Characterization of Circulating Protein Profiles in Individuals with Prader–Willi Syndrome and Individuals with Non-Syndromic Obesity
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Biochemical Parameters
2.3. Serum Collection for Proteomics
2.4. Circulating Proteome Profiling and Analysis
2.5. Bioinformatics Analysis
2.6. Statistical Methods
3. Results
3.1. Characteristics of the Participants
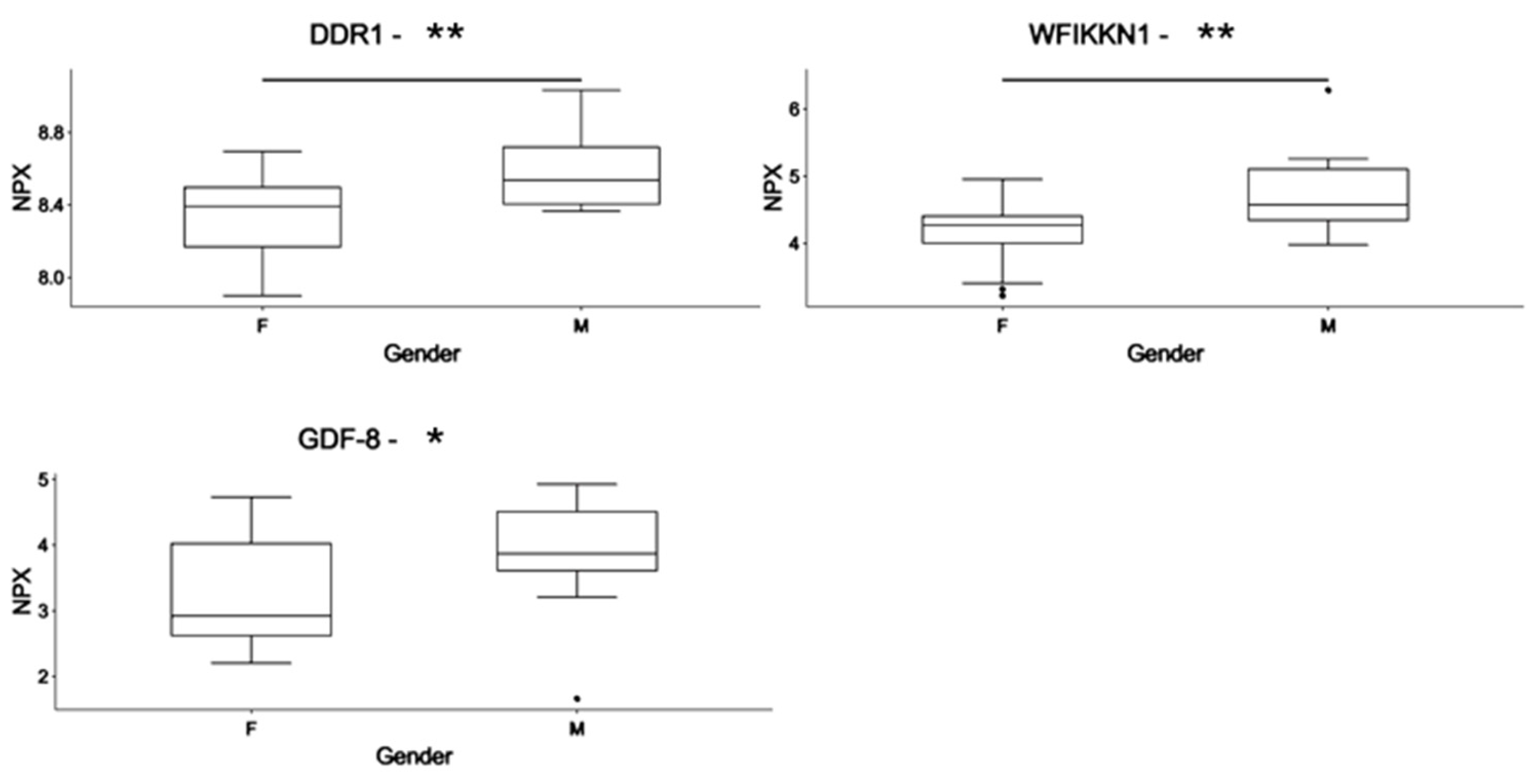
3.2. Differences in the Circulating Proteome in PWS: Identification of Gender-Associated Protein Markers
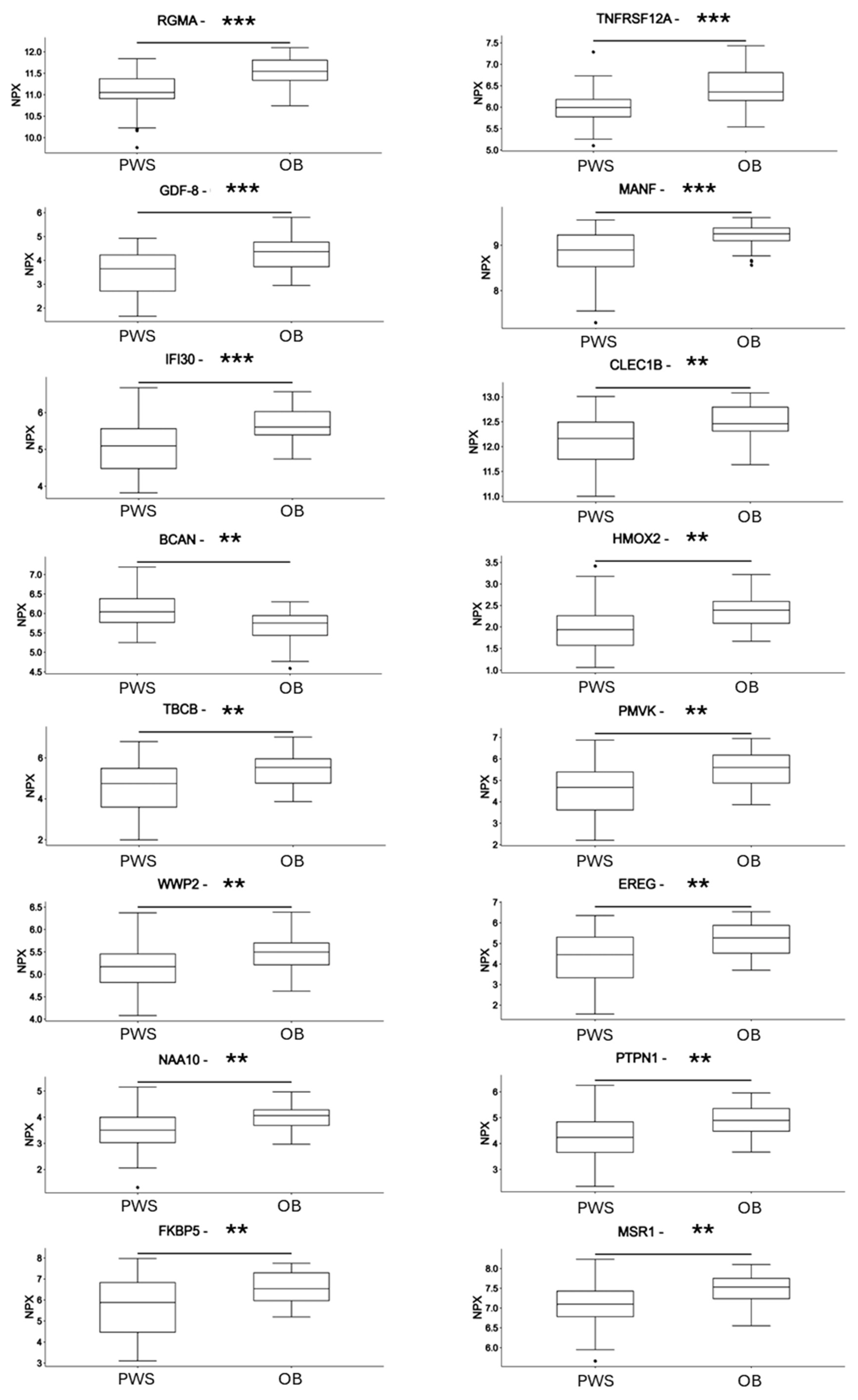
3.3. Differences in Circulating Protein Markers between Subjects with PWS and Subjects with Non-Syndromic Obesity
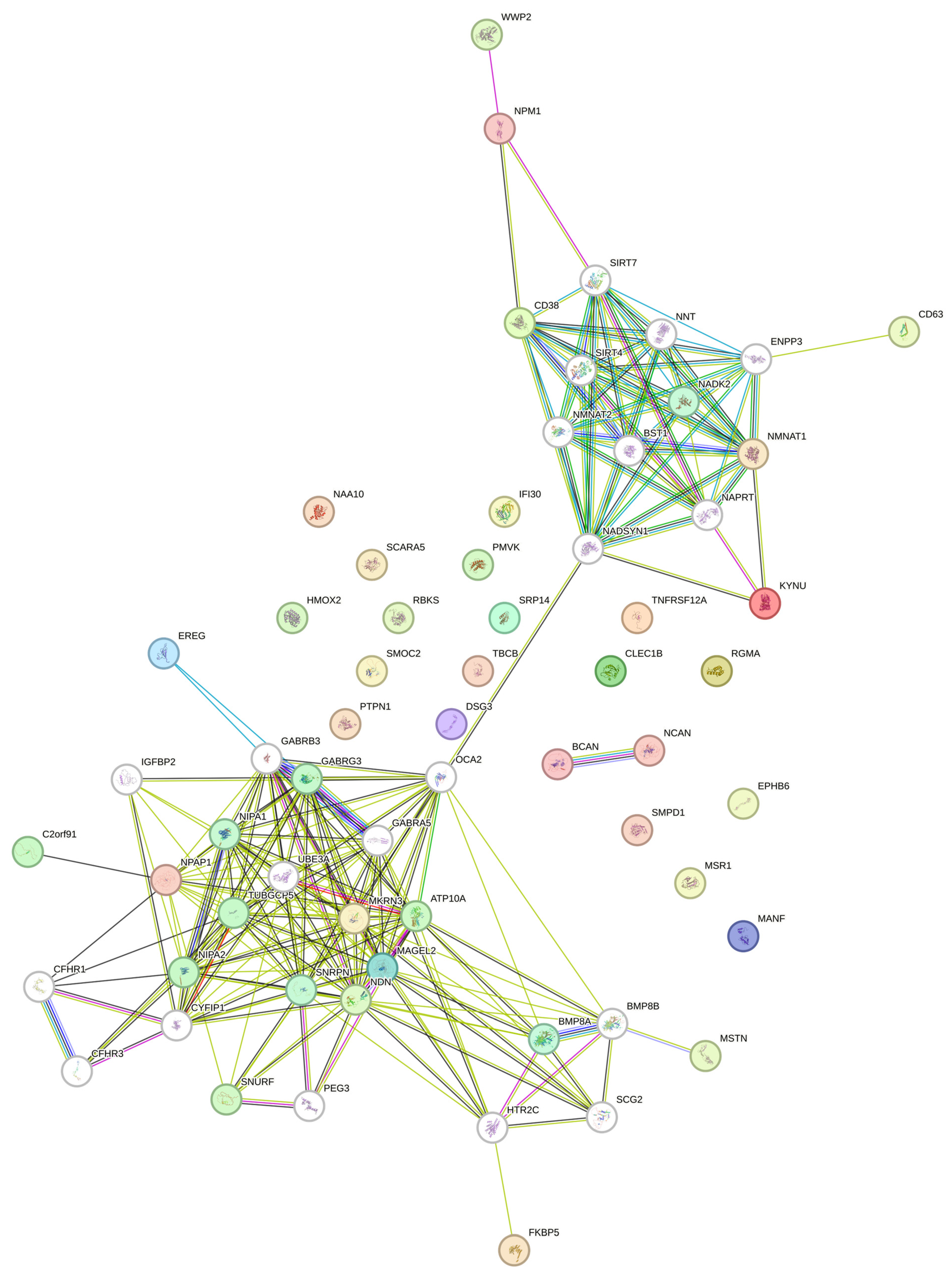
3.4. Exploring the Influence of Imprinted Genes on Circulating Protein Signatures in Prader–Willi Syndrome
| Author | Study | Model | Source | Type of Analysis | Number of Differently Exp. Genes/Prot. | Number of Shared Deregulated Genes/Prot. |
|---|---|---|---|---|---|---|
| Bittel DC, 2007 [44] | Lymphoblastoid cells were established from 4 subjects with PWS with 15q11-q13 deletion, three subjects with PWS with UPD subjects, and three controls with non-syndromic obesity | Human | cells | Transcriptome | Top 50 | none |
| Chen H, 2020 [43] | Tissues collected from Magel2p∆/m+ mice were analyzed through unbiased quantitative proteomic/mass spectrometry | Mouse | Hypothalamus | Proteome | 263 | none |
| Brainstem | Proteome | 254 | none | |||
| Adrenal gland | Proteome | 37 | none | |||
| WAT | Proteome | 34 | none | |||
| Liver | Proteome | 457 | MANF | |||
| Pituitary | Proteome | 184 | SRP14 | |||
| Bochukova EG, 2018 [30] | Through RNAseq, the post-mortem hypothalamic tissue of four subjects with PWS was compared with age and BMI-matched subjects with non-syndromic obesity | Human | Tissue | 574 | 2980 | CD63, FKBP5, NAA10, PTPN1, TNFRSF12A |
