. 2015, 22, 827–833. [Google Scholar] [CrossRef]
- Chudzinski, A.; Collinet, P.; Flamand, V.; Rubod, C. Ureterovesical Reimplantation for Ureteral Deep Infiltrating Endometriosis: A Retrospective Study. J. Gynecol. Obstet. Hum. Reprod. 2017, 46, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Alves, J.; Puga, M.; Fernandes, R.; Pinton, A.; Miranda, I.; Kovoor, E.; Wattiez, A. Laparoscopic Management of Ureteral Endometriosis and Hydronephrosis Associated With Endometriosis. J. Minim. Invasive Gynecol. 2017, 24, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Stepniewska, A.; Grosso, G.; Molon, A.; Caleffi, G.; Perin, E.; Scioscia, M.; Mainardi, P.; Minelli, L. Ureteral Endometriosis: Clinical and Radiological Follow-up after Laparoscopic Ureterocystoneostomy. Hum. Reprod. Oxf. Engl. 2011, 26, 112–116. [Google Scholar] [CrossRef]
- Ahn, J.H.; Han, J.-Y.; Nam, J.K.; Park, S.-W.; Lee, S.D.; Chung, M.K. Laparoscopic Ureteroneocystostomy: Modification of Current Techniques. Korean J. Urol. 2013, 54, 26–30. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Azioni, G.; Bracale, U.; Scala, A.; Capobianco, F.; Barone, M.; Rosati, M.; Pignata, G. Laparoscopic Ureteroneocystostomy and Vesicopsoas Hitch for Infiltrative Ureteral Endometriosis. Minim. Invasive Ther. Allied Technol. MITAT Off. J. Soc. Minim. Invasive Ther. 2010, 19, 292–297. [Google Scholar] [CrossRef]
- Mereu, L.; Gagliardi, M.L.; Clarizia, R.; Mainardi, P.; Landi, S.; Minelli, L. Laparoscopic Management of Ureteral Endometriosis in Case of Moderate-Severe Hydroureteronephrosis. Fertil. Steril. 2010, 93, 46–51. [Google Scholar] [CrossRef]
- Yang, C.; Jones, L.; Rivera, M.E.; Verlee, G.T.; Deane, L.A. Robotic-Assisted Ureteral Reimplantation with Boari Flap and Psoas Hitch: A Single-Institution Experience. J. Laparoendosc. Adv. Surg. Tech. A 2011, 21, 829–833. [Google Scholar] [CrossRef]
- Hung, Z.-C.; Hsu, T.-H.; Jiang, L.-Y.; Chao, W.-T.; Wang, P.-H.; Chen, W.-J.; Huang, E.Y.-H.; Chen, Y.-J.; Lin, A.T.L. Robot-Assisted Laparoscopic Ureteral Reconstruction for Ureter Endometriosis: Case Series and Literature Review. J. Chin. Med. Assoc. JCMA 2020, 83, 288–294. [Google Scholar] [CrossRef]
- Di Maida, F.; Mari, A.; Morselli, S.; Campi, R.; Sforza, S.; Cocci, A.; Tellini, R.; Tuccio, A.; Petraglia, F.; Masieri, L.; et al. Robotic Treatment for Urinary Tract Endometriosis: Preliminary Results and Surgical Details in a High-Volume Single-Institutional Cohort Study. Surg. Endosc. 2020, 34, 3236–3242. [Google Scholar] [CrossRef]
