Global Fight against Malaria: Goals and Achievements 1900–2022
Abstract
:1. Present Situation, Extent of the Disease in the Year 2022
2. Malaria in the History of Mankind before 1900
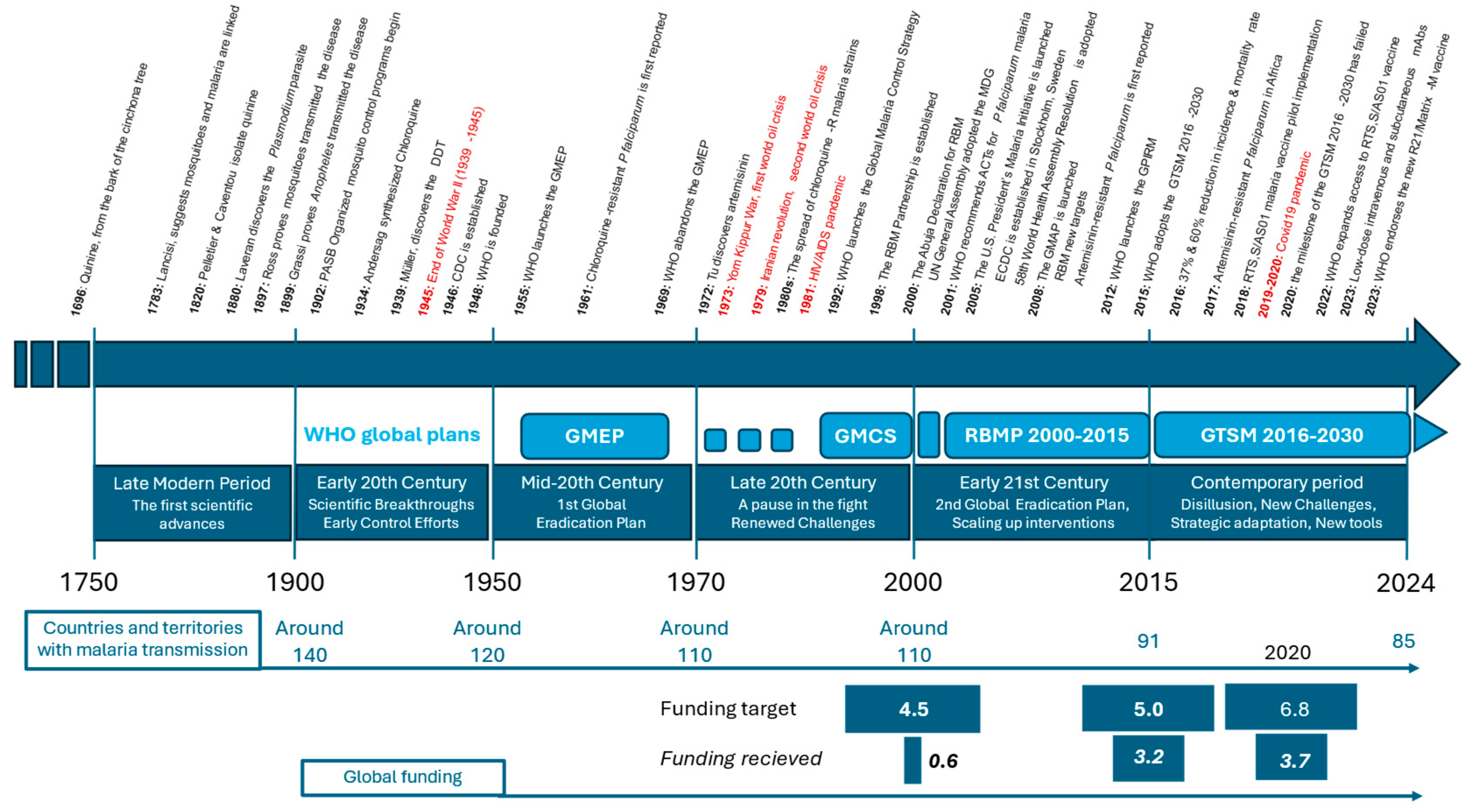
- ■
- 1696: Quinine, derived from the bark of the cinchona tree, is first used to treat malaria, becoming the primary treatment in Europe and the Americas.
- ■
- 1717: Giovanni Maria Lancisi, an Italian scientist, suggests a link between mosquitoes and malaria, theorizing that “poisonous vapors” from marshes (mal’aria) or bad air contribute to the disease. He recommended draining the marshes around Rome.
- ■
- 1740s: The use of cinchona bark for treating “ague” (malaria) becomes widespread in Europe.
- ■
- 1820: French chemists Pierre Joseph Pelletier and Joseph Bienaimé Caventou successfully isolate quinine from cinchona bark, making it the first alkaloid to be extracted for medicinal use. This breakthrough greatly enhances the effectiveness and availability of malaria treatment.
- ■
- 1880: Alphonse Laveran, a French Army doctor, discovers the Plasmodium parasite in the blood of a malaria patient in Algeria, identifying it as the cause of malaria. This work earns him the Nobel Prize in 1907.
- ■
- 1897: Sir Ronald Ross, a British Army doctor, proves that malaria is transmitted by mosquitoes, a discovery that earns him the Nobel Prize in 1902.
- ■
- 1899: Italian scientists Giuseppe Bastianelli, Amico Bignami, and Giovanni Battista Grassi proved that only mosquitoes of the genus Anopheles could transmit the disease.