| Burnett LC, 2017 [45] | Induced pluripotent stem cell-derived (iPSC-derived) neurons from 2 subjects with PWS with large deletion (LD) and two subjects with PWS with microdeletion (MD), iPSC-derived neurons were also differentiated from 9 unaffected individuals | Human | Cells | Transcriptome (GSE89991 Analyzed by GEO2R) | 100 (adj p-value) | none |
| Yazdi PG [46] | Skeletal (quadriceps) and whole brain samples from 3 mice with PWS-IC del and their WT age-matched littermates (n = 4) were analyzed by microarray | Mouse | Muscle | Transcriptome (GSE41759) | 16 (adj p-value) | none |
| Brain | Transcriptome (GSE41759) | 24 (adj p-value) | none | |||
| Salles J, 2021 [47] | Seven subjects (five with deletions of the PWS locus, one with a microdeletion of SNORD116, and one with a frameshift mutation of MAGEL2 with Schaaf–Yang syndrome), were compared to two control patients | Human | Blood | genome-wide methylation analysis | Top 50 hypomethylated and top 50 hypermethylated | none |
| Victor AK, 2023 [48] | Dental pulp stem cell (DPSC)-derived neurons from 4 neurotypical controls and 12 subjects with PWS (4 with deletion 8 with UPD) | Human | Cells | Transcriptome (GSE178687) | 250 (adj p-value) | none |
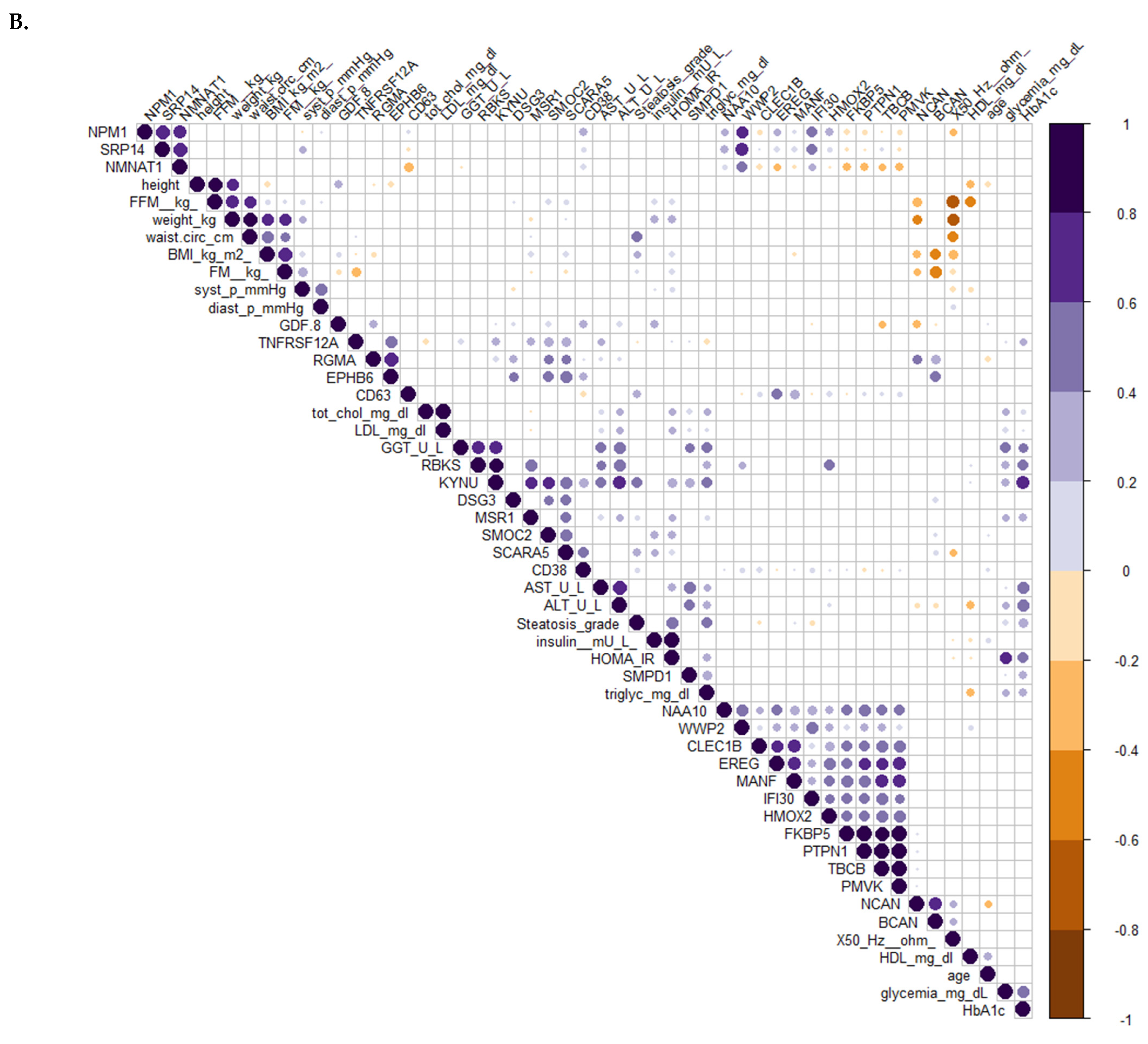
3.5. Difference in the Circulating Protein Profiles Associated with Variations in the Metabolic Profile between the Two Groups
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lionti, T.; Reid, S.M.; White, S.M.; Rowell, M.M. A Population-Based Profile of 160 Australians with Prader-Willi Syndrome: Trends in Diagnosis, Birth Prevalence and Birth Characteristics. Am. J. Med. Genet. A 2015, 167A, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.G.; Hartin, S.N.; Hossain, W.A.; Manzardo, A.M.; Kimonis, V.; Dykens, E.; Gold, J.A.; Kim, S.-J.; Weisensel, N.; Tamura, R.; et al. Molecular Genetic Classification in Prader-Willi Syndrome: A Multisite Cohort Study. J. Med. Genet. 2019, 56, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Angulo, M.A.; Butler, M.G.; Cataletto, M.E. Prader-Willi Syndrome: A Review of Clinical, Genetic, and Endocrine Findings. J. Endocrinol. Investig. 2015, 38, 1249–1263. [Google Scholar] [CrossRef] [PubMed]