- Mangalath, A.S.; Kumar, L.; Sawant, A.B.; Kesavan, R.; Ravindran, G.; Sunil, R. Comparison of Analgesic Requirements in Robot-Assisted versus Conventional Laparoscopic Abdominal Surgeries. J. Anaesthesiol. Clin. Pharmacol. 2021, 37, 79–84. [Google Scholar] [CrossRef]
- Kawka, M.; Fong, Y.; Gall, T.M.H. Laparoscopic versus Robotic Abdominal and Pelvic Surgery: A Systematic Review of Randomised Controlled Trials. Surg. Endosc. 2023, 37, 6672–6681. [Google Scholar] [CrossRef] [PubMed]
- Ng, A.P.; Sanaiha, Y.; Bakhtiyar, S.S.; Ebrahimian, S.; Branche, C.; Benharash, P. National Analysis of Cost Disparities in Robotic-Assisted versus Laparoscopic Abdominal Operations. Surgery 2023, 173, 1340–1345. [Google Scholar] [CrossRef] [PubMed]
- Soulier, V.; Scalabre, A.L.; Lopez, M.; Li, C.-Y.; Thach, S.; Vermersch, S.; Varlet, F.O. Laparoscopic Vesico-Ureteral Reimplantation with Lich-Gregoir Approach in Children: Medium Term Results of 159 Renal Units in 117 Children. World J. Urol. 2017, 35, 1791–1798. [Google Scholar] [CrossRef] [PubMed]
- Varlet, F.; Scalabre, A.; Vermersch, S. Vesico-Ureteric Reflux (VUR): Laparoscopic Lich–Gregoir Repair. In Minimally Invasive Techniques in Pediatric Urology: Endourology, Laparoscopy and Robotics; Esposito, C., Subramaniam, R., Varlet, F., Masieri, L., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 325–332. ISBN 978-3-030-99280-4. [Google Scholar]
- Bansal, A.; Sinha, R.J.; Jhanwar, A.; Prakash, G.; Purkait, B.; Singh, V. Laparoscopic Ureteral Reimplantation with Boari Flap for the Management of Long- Segment Ureteral Defect: A Case Series with Review of the Literature. Turk. J. Urol. 2017, 43, 313–318. [Google Scholar] [CrossRef]
- White, C.; Stifelman, M. Ureteral Reimplantation, Psoas Hitch, and Boari Flap. J. Endourol. 2020, 34, S-25. [Google Scholar] [CrossRef]
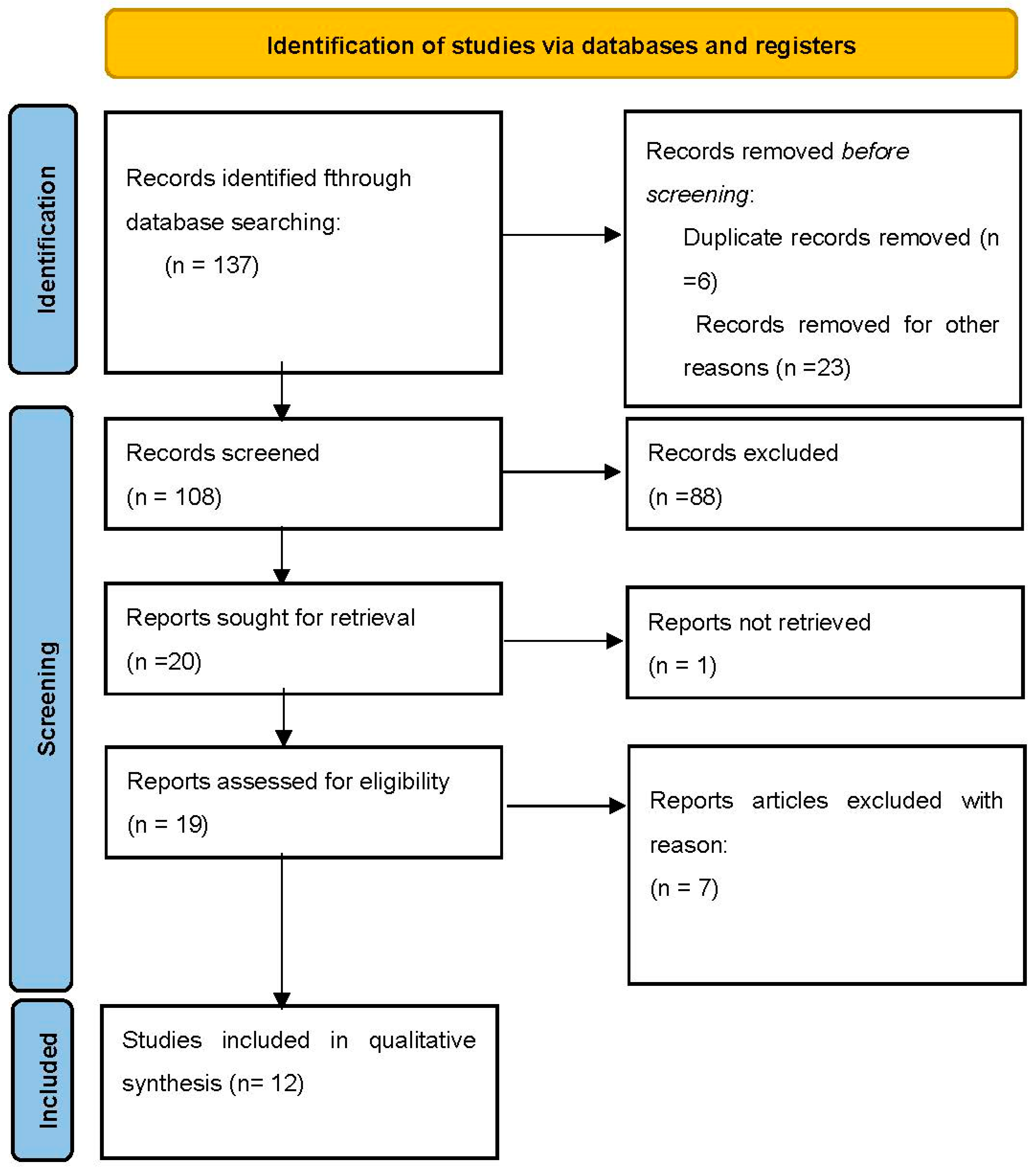
| Author and Year | Study Type | N. of Patients | Mean Age (Years, Mean) | Type of Endometriosis (Intrinsic/Extrinsic) | Surgical Technique | Duration of Surgery (Min-Mean)/and Ureteral Reimplantation (Mean) | Intraoperative Complications | Reintervention for Ureteral Reimplantation Complications | Postoperative Complications (Clavien–Dindo Grading System for Surgical Complications) | Follow-Up (Months) | Recurrence Rate (%) | Time of Hospitalization (Days, Mean) SD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ceccaroni et al., 2018 [28] | Prospective study | 160 | 36.1 | Intrinsic/ Extrinsic | Laparoscopic Ureteroneocystostomy 160/160 (100%) Lich–Gregoir or direct reimplantation with or without psoas hitch | 364.3/92.3 (120–600/30–180) | None | 3 bladder suture leakage (1 with associated pelvic abscess, 1 hemoperitoneum) | Grade I: 6 (3.7%) Grade II: 12 (7.5%) GradeIIIb: 7 (4.4%) | >6 months | 1.2% | 8 (7–18) |
| Schonman et al., 2013 [29] | Retrospective | 1 | 34.3 | Not specified | Not specified | Not specified | 2 laparotomy conversion after laparoscopic attempt | None required | None | 63 | 0% | Not specified |
| Bourdel et al., 2015 [30] | Retrospective | 3 | 32 | Extrinsic | Lich–Gregoir | 226.67 (120–480) | None | None required | Grade III: 1 (33.3%) | 22.5 | 0% | 12.6 (6–26) |
| Chudzinski et al., 2017 [31] | Retrospective | 3 | 28 | Not Specified | Psoas-hitch 70%, 3 vescical bipartition and 1 boari flap | 300 (174–426) | None | 1 | Grade IIIa: 1 (33.3%) | 48 | Not specified | 10.2 (4–16) |
| Alves et al., 2017 [32] | Retrospective | 13 | 32.1 | Intrinsic/ Extrinsic | 18 end-to-end anastomosis and 1 ureteral reimplantation (boari flap) | 157 (90–330) | Not specified | 1 (reimplantation after failed reanastomosis) | Grade II: 1 (7.6%) Grade IIIb: 2 (15.3%) | 2 months (longer follow-up was incomplete) | 4 (19%) but includes also 8 patients who underwent ureterolysis | Not specified |
| Stepniewska et al., 2010 [33] | Retrospective | 20 | 35 | Intrinsic/ Extrinsic | Lich–Gregoir or Boari flap when necessary | 313 (120–500) | Not specified | None required | Grade I: 4 (20%) Grade II: 10 (50%) Grade IIIa: 1 (5%) | 6 months | Not specified | 10 (7–17) |
| Ahn et al., 2013 [34] | Retrospective | 2 | 49.5 | Not specified | Lich–Gregoir with or without psoas hitch | 137 (104–228) | Not reported | None required | None | 12 | 0% | 7 (7–7) |
| Azioni et al., 2010 [35] | Retrospective | 6 | 33.6 | Intrinsic/ Extrinsic | Lich–Gregoir | 320 (250–440) | none | None required | None | none | 8.3 (7–10) | |
| Mereu et al., 2010 [36] | Retrospective | 17 | 32.7 | Not specified | 17 end-to-end ureteral anastomosis | 330 (60–540) | None | 2 persistent ureteral stenosis requiring further intervention of ureteroneocystostomy | Grade II: 4 (23.5%) | 21 | 12.5% | 8 (2–31) |
| Total | 8 retrospective 1 prospective studies | 225 patients | 34.8 | / | Lich–Gregoir ureteral reimplantation was the preferred technique with or without psoas-hitch | 271.1 | 2 laparotomy conversion | 7/225 (3.11%) | Grade I: 10 (4.4%) Grade II: 27 (12%) Grade IIIa/b: 12 (5.3%) | 22.56 | 2.95% | 9.1 |
| Author and Year | Study Type | N. of Patients | Mean Age (Years, Mean) | Type of Endometriosis (Intrinsic/Extrinsic) | Surgical Technique | Duration of Surgery (Min)/and Ureteral Reimplantation (Mean) | Intraoperative Complications | Reintervention for Ureteral Reimplantation Complications | Postoperative Complications | Follow-Up (Months, Mean) | Recurrence Rate (%) | Hospital Stay (Days, Mean) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chudzinski et al., 2017 [31] | Retrospective | 4 | 31 | Not Specified | Psoas-hitch 70%, 3 vescical bipartition and 1 boari flap | 321 (174–426) | None | 1 (25%) | Grade IIIb: 1 (25%) Grade II: 1 (25%) | 48 | 15% | 10.2 (4–16) |
| Yang et al., 2011 [37] | Retrospective | 1 | 28 | Not specified | Distal ureterectomy with psoas hitch | Not specified | None | None | none | 24 | none | 4 (4–6) |
| Hung et al., 2020 [38] | Retrospective | 4 | 36.2 | Not Specified | Terminoterminal ureteral anastomosis, ureteroneocystostomy | 299.8 (220–404) | None | None | None | 17 | none | 8.6 (7–11) |
| Di Maida et al., 2020 [39] | Retrospective | 15 | 34.7 | Not specified | 13 Lich–Gregoir ureteral reimplantation with psoas hitch, 2 end-to-end anastomosis | Not specified | Not specified for the subgroup | None | Not specified for the subgroup | 31.3 | 8.7% | 4 (4–6) |
| Total | 4 retrospective studies | 24 patients | 33.9 | / | 310.4 | None | 1/24 (4.1%) | Grade III: 1/9 (11.1%) Grade II: 1/9 (11.1%) | 30 | 5.9% | 6.7 |
| Study | Resolution of Symptoms | Restoration of Ureteral Function | No. of Patients with Ureteral Function Improvement |
|---|---|---|---|
| Ceccaroni et al., 2018 [28] | Improvement or complete resolution of pain and urinary symptoms in most patients. | Achieved effective drainage of the kidney without obstruction, confirmed through follow-up imaging studies. | 160/160 (100%); resolution of hydronephrosis observed on postoperative CT scans and ultrasounds. |
| Schonman et al., 2013 [29] | Significant improvement in pain and urinary symptoms. | Postoperative imaging confirmed effective ureteral function without obstruction. | 1/1 (100%); postoperative ultrasound confirmed no ureteral obstruction or hydronephrosis. |
| Bourdel et al., 2015 [30] | Improvement in pain and urinary symptoms for most patients. | Effective drainage of the kidney, confirmed via imaging studies. | 3/3 (100%); improvement in hydronephrosis based on postoperative ultrasound. |
| Chudzinski et al., 2017 [31] | A notable improvement in symptoms was observed. | Postoperative imaging indicated successful ureteral function | 3/4 (75%); resolution of hydronephrosis on postoperative CT scans and ultrasounds, creatinine levels were monitored in the early postoperative period. |
| Alves et al., 2017 [32] | Patients reported reduced pain and better urinary function. | Imaging studies confirmed effective kidney drainage without obstruction. | 13/13 (100%); resolution of hydronephrosis confirmed by CT and ultrasound. |
| Stepniewska et al., 2010 [33] | Significant reduction in symptoms for most patients. | Effective ureteral function confirmed by imaging | 19/20 (95%); postoperative CT and ultrasound indicated resolution of hydronephrosis. |
| Ahn et al., 2013 [34] | Improvement in pain and urinary symptoms. | Postoperative imaging showed effective kidney drainage. | 2/2 (100%) of patients, hydronephrosis resolved based on follow-up ultrasound and CT scans. |
| Azioni et al., 2010 [35] | Most patients experienced symptom relief. | Effective ureteral function as indicated by follow-up imaging. | 6/6 (100%); resolution of ureteral obstruction observed on follow-up ultrasound. |
| Mereu et al., 2010 [36] | Improvement in symptoms was observed. | Postoperative imaging confirmed effective ureteral function. | 15/17 (88%); follow-up ultrasound and CT scans showed improvement in hydronephrosis |
| Yang et al., 2011 [37] | Significant symptom reduction. | Imaging studies confirmed effective ureteral function. | 1/1 (100%); postoperative ultrasound and CT scans showed resolution of hydronephrosis. |
| Hung et al., 2020 [38] | Patients reported improvement in pain and urinary symptoms | Effective kidney drainage was confirmed through imaging | 4/4 (100%); hydronephrosis resolved as per postoperative CT and ultrasound findings. |
| Di Maida et al., 2020 [39] | Notable symptom relief was observed. | Postoperative imaging indicated successful ureteral function. | 13/15 (87%), follow-up CT and ultrasound showed restored ureteral function. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Michele, S.; Bramante, S.; Rosati, M. A Systematic Review of Ureteral Reimplantation Techniques in Endometriosis: Laparoscopic Versus Robotic-Assisted Approach. J. Clin. Med. 2024, 13, 5677. https://doi.org/10.3390/jcm13195677
Di Michele S, Bramante S, Rosati M. A Systematic Review of Ureteral Reimplantation Techniques in Endometriosis: Laparoscopic Versus Robotic-Assisted Approach. Journal of Clinical Medicine. 2024; 13(19):5677. https://doi.org/10.3390/jcm13195677
Chicago/Turabian StyleDi Michele, Stefano, Silvia Bramante, and Maurizio Rosati. 2024. "A Systematic Review of Ureteral Reimplantation Techniques in Endometriosis: Laparoscopic Versus Robotic-Assisted Approach" Journal of Clinical Medicine 13, no. 19: 5677. https://doi.org/10.3390/jcm13195677
APA StyleDi Michele, S., Bramante, S., & Rosati, M. (2024). A Systematic Review of Ureteral Reimplantation Techniques in Endometriosis: Laparoscopic Versus Robotic-Assisted Approach. Journal of Clinical Medicine, 13(19), 5677. https://doi.org/10.3390/jcm13195677