- ■
- 1902: Organized mosquito control programs begin, notably in the Panama Canal Zone, drastically reducing malaria cases.
- ■
- 1934: Chloroquine is synthesized by the scientist Johann Andersag at Bayer in Germany. It becomes widely used after World War II due to its effectiveness and low cost.
- ■
- 1939: Paul Hermann Müller, a Swiss chemist, discovers the insecticidal properties of DDT, leading to widespread mosquito control efforts. Müller receives the Nobel Prize in 1948.
- ■
- 1946: The U.S. Centers for Disease Control and Prevention (CDC) is established in Atlanta, Georgia, primarily to combat malaria in the United States.
- ■
- 1948: The World Health Organization (WHO) is founded, providing a global platform for coordinating health initiatives, including malaria control.
- ■
- 1951: The U.S. National Malaria Eradication Program successfully eliminates malaria as a significant public health issue.
- ■
- 1955: WHO launches the Global Malaria Eradication Program (GMEP), aiming to eliminate malaria worldwide through extensive use of DDT and chloroquine distribution.
- ■
- 1961: Chloroquine-resistant P. falciparum is first reported in Southeast Asia and South America, complicating global malaria control efforts.
- ■
- 1969: WHO abandons the GMEP due to logistical challenges, increasing resistance to insecticides and drugs, and difficulties in sustaining the program in sub-Saharan Africa.
- ■
- 1972: Chinese scientist Tu Youyou discovers artemisinin, derived from the sweet wormwood plant (Artemisia annua), which proves highly effective against malaria. This discovery lays the foundation for modern artemisinin-based therapies, and Tu Youyou was awarded the Nobel Prize in 2015.
- ■
- 1973: Artesunate, a derivative of artemisinin, is developed in China and becomes one of the most effective treatments for severe malaria.
- ■
- 1980s: The spread of chloroquine-resistant P. falciparum strains worsens the global malaria burden, particularly in Africa and Southeast Asia.
- ■
- 1992: WHO introduces the Global Malaria Control Strategy, shifting focus from eradication to control through improved diagnosis and treatment.
- ■
- 1998: The Roll Back Malaria (RBM) Partnership is established by WHO, UNICEF, UNDP, and the World Bank, aiming to halve malaria deaths by 2010 through coordinated global efforts.
- ■
- 2000: The Abuja Declaration is signed by African leaders, committing to reducing malaria mortality by 50% by 2010. This period sees significant increases in funding and political commitment to malaria control.
- ■
- 2001: WHO recommends artemisinin-based combination therapies (ACTs) as the first-line treatment for P. falciparum malaria, replacing chloroquine.
- ■
- 2005: The U.S. President’s Malaria Initiative (PMI) is launched, boosting U.S. support for malaria control in Africa, including funding for insecticide-treated bed nets, indoor residual spraying, and ACTs.
- ■
- 2005: The European Centre for Disease Prevention and Control (ECDC) is established in Stockholm, Sweden.
- ■
- 2008: The Global Malaria Action Plan (GMAP) is launched by the RBM Partnership, with targets to reduce global malaria cases by 75% and eliminate malaria in 8–10 countries by 2015.
- ■
- 2015: WHO adopts the Global Technical Strategy for Malaria 2016-2030, aiming to reduce malaria incidence and mortality by at least 90% by 2030. The strategy emphasizes sustained investment, innovation, and expanding access to prevention and treatment.
- ■
- 2016: the MDG indicators, Indicator 6.6 shows a 37% reduction in the incidence rate and a 60% reduction in the mortality rate by 2015 compared with 2000, preventing an estimated 6.8 million deaths, primarily among children under five in sub-Saharan Africa.
- ■
- 2018: The RTS,S/AS01 (Mosquirix) P. falciparum malaria vaccine, the first of its kind, begins pilot implementation in Ghana, Kenya, and Malawi. The vaccine is expected to complement existing malaria control measures.
- ■
- 2022: WHO expands access to the RTS,S/AS01 P. falciparum malaria vaccine, with plans to roll out the vaccine in more endemic countries in Africa.
- ■
- 2022-2023: Trials have shown the effectiveness of low-dose subcutaneous or intravenous monoclonal antibodies in preventing P. falciparum malaria.
- ■
- 2023: WHO endorses the new R21/Matrix-M P. falciparum malaria vaccine, providing an additional tool in the fight against malaria, particularly in regions with high transmission rates.
3. The Fight against Malaria before WHO Coordination: 1900–1950
4. The Fight against Malaria under WHO Coordination: From 1951 to the Present Day
4.1. The First Coordinated Action between 1951 and 1969
4.2. The 1955 Turning Point and the Launch of the First Global Malaria Eradication Program
4.3. Challenges and the End of the GMEP
4.4. A Pause in Coordinated Global Efforts to Eradicate Malaria, 1970–1999
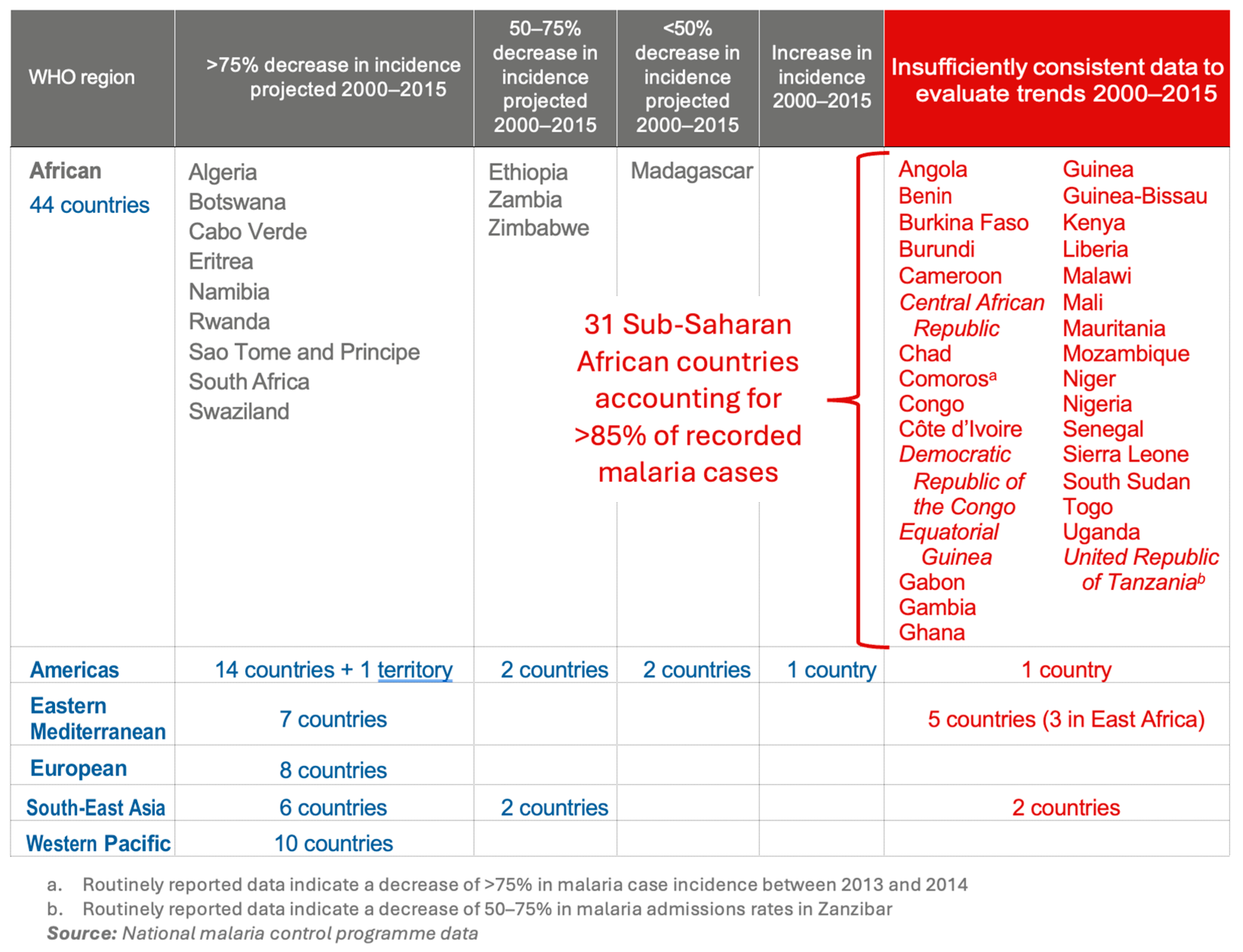
4.5. The Renewed Global Fight to Eradicate Malaria, from 1998 to Today
4.6. Roll Back Malaria: Definition of the Plan’s Objectives and Technical Implementation, 1998–2015
4.7. Global Technical Strategy for Malaria 2016–2030, Including Difficulties, New Challenges, and New Tools
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- White, N.J.; Pukrittayakamee, S.; Hien, T.T.; Faiz, M.A.; Mokuolu, O.A.; Dondorp, A.M. Malaria. Lancet 2014, 383, 723–735. [Google Scholar] [CrossRef] [PubMed]
- Snounou, G.; Sharp, P.M.; Culleton, R. The two parasite species formerly known as Plasmodium ovale. Trends Parasitol. 2024, 40, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, C.J.; Tanomsing, N.; Nolder, D.; Oguike, M.; Jennison, C.; Pukrittayakamee, S.; Dolecek, C.; Hien, T.T.; do Rosário, V.E.; Arez, A.P.; et al. Two nonrecombining sympatric forms of the human malaria parasite Plasmodium ovale occur globally. J. Infect. Dis. 2010, 201, 1544–1550. [Google Scholar] [CrossRef]
- Carter, R.; Mendis, K.N. Evolutionary and historical aspects of the burden of malaria. Clin. Microbiol. Rev. 2002, 15, 564–594. [Google Scholar] [CrossRef]
- Causes of Death. Our World in Data. Available online: https://ourworldindata.org/grapher/annual-number-of-deaths-by-cause (accessed on 18 August 2024).
- World Malaria Report 2023; World Health Organization: Geneva, Switzerland, 2023; p. 283. ISBN 978-92-4-008617-3. Available online: https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2023 (accessed on 22 June 2024).
- Lambert, M.-L.; van der Stuyft, P. Editorial: Global Health Fund or Global Fund to fight AIDS, Tuberculosis, and Malaria? Trop. Med. Int. Health 2002, 7, 557–558. [Google Scholar] [CrossRef] [PubMed]
- Global Technical Strategy for Malaria 2016–2030, 2021 Update. World Health Organization: Geneva, Switzerland, 2021. Available online: https://www.who.int/publications/i/item/9789240031357 (accessed on 18 August 2024).
- World Malaria Report 2021. World Health Organization: Geneva, Switzerland, 2021; p. 322. ISBN 978-92-4-004049-6. Available online: https://www.who.int/publications/i/item/9789240040496 (accessed on 10 June 2024).
- Shampo, M.A.; Kyle, R.A. Nei Ching—Oldest Known Medical Book. Mayo Clin. Proc. 1989, 64, 134. [Google Scholar] [CrossRef] [PubMed]
- Adams (Francis). The Genuine Works of Hippocrates. Of the Epidemics 1.6,7,24–26; Aphorisms 3.21,22;4.59,63; on Airs, Waters and Places c. 10. The Sydenham Society. London: C and J Adlard Printers. 1843. Available online: https://classicalliberalarts.com/resources/HIPPOCRATES_1.pdf (accessed on 10 June 2024).
- Arrow, K.J.; Panosian, C.; Gelband, H. (Eds.) A Brief History of Malaria. In Saving Lives, Buying Time: Economics of Malaria Drugs in an Age of Resistance; National Academies Press: Washington, DC, USA, 2004. [Google Scholar]
- Ambroise-Thomas, P. La petite et la grande histoire du paludisme (The short and long history of malaria). Bull. Acad. Natle Méd. 2007, 191, 1849–1857. [Google Scholar]
- Michel, M.; Skourtanioti, E.; Pierini, F.; Guevara, E.K.; Mötsch, A.; Kocher, A.; Barquera, R.; Bianco, R.A.; Carlhoff, S.; Coppola Bove, L.; et al. Ancient Plasmodium genomes shed light on the history of human malaria. Nature 2024, 631, 125–133. [Google Scholar] [CrossRef]
- Thompson, T. Ancient malaria genome from Roman skeleton hints at disease’s history. Nature 2024. [Google Scholar] [CrossRef]
- Hawass, Z.; Gad, Y.Z.; Ismail, S.; Khairat, R.; Fathalla, D.; Hasan, N.; Ahmed, A.; Elleithy, H.; Ball, M.; Gaballah, F.; et al. Ancestry and pathology in King Tutankhamun’s family. JAMA 2010, 303, 638–647. [Google Scholar] [CrossRef]
- Nerlich, A.G.; Schraut, B.; Dittrich, S.; Jelinek, T.; Zink, A.R. Plasmodium falciparum in ancient Egypt. Emerg. Infect. Dis. 2008, 14, 1317–1319. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P.D. Parasites in ancient Egypt and Nubia: Malaria, schistosomiasis and the pharaohs. Adv. Parasitol. 2024, 123, 23–49. [Google Scholar] [PubMed]
- Boualam, M.A.; Heitzmann, A.; Mousset, F.; Aboudharam, G.; Drancourt, M.; Pradines, B. Use of rapid diagnostic tests for the detection of ancient malaria infections in dental pulp from the sixth century in Versailles, France. Malar. J. 2023, 22, 151. [Google Scholar] [CrossRef] [PubMed]
- Laveran, A. Traité des Fièvres Palustres; Octave Doin: Paris, France, 1884. [Google Scholar]
- Buisson, Y. Laveran et l’Académie nationale de médecine [Laveran and the French Academy of Medicine]. Med. Trop. Sante Int. 2023, 3. [Google Scholar] [CrossRef]
- Ross, R. On some Peculiar Pigmented Cells Found in Two Mosquitos Fed on Malarial Blood. BMJ 1897, 2, 1786. [Google Scholar] [CrossRef]
- Grassi, B.; Bignami, A.; Bastianelli, G. Medical Zoology: Further Researches upon the Cycle of Human Malaria in the Body of the Mosquito. Ind. Med. Gaz. 1899, 34, 104–107. [Google Scholar]
- How Malaria Shaped Our History “Comment le paludisme a façonné notre histoire” with Pr Renaud Piarroux. Mecaniques des Epidemies Saison 5: Le Paludisme. Paris. 2022. Available online: https://www.radiofrance.fr/franceculture/podcasts/mecaniques-des-epidemies/comment-le-paludisme-a-faconne-notre-histoire-3885057 (accessed on 6 August 2024).
- Roersch van der Hoogte, A.; Pieters, T. Science in the service of colonial agro-industrialism: The case of cinchona cultivation in the Dutch and British East Indies, 1852–1900. Stud. Hist. Philos. Sci. Part C 2014, 47 Pt A, 12–22. [Google Scholar] [CrossRef]
- Greenwood, D. The quinine connection. J. Antimicrob. Chemother. 1992, 30, 417–427. [Google Scholar] [CrossRef]
- The First Ten Years of the World Health Organization; World Health Organization: Geneva, Switzerland, 1958. Available online: https://iris.who.int/bitstream/handle/10665/37089/a38153_eng_LR_part1.pdf?sequence=14 (accessed on 7 August 2024).
- Hay, S.I.; Guerra, C.A.; Tatem, A.J.; Noor, A.M.; Snow, R.W. The global distribution and population at risk of malaria: Past, present, and future. Lancet Infect. Dis. 2004, 4, 327–336. [Google Scholar] [CrossRef]
- Chen, T.T.; Ljungqvist, F.C.; Castenbrandt, H.; Hildebrandt, F.; Ingholt, M.M.; Hesson, J.C.; Ankarklev, J.; Seftigen, K.; Linderholm, H.W. The spatiotemporal distribution of historical malaria cases in Sweden: A climatic perspective. Malar. J. 2021, 20, 212. [Google Scholar] [CrossRef]
- Najera, J.A. Malaria and the work of WHO. Bull. World Health Organ. 1989, 67, 229–243. [Google Scholar] [PubMed]
- Litsios, S. Malaria control, the cold war, and the postwar reorganization of international assistance. Med. Anthropol. 1997, 17, 255–278. [Google Scholar] [CrossRef] [PubMed]
- Mertens, J.E. A History of Malaria and Conflict. Parasitol. Res. 2024, 123, 165. [Google Scholar] [CrossRef] [PubMed]
- Amelineau, F. Désastre sanitaire. Rev. Hist. Des Armées 1992, 186, 41–51. [Google Scholar] [CrossRef]
- Richard-Lenoble, D.; Danis, M.; Saliou, P. La médecine tropicale d’hier à aujourd’hui [Tropical medicine: Past and present]. Bull. Acad. Natl. Med. 2013, 197, 1353–1364. [Google Scholar]
- Faust, E.C. The History of Malaria in the United States. Am. Sci. 1951, 39, 121–130. [Google Scholar]
- Prinzi, A.; Rohde, R. The History of Malaria in the United States. 2023. Available online: https://asm.org/Articles/2023/September/The-History-of-Malaria-in-the-United-States (accessed on 16 September 2024).
- Carter, E.D. Malaria control in the Tennessee Valley Authority: Health, ecology, and metanarratives of development. J. Hist. Geogr. 2014, 43, 111–127. [Google Scholar] [CrossRef]
- Office of the Director, Epidemiology Program Office, CDC Historical Perspectives History of CDC. MMWR 1996, 45, 526–530.
- The Editors of Encyclopaedia Britannica. Industrial Revolution. Britannica. 2024. Available online: https://www.britannica.com/event/Industrial-Revolution (accessed on 20 July 2024).
- Piperaki, E.-T. Malaria Eradication in the European World: Historical Perspective and Imminent Threats. In Towards Malaria Elimination; Manguin, S., Dev, V., Eds.; IntechOpen: Rijeka, Croatia, 2018; Chapter 13; pp. 316–335. [Google Scholar] [CrossRef]
- Manguin, S.; Carnevale, P.; Mouchet, J.; Coosemans, M.; Julvez, J.; Richard-Lenoble, D.; Sircoulon, J. Biodiversity of Malaria in the World; John Libbey Eurotext; John Libbey: Montrouge, France, 2008. [Google Scholar]
- Najera, J.A. Malaria control: Present situation and need for historical research. Parassitologia 1990, 32, 215–229. [Google Scholar]
- Packard, R.M. Malaria dreams: Postwar visions of health and development in the Third World. Med. Anthropol. 1997, 17, 279–296. [Google Scholar] [CrossRef]
- Nájera, J.A.; González-Silva, M.; Alonso, P.L. Some lessons for the future from the Global Malaria Eradication Programme (1955–1969). PLoS Med. 2011, 8, e1000412. [Google Scholar] [CrossRef] [PubMed]
- Tognotti, E. Program to eradicate malaria in Sardinia, 1946–1950. Emerg. Infect. Dis. 2009, 15, 1460–1466. [Google Scholar] [CrossRef] [PubMed]
- Andrews, J.M.; Grant, J.S.; Fritz, R.F. Effects of suspended residual spraying and of imported malaria on malaria control in the USA. Bull. World Health Organ. 1954, 11, 839–848. [Google Scholar] [PubMed]
- Cavalié, P.; Mouchet, J. La Campagne Expérimentale d’Eradication du Paludisme dans le Nord de la République du Cameroun; World Health Organization: Geneva, Switzerland, 1961; p. 34, Report No.: WHO/Mal/323. Available online: https://iris.who.int/bitstream/handle/10665/64904/WHO_Mal_323_fre.pdf?sequence=1&isAllowed=y (accessed on 9 August 2024).
- Trigg, P.I.; Kondrachine, A.V. Commentary: Malaria control in the 1990s. Bull. World Health Organ. 1998, 76, 11–16. [Google Scholar] [PubMed]
- Nájera, J.A. Malaria control: Achievements, problems and strategies. Parassitologia 2001, 43, 1–89. [Google Scholar]
- Mulliken, D.L.; Zambone, J.D.; Rolph, C.G. DDT: A Persistent Lifesaver. Nat. Resour. Environ. 2005, 19, 3–7. [Google Scholar]
- Carter, R. The World Health Report 1999: Making a Difference, Chapter 4 Rolling Back Malaria, Box 4.1; World Health Organization: Geneva, Switzerland, 1999; p. 136. Available online: https://iris.who.int/bitstream/handle/10665/42167/WHR_1999.pdf?sequence=1&isAllowed=y (accessed on 16 August 2024).
- Beard, J. DDT and human health. Sci. Total Environ. 2006, 355, 78–89. [Google Scholar] [CrossRef]
- Roberts, D.R.; Tren, R. International advocacy against DDT and other public health insecticides for malaria control. Res. Rep. Trop. Med. 2011, 2, 23–30. [Google Scholar]
- Malaria Eradication: Benefits, Future Scenarios and Feasibility; World Health Organization: Geneva, Switzerland, 2020. Available online: https://www.who.int/publications/i/item/9789240003675 (accessed on 18 August 2024).
- Kettell, S. Oil Crisis. The Editors of Encyclopedia Britannica. Britannica. 2024. Available online: https://www.britannica.com/money/oil-crisis (accessed on 18 August 2024).
- Shilts, R. And the Band Played on: Politics, People, and the AIDS Epidemic; St. Martin’s Press: New York City, NY, USA, 1987. [Google Scholar]
- Rolling back malaria. TDR News 1999, 58, 1–2. Available online: https://pubmed.ncbi.nlm.nih.gov/12322120/ (accessed on 14 August 2024).
- Najera, J.A. Global Partnership to Roll Back Malaria. Malaria Control: Achievements, Problems and Strategies; World Health Organization: Geneva, Switzerland, 1999; p. 126, Report No.: WHO/MAL/99.1087. Available online: https://iris.who.int/handle/10665/66640 (accessed on 14 August 2024).
- World Health Assembly 46. Forty-Sixth World Health Assembly, Geneva, 3–14 May 1993: Resolutions and Decisions, Annexes; World Health Organization: Geneva, Switzerland, 1993. Available online: https://iris.who.int/handle/10665/176262 (accessed on 14 August 2024).
- White, N.J. Antimalarial drug resistance. J. Clin. Investig. 2004, 113, 1084–1092. [Google Scholar] [CrossRef]
- Pradines, B.; Dormoi, J.; Briolant, S.; Bogreau, H.; Rogier, C. La résistance aux antipaludiques. Rev. Francoph. Des Lab. 2010, 2010, 51–62. [Google Scholar] [CrossRef]
- Farooq, U.; Mahajan, R.C. Drug resistance in malaria. J. Vector Borne Dis. 2004, 41, 45–53. [Google Scholar] [PubMed]
- Murray, C.J.L.; Rosenfeld, L.C.; Lim, S.S.; Andrews, K.G.; Foreman, K.J.; Haring, D.; Fullman, N.; Naghavi, M.; Lozano, R.; Lopez, A.D. Global malaria mortality between 1980 and 2010: A systematic analysis. Lancet 2012, 379, 413–431. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Partnership to Roll Back Malaria; World Bank; United Nations Development Programme; African Summit on Roll Back Marlaria (2000: Abuja, N.; United Nations Children’s Fund (UNICEF). The Abuja Declaration and the Plan of Action: An Extract from the African Summit on Roll Back Marlaria, Abuja, 25 April 2000 (WHO/CDS/RBM/2000.17). 2003. Available online: https://iris.who.int/handle/10665/67816 (accessed on 14 August 2024).
- Roser, M.; Ritchie, H. Malaria. Our World in Data. 2024. Available online: https://ourworldindata.org/malaria (accessed on 15 August 2024).
- World Malaria Report 2016; World Health Organization: Geneva, Switzerland, 2016; p. 186. ISBN 978-92-4-151171-1. Available online: https://www.who.int/publications/i/item/9789241511711 (accessed on 22 July 2024).
- WHO/UNICEF report: Malaria MDG target achieved a mid-sharp drop in cases and mortality, but 3 billion people remain at risk. Neurosciences 2016, 21, 87–88.
- United Nations Millennium Declaration: General Assembly Resolution 55/2, Chap III Development and Poverty Eradication, Num. 19. United Nations, New York. 2000. Available online: https://www.ohchr.org/en/instruments-mechanisms/instruments/united-nations-millennium-declaration (accessed on 19 July 2024).
- World Health Assembly 58. Fifty-Eighth World Health Assembly, Geneva, 16–25 May 2005: Resolutions and Decisions: Annex; Annex: Global Immunization Vision and Strategy 2006–2015: Executive Summary; World Health Organization: Geneva, Switzerland, 2005; Report No.: WHA58/2005/REC/1. Available online: https://iris.who.int/handle/10665/20398 (accessed on 24 August 2024).
- The Global Malaria Action Plan For a Malaria Free World; Roll Back Malaria Partnership; World Health Organization: Geneva, Switzerland, 2008; p. 271. Available online: https://www.afro.who.int/publications/global-malaria-action-plan-malaria-free-world (accessed on 13 August 2024).
- Achieving the Malaria MDG Target: Reversing the Incidence of Malaria 2000–2015; World Health Organization and the United Nations Children’s Fund: Geneva, Switzerland, 2015; p. 34. ISBN 978-92-4-150944-2. Available online: https://www.who.int/publications/i/item/9789241509442 (accessed on 13 August 2024).
- Bhatt, S.; Weiss, D.J.; Cameron, E.; Bisanzio, D.; Mappin, B.; Dalrymple, U.; Battle, K.; Moyes, C.L.; Henry, A.; Eckhoff, P.A.; et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 2015, 526, 207–211. [Google Scholar] [CrossRef]
- World Malaria Report 2015; World Health Organization: Geneva, Switzerland, 2015; p. 243. ISBN 978-92-4-156515-8. Available online: https://www.who.int/publications/i/item/9789241565158 (accessed on 19 August 2024).
- Dondorp, A.M.; Nosten, F.; Yi, P.; Das, D.; Phyo, A.P.; Tarning, J.; Lwin, K.M.; Ariey, F.; Hanpithakpong, W.; Lee, S.J.; et al. Artemisinin resistance in Plasmodium falciparum malaria. N. Engl. J. Med. 2009, 361, 455–467. [Google Scholar] [CrossRef]
- Ariey, F.; Witkowski, B.; Amaratunga, C.; Beghain, J.; Langlois, A.-C.; Khim, N.; Kim, S.; Duru, V.; Bouchier, C.; Ma, L.; et al. A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature 2014, 505, 50–55. [Google Scholar] [CrossRef]
- Ranson, H.; Lissenden, N. Insecticide Resistance in African Anopheles Mosquitoes: A Worsening Situation that Needs Urgent Action to Maintain Malaria Control. Trends Parasitol. 2016, 32, 187–196. [Google Scholar] [CrossRef]
- Thiomela, R.F.; Tchouakui, M.; Menze, B.D.; Nchoutpouen, E.; Ngongang-Yipmo, E.S.; Wood, O.; Horstmann, S.; Mahob, R.J.; Fomena, A.; Wondji, C.S. Indoor residual spraying of experimental huts in Cameroon highlights the potential of Fludora® Fusion to control wild pyrethroid-resistant malaria vectors. BMC Infect. Dis. 2024, 24, 733. [Google Scholar] [CrossRef]
- Global Plan for Insecticide Resistance Management in Malaria Vectors: Executive Summary; World Health Organization: Geneva, Switzerland, 2012; p. 22, Report No.: WHO/HTM/GMP/2012.5. Available online: https://www.who.int/publications/i/item/WHO-HTM-GMP-2012.5 (accessed on 25 August 2024).
- Dhorda, M.; Kaneko, A.; Komatsu, R.; Kc, A.; Mshamu, S.; Gesase, S.; Kapologwe, N.; Assefa, A.; Opigo, J.; Adoke, Y.; et al. Artemisinin-resistant malaria in Africa demands urgent action. Science 2024, 385, 252–254. [Google Scholar] [CrossRef]
- Uwimana, A.; Legrand, E.; Stokes, B.H.; Ndikumana, J.-L.M.; Warsame, M.; Umulisa, N.; Ngamije, D.; Munyaneza, T.; Mazarati, J.-B.; Munguti, K.; et al. Emergence and clonal expansion of in vitro artemisinin-resistant Plasmodium falciparum kelch13 R561H mutant parasites in Rwanda. Nat. Med. 2020, 26, 1602–1608. [Google Scholar] [CrossRef] [PubMed]
- Conrad, M.D.; Asua, V.; Garg, S.; Giesbrecht, D.; Niaré, K.; Smith, S.; Namuganga, J.F.; Katairo, T.; Legac, J.; Crudale, R.M.; et al. Evolution of Partial Resistance to Artemisinins in Malaria Parasites in Uganda. N. Engl. J. Med. 2023, 389, 722–732. [Google Scholar] [CrossRef] [PubMed]
- Jeang, B.; Zhong, D.; Lee, M.-C.; Atieli, H.; Yewhalaw, D.; Yan, G. Molecular surveillance of Kelch 13 polymorphisms in Plasmodium falciparum isolates from Kenya and Ethiopia. Malar. J. 2024, 23, 36. [Google Scholar] [CrossRef]
- Taylor, R.; Messenger, L.A.; Abeku, T.A.; Clarke, S.E.; Yadav, R.S.; Lines, J. Invasive Anopheles stephensi in Africa: Insights from Asia. Trends Parasitol. 2024, 40, 731–743. [Google Scholar] [CrossRef]
- Faulde, M.K.; Rueda, L.M.; Khaireh, B.A. First record of the Asian malaria vector Anopheles stephensi and its possible role in the resurgence of malaria in Djibouti, Horn of Africa. Acta Trop. 2014, 139, 39–43. [Google Scholar] [CrossRef]
- de Santi, V.P.; Khaireh, B.A.; Chiniard, T.; Pradines, B.; Taudon, N.; Larréché, S.; Mohamed, A.B.; de Laval, F.; Berger, F.; Gala, F.; et al. Role of Anopheles stephensi Mosquitoes in Malaria Outbreak, Djibouti, 2019. Emerg. Infect. Dis. 2021, 27, 1697–1700. [Google Scholar] [CrossRef]
- Carter, T.E.; Yared, S.; Gebresilassie, A.; Bonnell, V.; Damodaran, L.; Lopez, K.; Ibrahim, M.; Mohammed, S.; Janies, D. First detection of Anopheles stephensi Liston, 1901 (Diptera: Culicidae) in Ethiopia using molecular and morphological approaches. Acta Trop. 2018, 188, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Emiru, T.; Getachew, D.; Murphy, M.; Sedda, L.; Ejigu, L.A.; Bulto, M.G.; Byrne, I.; Demisse, M.; Abdo, M.; Chali, W.; et al. Evidence for a role of Anopheles stephensi in the spread of drug- and diagnosis-resistant malaria in Africa. Nat. Med. 2023, 29, 3203–3211. [Google Scholar] [CrossRef]
- Ochomo, E.O.; Milanoi, S.; Abong’o, B.; Onyango, B.; Muchoki, M.; Omoke, D.; Olanga, E.; Njoroge, L.; Juma, E.O.; Otieno, J.D.; et al. Detection of Anopheles stephensi Mosquitoes by Molecular Surveillance, Kenya. Emerg. Infect. Dis. 2023, 29, 2498–2508. [Google Scholar] [CrossRef]
- Afrane, Y.A.; Abdulai, A.; Mohammed, A.R.; Akuamoah-Boateng, Y.; Owusu-Asenso, C.M.; Sraku, I.K.; Yanney, S.A.; Malm, K.; Lobo, N.F. First detection of Anopheles stephensi in Ghana using molecular surveillance. Biorxiv 2023. [Google Scholar] [CrossRef]
- World Urbanization Prospects: The 2018 Revision; Report No.: ST/ESA/SER.A/420; Department of Economic and Social Affairs, Population Division, United Nations: New York, NY, USA, 2019; p. 126. Available online: https://population.un.org/wup/Publications/Files/WUP2018-Report.pdf (accessed on 25 August 2024).
- Vector Alert: Anopheles Stephensi Invasion and Spread: Horn of Africa, the Republic of the Sudan and Surrounding Geographical Areas, and Sri Lanka: Information Note; Report No.: WHO/HTM/GMP/2019.09; World Health Organization: Geneva, Switzerland, 2019; p. 4. Available online: https://iris.who.int/handle/10665/326595 (accessed on 25 August 2024).
- Vector Alert: Anopheles Stephensi Invasion and Spread in Africa and Sri Lanka; World Health Organization: Geneva, Switzerland, 2023; p. 4. Available online: https://www.who.int/publications/i/item/9789240067714 (accessed on 25 August 2024).
- Grillet, M.E.; Villegas, L.; Oletta, J.F.; Tami, A.; Conn, J.E. Malaria in Venezuela requires response. Science 2018, 359, 528. [Google Scholar] [CrossRef] [PubMed]
- World Malaria Report 2022; World Health Organization: Geneva, Switzerland, 2022; p. 293. ISBN 978-92-4-006489-8. Available online: https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2022 (accessed on 22 July 2024).
- WHO. Malaria vaccine: WHO position paper—May 2024. WER 2024, 99, 225–248. [Google Scholar]
- Lancet, T. Malaria vaccine approval: A step change for global health. Lancet 2021, 398, 1381. [Google Scholar] [CrossRef] [PubMed]
- Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: Final results of a phase 3, individually randomised, controlled trial. Lancet 2015, 386, 31–45. [CrossRef]
- Datoo, M.S.; Dicko, A.; Tinto, H.; Ouédraogo, J.-B.; Hamaluba, M.; Olotu, A.; Beaumont, E.; Ramos Lopez, F.; Natama, H.M.; Weston, S.; et al. Safety and efficacy of malaria vaccine candidate R21/Matrix-M in African children: A multicentre, double-blind, randomised, phase 3 trial. Lancet 2024, 403, 533–544. [Google Scholar] [CrossRef]
- Laurens, M.B. Novel malaria vaccines. Hum. Vaccin. Immunother. 2021, 17, 4549–4552. [Google Scholar] [CrossRef]
- Miura, K.; Flores-Garcia, Y.; Long, C.A.; Zavala, F. Vaccines and monoclonal antibodies: New tools for malaria control. Clin. Microbiol. Rev. 2024, 37, e0007123. [Google Scholar] [CrossRef]
- Schmit, N.; Topazian, H.M.; Natama, H.M.; Bellamy, D.; Traoré, O.; Somé, M.A.; Rouamba, T.; Tahita, M.C.; Bonko, M.D.A.; Sourabié, A.; et al. The public health impact and cost-effectiveness of the R21/Matrix-M malaria vaccine: A mathematical modelling study. Lancet Infect. Dis. 2024, 24, 465–475. [Google Scholar] [CrossRef]
- Monoclonal Antibodies for Malaria Prevention: Preferred Product Characteristics and Clinical Development Considerations; World Health Organization: Geneva, Switzerland, 2023; p. 24. Available online: https://www.who.int/publications/i/item/9789240070981 (accessed on 26 August 2024).
- Gaudinski, M.R.; Berkowitz, N.M.; Idris, A.H.; Coates, E.E.; Holman, L.A.; Mendoza, F.; Gordon, I.J.; Plummer, S.H.; Trofymenko, O.; Hu, Z.; et al. A Monoclonal Antibody for Malaria Prevention. N. Engl. J. Med. 2021, 385, 803–814. [Google Scholar] [CrossRef]
- Lyke, K.E.; Berry, A.A.; Mason, K.; Idris, A.H.; O’Callahan, M.; Happe, M.; Strom, L.; Berkowitz, N.M.; Guech, M.; Hu, Z.; et al. Low-dose intravenous and subcutaneous CIS43LS monoclonal antibody for protection against malaria (VRC 612 Part C): A phase 1, adaptive trial. Lancet Infect. Dis. 2023, 23, 578–588. [Google Scholar] [CrossRef]
- Wu, R.L.; Idris, A.H.; Berkowitz, N.M.; Happe, M.; Gaudinski, M.R.; Buettner, C.; Strom, L.; Awan, S.F.; Holman, L.A.; Mendoza, F.; et al. Low-Dose Subcutaneous or Intravenous Monoclonal Antibody to Prevent Malaria. N. Engl. J. Med. 2022, 387, 397–407. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thellier, M.; Gemegah, A.A.J.; Tantaoui, I. Global Fight against Malaria: Goals and Achievements 1900–2022. J. Clin. Med. 2024, 13, 5680. https://doi.org/10.3390/jcm13195680
Thellier M, Gemegah AAJ, Tantaoui I. Global Fight against Malaria: Goals and Achievements 1900–2022. Journal of Clinical Medicine. 2024; 13(19):5680. https://doi.org/10.3390/jcm13195680
Chicago/Turabian StyleThellier, Marc, Ayawovi Arlene Jessicka Gemegah, and Ilhame Tantaoui. 2024. "Global Fight against Malaria: Goals and Achievements 1900–2022" Journal of Clinical Medicine 13, no. 19: 5680. https://doi.org/10.3390/jcm13195680