- Pacoricona Alfaro, D.L.; Lemoine, P.; Ehlinger, V.; Molinas, C.; Diene, G.; Valette, M.; Pinto, G.; Coupaye, M.; Poitou-Bernert, C.; Thuilleaux, D.; et al. Causes of Death in Prader-Willi Syndrome: Lessons from 11 Years’ Experience of a National Reference Center. Orphanet J. Rare Dis. 2019, 14, 238. [Google Scholar] [CrossRef]
- Cassidy, S.B.; Schwartz, S.; Miller, J.L.; Driscoll, D.J. Prader-Willi Syndrome. Genet. Med. 2012, 14, 10–26. [Google Scholar] [CrossRef]
- Tauber, M.; Hoybye, C. Endocrine Disorders in Prader-Willi Syndrome: A Model to Understand and Treat Hypothalamic Dysfunction. Lancet Diabetes Endocrinol. 2021, 9, 235–246. [Google Scholar] [CrossRef]
- Heksch, R.; Kamboj, M.; Anglin, K.; Obrynba, K. Review of Prader-Willi Syndrome: The Endocrine Approach. Transl. Pediatr. 2017, 6, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, X.; Zhao, Y.; Wang, Q.; Zhang, Y.; Gao, H.; Zhang, B.; Cui, W.; Zhao, Y. Genetic Subtypes and Phenotypic Characteristics of 110 Patients with Prader-Willi Syndrome. Ital. J. Pediatr. 2022, 48, 121. [Google Scholar] [CrossRef]
- Ozçelik, T.; Leff, S.; Robinson, W.; Donlon, T.; Lalande, M.; Sanjines, E.; Schinzel, A.; Francke, U. Small Nuclear Ribonucleoprotein Polypeptide N (SNRPN), an Expressed Gene in the Prader-Willi Syndrome Critical Region. Nat. Genet. 1992, 2, 265–269. [Google Scholar] [CrossRef]
- Boccaccio, I.; Glatt-Deeley, H.; Watrin, F.; Roëckel, N.; Lalande, M.; Muscatelli, F. The Human MAGEL2 Gene and Its Mouse Homologue Are Paternally Expressed and Mapped to the Prader-Willi Region. Hum. Mol. Genet. 1999, 8, 2497–2505. [Google Scholar] [CrossRef]
- Butler, M.G.; Manzardo, A.M.; Forster, J.L. Prader-Willi Syndrome: Clinical Genetics and Diagnostic Aspects with Treatment Approaches. Curr. Pediatr. Rev. 2016, 12, 136–166. [Google Scholar] [CrossRef] [PubMed]
- Neumann, L.C.; Markaki, Y.; Mladenov, E.; Hoffmann, D.; Buiting, K.; Horsthemke, B. The Imprinted NPAP1/C15orf2 Gene in the Prader-Willi Syndrome Region Encodes a Nuclear Pore Complex Associated Protein. Hum. Mol. Genet. 2012, 21, 4038–4048. [Google Scholar] [CrossRef] [PubMed]
- Glenn, C.C.; Porter, K.A.; Jong, M.T.; Nicholls, R.D.; Driscoll, D.J. Functional Imprinting and Epigenetic Modification of the Human SNRPN Gene. Hum. Mol. Genet. 1993, 2, 2001–2005. [Google Scholar] [CrossRef]
- Buiting, K.; Nazlican, H.; Galetzka, D.; Wawrzik, M.; Gross, S.; Horsthemke, B. C15orf2 and a Novel Noncoding Transcript from the Prader-Willi/Angelman Syndrome Region Show Monoallelic Expression in Fetal Brain. Genomics 2007, 89, 588–595. [Google Scholar] [CrossRef][Green Version]
- Sutcliffe, J.S.; Nakao, M.; Christian, S.; Orstavik, K.H.; Tommerup, N.; Ledbetter, D.H.; Beaudet, A.L. Deletions of a Differentially Methylated CpG Island at the SNRPN Gene Define a Putative Imprinting Control Region. Nat. Genet. 1994, 8, 52–58. [Google Scholar] [CrossRef]
- Jiang, Y.; Wauki, K.; Liu, Q.; Bressler, J.; Pan, Y.; Kashork, C.D.; Shaffer, L.G.; Beaudet, A.L. Genomic Analysis of the Chromosome 15q11-Q13 Prader-Willi Syndrome Region and Characterization of Transcripts for GOLGA8E and WHCD1L1 from the Proximal Breakpoint Region. BMC Genom. 2008, 9, 50. [Google Scholar] [CrossRef] [PubMed]
- Sledziowska, M.; Winczura, K.; Jones, M.; Almaghrabi, R.; Mischo, H.; Hebenstreit, D.; Garcia, P.; Grzechnik, P. Non-Coding RNAs Associated with Prader-Willi Syndrome Regulate Transcription of Neurodevelopmental Genes in Human Induced Pluripotent Stem Cells. Hum. Mol. Genet. 2023, 32, 608–620. [Google Scholar] [CrossRef] [PubMed]
- Miller, N.L.G.; Wevrick, R.; Mellon, P.L. Necdin, a Prader-Willi Syndrome Candidate Gene, Regulates Gonadotropin-Releasing Hormone Neurons during Development. Hum. Mol. Genet. 2009, 18, 248–260. [Google Scholar] [CrossRef]
- Fujiwara, K.; Hasegawa, K.; Ohkumo, T.; Miyoshi, H.; Tseng, Y.-H.; Yoshikawa, K. Necdin Controls Proliferation of White Adipocyte Progenitor Cells. PLoS ONE 2012, 7, e30948. [Google Scholar] [CrossRef]
- Dong, Y.; Lu, R.; Cao, H.; Zhang, J.; Wu, X.; Deng, Y.; Li, J.-D. Deficiency in Prader-Willi Syndrome Gene Necdin Leads to Attenuated Cardiac Contractility. iScience 2024, 27, 109974. [Google Scholar] [CrossRef]
- Mercer, R.E.; Wevrick, R. Loss of Magel2, a Candidate Gene for Features of Prader-Willi Syndrome, Impairs Reproductive Function in Mice. PLoS ONE 2009, 4, e4291. [Google Scholar] [CrossRef] [PubMed]
- Tennese, A.A.; Wevrick, R. Impaired Hypothalamic Regulation of Endocrine Function and Delayed Counterregulatory Response to Hypoglycemia in Magel2-Null Mice. Endocrinology 2011, 152, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, A.; Copping, N.A.; Onaga, B.; Pride, M.C.; Coulson, R.L.; Yang, M.; Yasui, D.H.; LaSalle, J.M.; Silverman, J.L. Cognitive Deficits in the Snord116 Deletion Mouse Model for Prader-Willi Syndrome. Neurobiol. Learn. Mem. 2019, 165, 106874. [Google Scholar] [CrossRef] [PubMed]
- Polex-Wolf, J.; Lam, B.Y.H.; Larder, R.; Tadross, J.; Rimmington, D.; Bosch, F.; Cenzano, V.J.; Ayuso, E.; Ma, M.K.L.; Rainbow, K.; et al. Hypothalamic Loss of Snord116 Recapitulates the Hyperphagia of Prader-Willi Syndrome. J. Clin. Investig. 2018, 128, 960–969. [Google Scholar] [CrossRef]
- Lee, M.-S.; Lin, Y.-S.; Deng, Y.-F.; Hsu, W.-T.; Shen, C.-C.; Cheng, Y.-H.; Huang, Y.-T.; Li, C. Modulation of Alternative Splicing by Expression of Small Nuclear Ribonucleoprotein Polypeptide N. FEBS J. 2014, 281, 5194–5207. [Google Scholar] [CrossRef]
- Saltzman, A.L.; Pan, Q.; Blencowe, B.J. Regulation of Alternative Splicing by the Core Spliceosomal Machinery. Genes. Dev. 2011, 25, 373–384. [Google Scholar] [CrossRef]
- Schmauss, C.; Brines, M.L.; Lerner, M.R. The Gene Encoding the Small Nuclear Ribonucleoprotein-Associated Protein N Is Expressed at High Levels in Neurons. J. Biol. Chem. 1992, 267, 8521–8529. [Google Scholar] [CrossRef]
- Li, H.; Zhao, P.; Xu, Q.; Shan, S.; Hu, C.; Qiu, Z.; Xu, X. The Autism-Related Gene SNRPN Regulates Cortical and Spine Development via Controlling Nuclear Receptor Nr4a1. Sci. Rep. 2016, 6, 29878. [Google Scholar] [CrossRef]
- Safe, S.; Jin, U.-H.; Morpurgo, B.; Abudayyeh, A.; Singh, M.; Tjalkens, R.B. Nuclear Receptor 4A (NR4A) Family—Orphans No More. J. Steroid Biochem. Mol. Biol. 2016, 157, 48–60. [Google Scholar] [CrossRef]
- Bochukova, E.G.; Lawler, K.; Croizier, S.; Keogh, J.M.; Patel, N.; Strohbehn, G.; Lo, K.K.; Humphrey, J.; Hokken-Koelega, A.; Damen, L.; et al. A Transcriptomic Signature of the Hypothalamic Response to Fasting and BDNF Deficiency in Prader-Willi Syndrome. Cell Rep. 2018, 22, 3401–3408. [Google Scholar] [CrossRef]
- Lukaski, H.C. Methods for the Assessment of Human Body Composition: Traditional and New. Am. J. Clin. Nutr. 1987, 46, 537–556. [Google Scholar] [CrossRef] [PubMed]
- Bedogni, G.; Agosti, F.; De Col, A.; Marazzi, N.; Tagliaferri, A.; Sartorio, A. Comparison of Dual-Energy X-Ray Absorptiometry, Air Displacement Plethysmography and Bioelectrical Impedance Analysis for the Assessment of Body Composition in Morbidly Obese Women. Eur. J. Clin. Nutr. 2013, 67, 1129–1132. [Google Scholar] [CrossRef]
- Wallace, T.M.; Levy, J.C.; Matthews, D.R. Use and Abuse of HOMA Modeling. Diabetes Care 2004, 27, 1487–1495. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis Model Assessment: Insulin Resistance and β-Cell Function from Fasting Plasma Glucose and Insulin Concentrations in Man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape Provides a Biologist-Oriented Resource for the Analysis of Systems-Level Datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef]
- Raudvere, U.; Kolberg, L.; Kuzmin, I.; Arak, T.; Adler, P.; Peterson, H.; Vilo, J. G:Profiler: A Web Server for Functional Enrichment Analysis and Conversions of Gene Lists (2019 Update). Nucleic Acids Res. 2019, 47, W191–W198. [Google Scholar] [CrossRef]
- Piñero, J.; Bravo, À.; Queralt-Rosinach, N.; Gutiérrez-Sacristán, A.; Deu-Pons, J.; Centeno, E.; García-García, J.; Sanz, F.; Furlong, L.I. DisGeNET: A Comprehensive Platform Integrating Information on Human Disease-Associated Genes and Variants. Nucleic Acids Res. 2017, 45, D833–D839. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING Database in 2023: Protein-Protein Association Networks and Functional Enrichment Analyses for Any Sequenced Genome of Interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef] [PubMed]
- Heberle, H.; Meirelles, G.V.; da Silva, F.R.; Telles, G.P.; Minghim, R. InteractiVenn: A Web-Based Tool for the Analysis of Sets through Venn Diagrams. BMC Bioinform. 2015, 16, 169. [Google Scholar] [CrossRef]
- Schemper, M. Predictive Accuracy and Explained Variation. Stat. Med. 2003, 22, 2299–2308. [Google Scholar] [CrossRef]
- Carl, R. Bacon Practical Portfolio Performance Measurement and Attribution, 2nd ed.; John Wiley & Sons: Chicester, UK, 2008; ISBN 978-0-470-05928-9. [Google Scholar]
- Friendly, M. Corrgrams. Am. Stat. 2002, 56, 316–324. [Google Scholar] [CrossRef]
- Chen, H.; Victor, A.K.; Klein, J.; Tacer, K.F.; Tai, D.J.C.; de Esch, C.; Nuttle, A.; Temirov, J.; Burnett, L.C.; Rosenbaum, M.; et al. Loss of MAGEL2 in Prader-Willi Syndrome Leads to Decreased Secretory Granule and Neuropeptide Production. JCI Insight 2020, 5, 138576. [Google Scholar] [CrossRef]
- Bittel, D.C.; Kibiryeva, N.; Sell, S.M.; Strong, T.V.; Butler, M.G. Whole Genome Microarray Analysis of Gene Expression in Prader–Willi Syndrome. Am. J. Med. Genet. A 2007, 143A, 430–442. [Google Scholar] [CrossRef]
- Burnett, L.C.; LeDuc, C.A.; Sulsona, C.R.; Paull, D.; Rausch, R.; Eddiry, S.; Carli, J.F.M.; Morabito, M.V.; Skowronski, A.A.; Hubner, G.; et al. Deficiency in Prohormone Convertase PC1 Impairs Prohormone Processing in Prader-Willi Syndrome. J. Clin. Investig. 2017, 127, 293–305. [Google Scholar] [CrossRef]
- Yazdi, P.G.; Su, H.; Ghimbovschi, S.; Fan, W.; Coskun, P.E.; Nalbandian, A.; Knoblach, S.; Resnick, J.L.; Hoffman, E.; Wallace, D.C.; et al. Differential Gene Expression Reveals Mitochondrial Dysfunction in an Imprinting Center Deletion Mouse Model of Prader-Willi Syndrome. Clin. Transl. Sci. 2013, 6, 347–355. [Google Scholar] [CrossRef]
- Salles, J.; Eddiry, S.; Lacassagne, E.; Laurier, V.; Molinas, C.; Bieth, É.; Franchitto, N.; Salles, J.-P.; Tauber, M. Patients with PWS and Related Syndromes Display Differentially Methylated Regions Involved in Neurodevelopmental and Nutritional Trajectory. Clin. Epigenetics 2021, 13, 159. [Google Scholar] [CrossRef] [PubMed]
- Victor, A.K.; Hedgecock, T.; Donaldson, M.; Johnson, D.; Rand, C.M.; Weese-Mayer, D.E.; Reiter, L.T. Analysis and Comparisons of Gene Expression Changes in Patient- Derived Neurons from ROHHAD, CCHS, and PWS. Front. Pediatr. 2023, 11, 1090084. [Google Scholar] [CrossRef] [PubMed]
- Szláma, G.; Kondás, K.; Trexler, M.; Patthy, L. WFIKKN1 and WFIKKN2 Bind Growth Factors TGFβ1, BMP2 and BMP4 but Do Not Inhibit Their Signalling Activity. FEBS J. 2010, 277, 5040–5050. [Google Scholar] [CrossRef]
- Kondás, K.; Szláma, G.; Nagy, A.; Trexler, M.; Patthy, L. Biological Functions of the WAP Domain-Containing Multidomain Proteins WFIKKN1 and WFIKKN2. Biochem. Soc. Trans. 2011, 39, 1416–1420. [Google Scholar] [CrossRef]
- Kondás, K.; Szláma, G.; Trexler, M.; Patthy, L. Both WFIKKN1 and WFIKKN2 Have High Affinity for Growth and Differentiation Factors 8 and 11*. J. Biol. Chem. 2008, 283, 23677–23684. [Google Scholar] [CrossRef]
- Aoki, M.S.; Soares, A.G.; Miyabara, E.H.; Baptista, I.L.; Moriscot, A.S. Expression of Genes Related to Myostatin Signaling during Rat Skeletal Muscle Longitudinal Growth. Muscle Nerve 2009, 40, 992–999. [Google Scholar] [CrossRef] [PubMed]
- Semba, R.D.; Zhang, P.; Zhu, M.; Fabbri, E.; Gonzalez-Freire, M.; Carlson, O.D.; