Volumetry as a Criterion for Suboccipital Craniectomy after Cerebellar Infarction
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Inclusion and Exclusion Criteria
2.3. Data Collection
2.4. Treatment Procedures
2.5. Neuroradiological Analysis
2.6. Outcome
2.7. Volumetry
2.8. Statistical Evaluation
3. Results
3.1. Patients
3.2. Outcome
3.3. Volumetric Evaluation
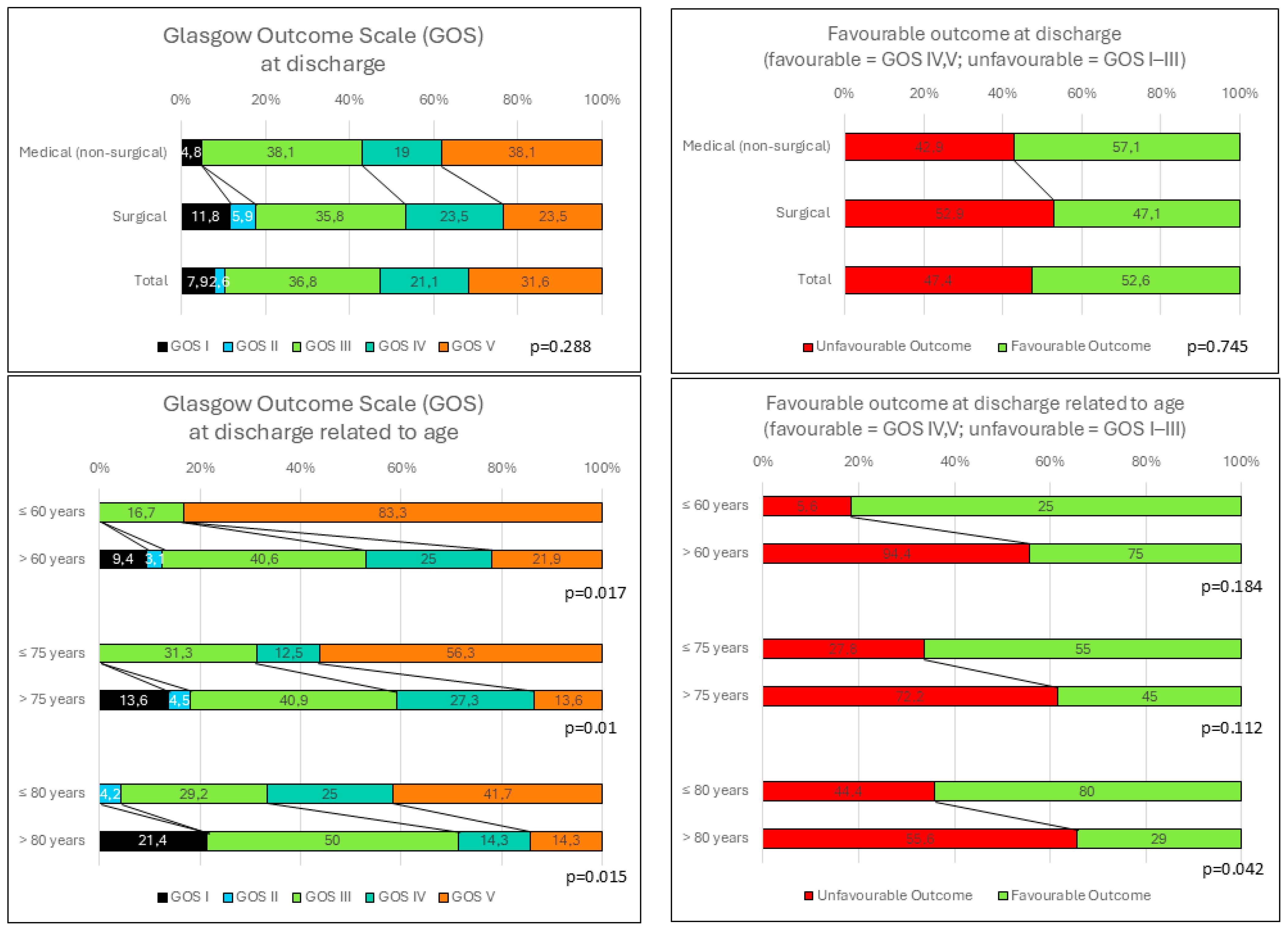
3.4. Predictive Factors and the Glasgow Outcome Scale at Discharge
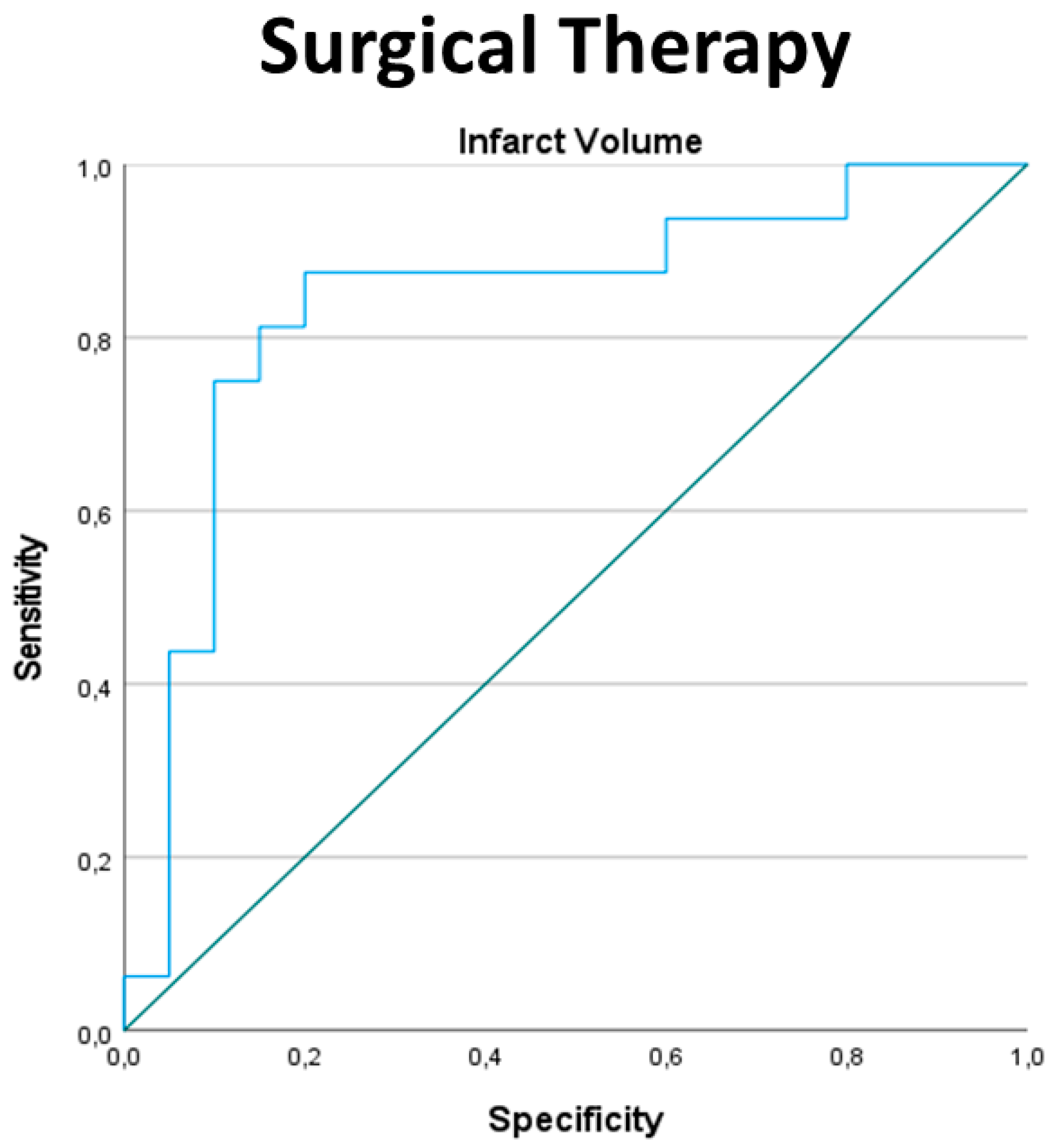
3.5. Indication for Surgical Therapy
3.6. Age-Dependent Analysis
4. Discussion
4.1. Outcome after Cerebellar Infarction
4.2. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, J.; Smith, J.P. Health, Economic Status, and Aging in High-Income Countries. In Future Directions for the Demography of Aging: Proceedings of a Workshop; Hayward, M.D., Majmundar, M.K., Eds.; The National Academies Press: Washinton, DC, USA, 2018. [Google Scholar]
- Smith, S.; Horgan, F.; Sexton, E.; Cowman, S.; Hickey, A.; Kelly, P.; McGee, H.; Murphy, S.; O’Neill, D.; Royston, M.; et al. The future cost of stroke in Ireland: An analysis of the potential impact of demographic change and implementation of evidence-based therapies. Age Ageing 2013, 42, 299–306. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hernandez-Duran, S.; Wolfert, C.; Rohde, V.; Mielke, D. Cerebellar Necrosectomy Instead of Suboccipital Decompression: A Suitable Alternative for Patients with Space-Occupying Cerebellar Infarction. World Neurosurg. 2020, 144, e723–e733. [Google Scholar] [CrossRef] [PubMed]
- Calic, Z.; Cappelen-Smith, C.; Cuganesan, R.; Anderson, C.S.; Welgampola, M.; Cordato, D.J. Frequency, Aetiology, and Outcome of Small Cerebellar Infarction. Cerebrovasc. Dis. Extra. 2017, 7, 173–180. [Google Scholar] [CrossRef]
- Calic, Z.; Cappelen-Smith, C.; Anderson, C.S.; Xuan, W.; Cordato, D.J. Cerebellar Infarction and Factors Associated with Delayed Presentation and Misdiagnosis. Cerebrovasc. Dis. 2016, 42, 476–484. [Google Scholar] [CrossRef]
- Edlow, J.A.; Newman-Toker, D.E.; Savitz, S.I. Diagnosis and initial management of cerebellar infarction. Lancet Neurol. 2008, 7, 951–964. [Google Scholar] [CrossRef]
- Wang, Y.; Binkley, M.M.; Qiao, M.; Pardon, A.; Keyrouz, S.; Dhar, R.; Ford, A.L. Rate of Infarct-Edema Growth on CT Predicts Need for Surgical Intervention and Clinical Outcome in Patients with Cerebellar Infarction. Neurocrit. Care 2022, 36, 1011–1021. [Google Scholar] [CrossRef] [PubMed]
- Wijdicks, E.F.; Sheth, K.N.; Carter, B.S.; Greer, D.M.; Kasner, S.E.; Kimberly, W.T.; Schwab, S.; Smith, E.E.; Tamargo, R.J.; Wintermark, M.; et al. Recommendations for the management of cerebral and cerebellar infarction with swelling: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014, 45, 1222–1238. [Google Scholar] [CrossRef]
- Juttler, E.; Schweickert, S.; Ringleb, P.A.; Huttner, H.B.; Kohrmann, M.; Aschoff, A. Long-term outcome after surgical treatment for space-occupying cerebellar infarction: Experience in 56 patients. Stroke 2009, 40, 3060–3066. [Google Scholar] [CrossRef]
- Hornig, C.R.; Rust, D.S.; Busse, O.; Jauss, M.; Laun, A. Space-occupying cerebellar infarction. Clinical course and prognosis. Stroke 1994, 25, 372–374. [Google Scholar] [CrossRef]
- Ayling, O.G.S.; Alotaibi, N.M.; Wang, J.Z.; Fatehi, M.; Ibrahim, G.M.; Benavente, O.; Field, T.S.; Gooderham, P.A.; Macdonald, R.L. Suboccipital Decompressive Craniectomy for Cerebellar Infarction: A Systematic Review and Meta-Analysis. World Neurosurg. 2018, 110, 450–459 e455. [Google Scholar] [CrossRef]
- Lindeskog, D.; Lilja-Cyron, A.; Kelsen, J.; Juhler, M. Long-term functional outcome after decompressive suboccipital craniectomy for space-occupying cerebellar infarction. Clin. Neurol. Neurosurg. 2019, 176, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Feely, M.P. Cerebellar infarction. Neurosurgery 1979, 4, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Heros, R.C. Surgical treatment of cerebellar infarction. Stroke 1992, 23, 937–938. [Google Scholar] [CrossRef]
- Jauss, M.; Krieger, D.; Hornig, C.; Schramm, J.; Busse, O. Surgical and medical management of patients with massive cerebellar infarctions: Results of the German-Austrian Cerebellar Infarction Study. J. Neurol. 1999, 246, 257–264. [Google Scholar] [CrossRef]
- Suyama, Y.; Wakabayashi, S.; Aihara, H.; Ebiko, Y.; Kajikawa, H.; Nakahara, I. Evaluation of clinical significance of decompressive suboccipital craniectomy on the prognosis of cerebellar infarction. Fujita Med. J. 2019, 5, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Duran, S.; Walter, J.; Behmanesh, B.; Bernstock, J.D.; Czabanka, M.; Dinc, N.; Dubinski, D.; Freiman, T.M.; Gunther, A.; Hellmuth, K.; et al. Necrosectomy Versus Stand-Alone Suboccipital Decompressive Craniectomy for the Management of Space-Occupying Cerebellar Infarctions-A Retrospective Multicenter Study. Neurosurgery 2024, 94, 559–566. [Google Scholar] [CrossRef]
- Hernandez-Duran, S.; Walter, J.; Behmanesh, B.; Bernstock, J.D.; Czabanka, M.; Dinc, N.; Dubinski, D.; Freiman, T.M.; Konczalla, J.; Melkonian, R.; et al. Surgical infarct volume reduction and functional outcomes in patients with ischemic cerebellar stroke: Results from a multicentric retrospective study. J. Neurosurg. 2024, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kudo, H.; Kawaguchi, T.; Minami, H.; Kuwamura, K.; Miyata, M.; Kohmura, E. Controversy of surgical treatment for severe cerebellar infarction. J. Stroke Cerebrovasc. Dis. 2007, 16, 259–262. [Google Scholar] [CrossRef] [PubMed]
- Pfefferkorn, T.; Eppinger, U.; Linn, J.; Birnbaum, T.; Herzog, J.; Straube, A.; Dichgans, M.; Grau, S. Long-term outcome after suboccipital decompressive craniectomy for malignant cerebellar infarction. Stroke 2009, 40, 3045–3050. [Google Scholar] [CrossRef]
- Won, S.Y.; Hernandez-Duran, S.; Behmanesh, B.; Bernstock, J.D.; Czabanka, M.; Dinc, N.; Dubinski, D.; Freiman, T.M.; Gunther, A.; Hellmuth, K.; et al. Functional Outcomes in Conservatively vs Surgically Treated Cerebellar Infarcts. JAMA Neurol. 2024, 81, 384–393. [Google Scholar] [CrossRef]
- Jauch, E.C.; Saver, J.L.; Adams, H.P., Jr.; Bruno, A.; Connors, J.J.; Demaerschalk, B.M.; Khatri, P.; McMullan, P.W., Jr.; Qureshi, A.I.; Rosenfield, K.; et al. Guidelines for the early management of patients with acute ischemic stroke: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013, 44, 870–947. [Google Scholar] [CrossRef] [PubMed]
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2019, 50, e344–e418. [Google Scholar] [CrossRef]
- van der Worp, H.B.; Hofmeijer, J.; Juttler, E.; Lal, A.; Michel, P.; Santalucia, P.; Schonenberger, S.; Steiner, T.; Thomalla, G. European Stroke Organisation (ESO) guidelines on the management of space-occupying brain infarction. Eur. Stroke J. 2021, 6, XC–CX. [Google Scholar] [CrossRef]
- Tchopev, Z.; Hiller, M.; Zhuo, J.; Betz, J.; Gullapalli, R.; Sheth, K.N. Prediction of poor outcome in cerebellar infarction by diffusion MRI. Neurocrit. Care. 2013, 19, 276–282. [Google Scholar] [CrossRef]
- Mourand, I.; Mahmoudi, M.; Dargazanli, C.; Pavillard, F.; Arquizan, C.; Labreuche, J.; Derraz, I.; Gaillard, N.; Blanchet-Fourcade, G.; Lefevre, P.H.; et al. DWI cerebellar infarct volume as predictor of outcomes after endovascular treatment of acute basilar artery occlusion. J. Neurointerv. Surg. 2021, 13, 995–1001. [Google Scholar] [CrossRef] [PubMed]
- Lakatos, L.; Bolognese, M.; Muller, M.; Osterreich, M.; von Hessling, A. Automated Supra- and Infratentorial Brain Infarct Volume Estimation on Diffusion Weighted Imaging Using the RAPID Software. Front. Neurol. 2022, 13, 907151. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.R.; Basma, J.; Jones, G.M.; Lillard, J.; Wallace, D.; Ajmera, S.; Gienapp, A.J.; Michael, L.M., 2nd. Predicting Surgical Intervention in Cerebellar Stroke: A Quantitative Retrospective Analysis. World Neurosurg. 2020, 142, e160–e172. [Google Scholar] [CrossRef]
- Kim, M.J.; Park, S.K.; Song, J.; Oh, S.Y.; Lim, Y.C.; Sim, S.Y.; Shin, Y.S.; Chung, J. Preventive Suboccipital Decompressive Craniectomy for Cerebellar Infarction: A Retrospective-Matched Case-Control Study. Stroke 2016, 47, 2565–2573. [Google Scholar] [CrossRef]
- Lucia, K.; Reitz, S.; Hattingen, E.; Steinmetz, H.; Seifert, V.; Czabanka, M. Predictors of clinical outcomes in space-occupying cerebellar infarction undergoing suboccipital decompressive craniectomy. Front. Neurol. 2023, 14, 1165258. [Google Scholar] [CrossRef]
- Goulin Lippi Fernandes, E.; Ridwan, S.; Greeve, I.; Schabitz, W.R.; Grote, A.; Simon, M. Clinical and Computerized Volumetric Analysis of Posterior Fossa Decompression for Space-Occupying Cerebellar Infarction. Front. Neurol. 2022, 13, 840212. [Google Scholar] [CrossRef]
- Khalsa, S.S.S.; Siu, A.; DeFreitas, T.A.; Cappuzzo, J.M.; Myseros, J.S.; Magge, S.N.; Oluigbo, C.O.; Keating, R.F. Comparison of posterior fossa volumes and clinical outcomes after decompression of Chiari malformation Type I. J. Neurosurg. Pediatr. 2017, 19, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Trigylidas, T.; Baronia, B.; Vassilyadi, M.; Ventureyra, E.C. Posterior fossa dimension and volume estimates in pediatric patients with Chiari I malformations. Childs. Nerv. Syst. 2008, 24, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.B.; St Louis, E.K. Management of acute cerebellar stroke. Arch. Neurol. 2005, 62, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Kirollos, R.W.; Tyagi, A.K.; Ross, S.A.; van Hille, P.T.; Marks, P.V. Management of spontaneous cerebellar hematomas: A prospective treatment protocol. Neurosurgery 2001, 49, 1378–1386, discussion 1386–1377. [Google Scholar] [CrossRef]
- Amarenco, P.; Levy, C.; Cohen, A.; Touboul, P.J.; Roullet, E.; Bousser, M.G. Causes and mechanisms of territorial and nonterritorial cerebellar infarcts in 115 consecutive patients. Stroke 1994, 25, 105–112. [Google Scholar] [CrossRef]
- Teasdale, G.; Jennett, B. Assessment of coma and impaired consciousness. A practical scale. Lancet 1974, 2, 81–84. [Google Scholar] [CrossRef]
- Pallicino, P.; Snyder, W.; Granger, C. The NIH stroke scale and the FIM in stroke rehabilitation. Stroke 1992, 23, 919. [Google Scholar] [CrossRef]
- Teasdale, G.M.; Pettigrew, L.E.; Wilson, J.T.; Murray, G.; Jennett, B. Analyzing outcome of treatment of severe head injury: A review and update on advancing the use of the Glasgow Outcome Scale. J. Neurotrauma 1998, 15, 587–597. [Google Scholar] [CrossRef]
- Kim, S.Y. The Definition of Obesity. Korean J. Fam. Med. 2016, 37, 309. [Google Scholar] [CrossRef][Green Version]
- Wendel, M.P.; Bane, H.; Frankowski, S.; Magann, E.F. Elevated Blood Pressure and Stage 1 Hypertension in Pregnancy: A Review of the Literature. Obstet. Gynecol. Surv. 2022, 77, 415–422. [Google Scholar] [CrossRef]
- European Stroke Initiative Executive Committee; Committee, E.W.; Olsen, T.S.; Langhorne, P.; Diener, H.C.; Hennerici, M.; Ferro, J.; Sivenius, J.; Wahlgren, N.G.; Bath, P. European Stroke Initiative Recommendations for Stroke Management-update 2003. Cerebrovasc. Dis. 2003, 16, 311–337. [Google Scholar] [CrossRef]
- Neugebauer, H.; Witsch, J.; Zweckberger, K.; Juttler, E. Space-occupying cerebellar infarction: Complications, treatment, and outcome. Neurosurg. Focus. 2013, 34, E8. [Google Scholar] [CrossRef] [PubMed]
- Althaus, K.; Dreyhaupt, J.; Hyrenbach, S.; Pinkhardt, E.H.; Kassubek, J.; Ludolph, A.C. MRI as a first-line imaging modality in acute ischemic stroke: A sustainable concept. Ther. Adv. Neurol. Disord. 2021, 14, 17562864211030363. [Google Scholar] [CrossRef] [PubMed]
- Dupont, S.; Rabinstein, A.A. CT evaluation of lateral ventricular dilatation after subarachnoid hemorrhage: Baseline bicaudate index values [correction of balues]. Neurol. Res. 2013, 35, 103–106. [Google Scholar] [CrossRef]
- Brand, C.; Pala, A.; Kielhorn, W.; Wirtz, C.R.; Kapapa, T. Do Complication Rates of Ventricular Drain Placement Differ between Twist Drill and Burr Hole in Acute Hydrocephalus? J. Neurol. Surg. A Cent. Eur. Neurosurg. 2019, 80, 277–284. [Google Scholar] [CrossRef]
- Kapapa, T.; Brand, C.; Wirtz, C.R.; Woischneck, D. Outcome after Decompressive Craniectomy in Different Pathologies. World Neurosurg. 2016, 93, 389–397. [Google Scholar] [CrossRef]
- Chen, H.J.; Lee, T.C.; Wei, C.P. Treatment of cerebellar infarction by decompressive suboccipital craniectomy. Stroke 1992, 23, 957–961. [Google Scholar] [CrossRef]
- De Cocker, L.J.; Lovblad, K.O.; Hendrikse, J. MRI of Cerebellar Infarction. Eur. Neurol. 2017, 77, 137–146. [Google Scholar] [CrossRef] [PubMed]
- De Cocker, L.J.; Geerlings, M.I.; Hartkamp, N.S.; Grool, A.M.; Mali, W.P.; Van der Graaf, Y.; Kloppenborg, R.P.; Hendrikse, J.; SMART Study Group. Cerebellar infarct patterns: The SMART-Medea study. Neuroimage Clin. 2015, 8, 314–321. [Google Scholar] [CrossRef][Green Version]
- Savitz, S.I.; Caplan, L.R.; Edlow, J.A. Pitfalls in the diagnosis of cerebellar infarction. Acad. Emerg. Med. 2007, 14, 63–68. [Google Scholar] [CrossRef]
- Roh, J.K.; Kim, K.K.; Han, M.H.; Chang, K.H.; Kim, H.J.; Lee, S.B.; Myung, H. Magnetic resonance imaging in brainstem ischemic stroke. J. Korean Med. Sci. 1991, 6, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Etgen, T.; Grafin von Einsiedel, H.; Rottinger, M.; Winbeck, K.; Conrad, B.; Sander, D. Detection of acute brainstem infarction by using DWI/MRI. Eur. Neurol. 2004, 52, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Steffen, P.; Beyer, L.S.; McDonough, R.; Thaler, C.; Faizy, T.; Fiehler, J.; Gbadamosi, J.; Habermann, C.R.; Schonfeld, M.H. Improved Detectability of Brain Stem Ischemia by Combining Axial and Coronal Diffusion-Weighted Imaging. Stroke 2021, 52, 1843–1846. [Google Scholar] [CrossRef]
- Sananmuang, T.; Dejsiripongsa, T.; Keandoungchun, J.; Apirakkan, M. Reliability of ABC/2 Method in Measuring of Infarct Volume in Magnetic Resonance Diffusion-Weighted Image. Asian J. Neurosurg. 2019, 14, 801–807. [Google Scholar] [CrossRef]
- Nouraee, C.; Fisher, M.; Di Napoli, M.; Salazar, P.; Farr, T.D.; Jafarli, A.; Divani, A.A. A Brief Review of Edema-Adjusted Infarct Volume Measurement Techniques for Rodent Focal Cerebral Ischemia Models with Practical Recommendations. J. Vasc. Interv. Neurol. 2019, 10, 38–45. [Google Scholar] [PubMed]
- Hofmeijer, J.; Amelink, G.J.; Algra, A.; van Gijn, J.; Macleod, M.R.; Kappelle, L.J.; van der Worp, H.B.; HAMLET investigators. Hemicraniectomy after middle cerebral artery infarction with life-threatening Edema trial (HAMLET). Protocol for a randomised controlled trial of decompressive surgery in space-occupying hemispheric infarction. Trials 2006, 7, 29. [Google Scholar] [CrossRef]
- Juttler, E.; Schwab, S.; Schmiedek, P.; Unterberg, A.; Hennerici, M.; Woitzik, J.; Witte, S.; Jenetzky, E.; Hacke, W.; Group, D.S. Decompressive Surgery for the Treatment of Malignant Infarction of the Middle Cerebral Artery (DESTINY): A randomized, controlled trial. Stroke 2007, 38, 2518–2525. [Google Scholar] [CrossRef]
- Vahedi, K.; Vicaut, E.; Mateo, J.; Kurtz, A.; Orabi, M.; Guichard, J.P.; Boutron, C.; Couvreur, G.; Rouanet, F.; Touze, E.; et al. Sequential-design, multicenter, randomized, controlled trial of early decompressive craniectomy in malignant middle cerebral artery infarction (DECIMAL Trial). Stroke 2007, 38, 2506–2517. [Google Scholar] [CrossRef]
- Şeker, A.; Rhoton, A.L. The Anatomy of the Posterior Cranial Fossa. In Posterior Fossa Tumors in Children; Özek, M., Cinalli, G., Maixner, W., Sainte-Rose, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar] [CrossRef]
- Koh, M.G.; Phan, T.G.; Atkinson, J.L.; Wijdicks, E.F. Neuroimaging in deteriorating patients with cerebellar infarcts and mass effect. Stroke 2000, 31, 2062–2067. [Google Scholar] [CrossRef]
- Tohgi, H.; Takahashi, S.; Chiba, K.; Hirata, Y. Cerebellar infarction. Clinical and neuroimaging analysis in 293 patients. The Tohoku Cerebellar Infarction Study Group. Stroke 1993, 24, 1697–1701. [Google Scholar] [CrossRef]
- Kase, C.S.; Norrving, B.; Levine, S.R.; Babikian, V.L.; Chodosh, E.H.; Wolf, P.A.; Welch, K.M. Cerebellar infarction. Clinical and anatomic observations in 66 cases. Stroke 1993, 24, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.; Huang, C.; Strbian, D.; Sundararajan, S. Diagnosis and Management of acute cerebllar infarction. Stroke A J. Cereb. Circ. 2014, 45, 56–58. [Google Scholar] [CrossRef]
- Datar, S.; Rabinstein, A.A. Cerebellar infarction. Neurol. Clin. 2014, 32, 979–991. [Google Scholar] [CrossRef] [PubMed]
- Feigin, V.L.; Lawes, C.M.; Bennett, D.A.; Barker-Collo, S.L.; Parag, V. Worldwide stroke incidence and early case fatality reported in 56 population-based studies: A systematic review. Lancet Neurol. 2009, 8, 355–369. [Google Scholar] [CrossRef] [PubMed]
- Macdonell, R.A.; Kalnins, R.M.; Donnan, G.A. Cerebellar infarction: Natural history, prognosis, and pathology. Stroke 1987, 18, 849–855. [Google Scholar] [CrossRef]
- Tsitsopoulos, P.P.; Tobieson, L.; Enblad, P.; Marklund, N. Clinical outcome following surgical treatment for bilateral cerebellar infarction. Acta. Neurol. Scand. 2011, 123, 345–351. [Google Scholar] [CrossRef]
| n = 38 | Medical (n = 21, 55%) | Surgery (n = 17, 45%) | Total (n = 38, 100%) | p |
|---|---|---|---|---|
| Age (years, SD) | 80.1 (12.94) | 69.5 (13.15) | 75.4 (13.93) | 0.017 |
| Gender | ||||
| Female | 9 (43) | 7 (41) | 16 (42) | 1.000 |
| Imaging on admission | ||||
| Magnet Resonance Imaging | 10 (48) | 5 (29) | 15 (40) | 0.326 |
| Computed Tomography | 11 (52) | 12 (71) | 23 (60) | |
| Imaging pre-surgery | ||||
| Magnet Resonance Imaging | 2 (12) | |||
| Computed Tomography | 15 (88) | |||
| Imaging for follow-up/post-surgery | ||||
| Magnet Resonance Imaging | 11 (52) | 6 (35) | 17 (45) | 0.342 |
| Computed Tomography | 10 (48) | 11 (65) | 21 (55) | |
| Clinical symptoms n (%) | ||||
| Cephalgia | 6 (30) | 12 (70) | 18 (49) | 0.022 |
| Disturbance of consciousness | 4 (19) | 11 (65) | 15 (40) | 0.007 |
| Dizziness | 16 (80) | 13 (77) | 29 (78) | 1.000 |
| Nausea/vomiting | 14 (70) | 10 (59) | 24 (65) | 0.512 |
| Facial paresis | 3 (15) | 1 (6) | 4 (11) | 0.609 |
| Speech disorders | 10 (48) | 8 (47) | 18 (47) | 1.000 |
| Gait disturbances | 13 (62) | 9 (53) | 22 (58) | 0.743 |
| Ocular motility disorder | 8 (40) | 8 (47) | 16 (43) | 0.746 |
| Disturbances of coordination | 15 (75) | 7 (44) | 22 (61) | 0.087 |
| Initial NIHSS on admission, Mean (SD) | 4.4 (6.89) | 5.7 (6.56) | 5 (6.65) | 0.398 |
| Median (Range) | 2 (0–25) | 3 (0–18) | 2 (0–25) | |
| GCS pre-interventional, Mean (SD) | 13.3 (3.39) | 10.5 (4.83) | 12.1 (4.26) | 0.006 |
| Median (Range) | 15 (4–15) | 14 (3–15) | 15 (3–15) | |
| GCS on admission, Mean (SD) | 14.2 (2.68) | 14.4 (1.97) | 14.3 (2.36) | 0.532 |
| Median (Range) | 15 (4–15) | 15 (7–15) | 15 (4–15) | |
| Anisocoria (%) | 4 (19) | 1 (6) | 5 (13) | 0.355 |
| ICU stay in days, Mean (SD) | 4.8 (8.35) | 15.1 (13.1) | 9.2 (11.7) | 0.002 |
| Artificial ventilation in hours, Mean (SD) | 43.1 (102.0) | 48.5 (67.77) | 45.5 (87.31) | 0.003 |
| Risk factors n (%) | ||||
| Diabetes mellitus | 3 (14) | 7 (41) | 10 (26) | 0.078 |
| Nicotine abuse | 3 (14) | 6 (35) | 9 (24) | 0.249 |
| Arterial hypertension | 17 (81) | 16 (94) | 33 (87) | 0.355 |
| History of vascular events | 6 (29) | 7 (41) | 13 (34) | 0.502 |
| Coronary heart disease | 0 (0) | 6 (35) | 6 (16) | 0.004 |
| Obesity | 5 (24) | 8 (47) | 13 (34) | 0.178 |
| Duration between symptom onset and surgery in hours, Mean (SD) | 48.53 (32.51) | |||
| Vascular territory n (%) | ||||
| Vertebral artery | 4 (19) | 2 (12) | 6 (16) | 0.801 |
| Posterior inferior cerebellar artery | 11 (52) | 12 (71) | 23 (61) | |
| Anterior inferior cerebellar artery | 2 (10) | 1 (6) | 3 (8) | |
| Superior cerebellar artery | 3 (14) | 1 (6) | 4 (11) | |
| Unknown | 1 (5) | 1 (6) | 2 (5) | |
| GOS at discharge | ||||
| Mean (SD) | 3.9 (1.11) | 3.4 (1.28) | 3.7 (1.19) | 0.288 |
| Median (Range) | 4 (1–5) | 3 (1–5) | 4 (1–5) | |
| In-hospital outcome n (%) | ||||
| Death | 1 (5) | 2 (12) | 3 (8) | 0.577 |
| Volumetric data | ||||
| Cerebellar volume (cm3) (SD) | 136.41 (22.90) | 146.0 (19.47) | 140.67 (21.69) | 0.161 |
| Volume cerebellar infarction (cm3) (SD) | 25.78 (20.60) | 52.79 (22.73) | 37.79 (25.24) | <0.001 |
| Volume cranial posterior fossa (cm3) (SD) | 184.14 (29.59) | 183.79 (26.33) | 183.98 (27.80) | 0.762 |
| Volume IV ventricle (pre-surgery) (cm3) (SD) | 0.79 (0.64) | 0.80 (0.71) | 0.79 (0.66) | 0.883 |
| Volume IV ventricle (post-surgery) (cm3) (SD) | 0.96 (0.66) | 0.35 (0.19) | 0.66 (0.57) | 0.001 |
| Midline shift (pre-surgery) (mm) (SD) | 2.19 (2.19) | 3,27 (1.72) | 2.59 (2.06) | 0.102 |
| Ratio posterior fossa: infarct volume (SD) | 12.05 (9.09) | 5.13 (5.65) | 8.97 (8.41) | <0.001 |
| Ratio cerebellar: infarct volume (SD) | 8.55 (5.97) | 3.82 (3.39) | 6.45 (5.47) | <0.001 |
| Univariate | ||||
|---|---|---|---|---|
| Factor | Odds Ratio | 95% Confidence Interval | p | |
| Lower | Upper | |||
| Stay in ICU (days) | 0.914 | 0.84 | 0.996 | 0.04 |
| Age > 80 years | 0.250 | 0.06 | 1.048 | 0.05 |
| Age | 0.943 | 0.891 | 0.999 | 0.05 |
| Multivariate | ||||
| Factor | Odds Ratio | 95% Confidence Interval | p | |
| Lower | Upper | |||
| Stay in ICU (days) | 0.873 | 0.776 | 0.981 | 0.02 |
| Univariate | ||||
|---|---|---|---|---|
| Factor | Odds Ratio | 95% Confidence Interval | p | |
| Lower | Upper | |||
| Age | 1.067 | 1.006 | 1.131 | 0.03 |
| GCS at admission | 1.184 | 0.997 | 1.406 | 0.05 |
| Infarct volume | 0.942 | 0.904 | 0.982 | 0.005 |
| Ratio of posterior fossa volume to infarct volume | 1.171 | 1.007 | 1.361 | 0.04 |
| Ratio of cerebellar volume to infarct volume | 1.306 | 1.027 | 1.661 | 0.029 |
| In-hospital stay in days | 1.166 | 1.018 | 1.336 | 0.027 |
| Stay in ICU (days) | 0.888 | 0.808 | 0.975 | 0.013 |
| Infarct volume ≥ 40 cm3 | 0.058 | 0.011 | 0.305 | <0.001 |
| Infarct volume ≥ 50 cm3 | 0.067 | 0.011 | 0.394 | 0.003 |
| Infarct volume ≥ 55 cm3 | 0.111 | 0.019 | 0.645 | 0.014 |
| Infarct volume > 59 cm3 | 0.068 | 0.007 | 0.636 | 0.018 |
| Multivariate | ||||
| Factor | Odds Ratio | 95% Confidence Interval | p | |
| Lower | Upper | |||
| Ratio of posterior fossa volume to infarct volume > 4:1 | 1.162 | 1.007 | 1.341 | 0.04 |
| ROC Curve Analysis for All Patients, Surgical Therapy | Infarction Volume cm3 | Ratio of Posterior Fossa Volume to Infarction Volume | Ratio of Cerebellar to Infarction Volume |
|---|---|---|---|
| AUC | 0.841 | 0.841 | 0.838 |
| Standard error | 0.73 | 0.076 | 0.076 |
| p | 0.001 | 0.001 | 0.001 |
| CI 95%: lower | 0.698 | 0.692 | 0.688 |
| CI 95%: upper | 0.983 | 0.989 | 0.987 |
| Cut-off | 31.35 | 5.2127 | 3.7957 |
| Sensitivity | 0.875 | 0.900 | 0.900 |
| Specificity | 0.200 | 0.125 | 0.125 |
| Age Classification (Years) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| ≤60 | >60 | p | ≤75 | >75 | p | ≤80 | >80 | p | |
| n(%) | 6 (10.7) | 32 (89.3) | <0.001 | 16 (35.2) | 22 (64.8) | <0.001 | 24 (56.8) | 14 (43.2) | <0.001 |
| Age | |||||||||
| Mean (SD) | 51.0 (6.92) | 79.9 (9.32) | <0.001 | 63.1 (11.07) | 84.4 (7.35) | <0.001 | 67.8 (11.3) | 88.4 (6.28) | <0.001 |
| Median (range) | 54 (40–58) | 78 (61–96) | 66 (40–75) | 82 (76–96) | 73 (40–79) | 87.5 (80–96) | |||
| Glasgow Coma Score | |||||||||
| Mean (SD) | 10.5 (5.86) | 12.4 (3.93) | 0.225 | 11.94 (4.85) | 12.2 (3.87) | 0.728 | 12.1 (4.46) | 12.1 (4.03) | 0.891 |
| Median (range) | 13.5 (3–15) | 15 (3–15) | 14.5 (3–15) | 15 (3–15) | 15 (3–15) | 15 (3–15) | |||
| Surgery n (%) | 4 (66.7) | 13 (40.6) | 0.378 | 10 (62.5) | 7 (31.8) | 0.099 | 15 (62.5) | 2 (14.3) | 0.006 |
| Medical n (%) | 2 (33.3) | 19 (59.4) | 6 (37.5) | 15 (68.2) | 9 (37.5) | 12 (85.7) | |||
| Stay in ICU: Mean (SD) | 7.17 (6.08) | 9.65 (12.53) | 0.966 | 11.63 (14.76) | 7.43 (8.67) | 0.471 | 10.22 (12.94) | 7.64 (9.56) | 0.564 |
| Tracheostoma (n =5, 13.16%): n(%) | 0 (0) | 5 (100) | 0.401 | 2 (40) | 3 (60) | 0.654 | 4 (80) | 1 (20) | 0.381 |
| Anisocoria (n =5, 13.16%): n(%) | 1 (20) | 4 (80) | 0.599 | 1 (20) | 4 (80) | 0.286 | 3 (60) | 2 (40) | 0.619 |
| Infarct volume in cm3, Mean (SD) | 39.6 (19.27) | 37.49 (26.33) | 0.599 | 45.30 (22.91) | 33.0 (25.98) | 0.077 | 42.77 (28.09) | 29.97 (18.27) | 0.178 |
| MLS in mm, Mean (SD) | 2.46 (1.76) | 2.61 (2.13) | 1 | 3.12 (2.18) | 2.24 (1.95) | 0.242 | 2.64 (2.12) | 2.54 (2.07) | 0.864 |
| Ratio of posterior fossa volume to infarct volume, Mean (SD) | 7.97 (7.98) | 9.14 (8.59) | 0.945 | 7.49 (9.03) | 9.90 (8.05) | 0.136 | 8.88 (9.13) | 9.13 (7.46) | 0.284 |
| Ratio of cerebellar to infarct volume, Mean (SD) | 5.40 (4.30) | 6.61 (5.67) | 0.982 | 5.37 (5.90) | 7.16 (5.19) | 0.144 | 6.33 (5.96) | 6.64 (4.79) | 0.284 |
| Glasgow Outcome Scale | |||||||||
| Mean (SD) | 4.67 (0.82) | 3.47 (1.16) | 0.017 | 4.25 (0.931) | 3.23 (1.19) | 0.01 | 4.04 (0.96) | 3 (1.3) | 0.015 |
| Median (range) | 5 (3–5) | 4 (1–5) | 5 (3–5) | 3 (1–5) | 4 (2–5) | 3 (1–5) | |||
| Unfavorable outcome n (%) | 1 (16.7) | 17 (53.1) | 0.184 | 5 (31.3) | 13 (59.1) | 0.112 | 8 (33.3) | 10 (71.4) | 0.042 |
| Favorable outcome n (%) | 5 (83.3) | 15 (46.9) | 11 (68.8) | 9 (40.9) | 16 (66.7) | 4 (28.6) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kapapa, T.; Pala, A.; Alber, B.; Mauer, U.M.; Harth, A.; Neugebauer, H.; Sailer, L.; Kreiser, K.; Schmitz, B.; Althaus, K. Volumetry as a Criterion for Suboccipital Craniectomy after Cerebellar Infarction. J. Clin. Med. 2024, 13, 5689. https://doi.org/10.3390/jcm13195689
Kapapa T, Pala A, Alber B, Mauer UM, Harth A, Neugebauer H, Sailer L, Kreiser K, Schmitz B, Althaus K. Volumetry as a Criterion for Suboccipital Craniectomy after Cerebellar Infarction. Journal of Clinical Medicine. 2024; 13(19):5689. https://doi.org/10.3390/jcm13195689
Chicago/Turabian StyleKapapa, Thomas, Andrej Pala, Burkhard Alber, Uwe Max Mauer, Andreas Harth, Hermann Neugebauer, Lisa Sailer, Kornelia Kreiser, Bernd Schmitz, and Katharina Althaus. 2024. "Volumetry as a Criterion for Suboccipital Craniectomy after Cerebellar Infarction" Journal of Clinical Medicine 13, no. 19: 5689. https://doi.org/10.3390/jcm13195689
APA StyleKapapa, T., Pala, A., Alber, B., Mauer, U. M., Harth, A., Neugebauer, H., Sailer, L., Kreiser, K., Schmitz, B., & Althaus, K. (2024). Volumetry as a Criterion for Suboccipital Craniectomy after Cerebellar Infarction. Journal of Clinical Medicine, 13(19), 5689. https://doi.org/10.3390/jcm13195689






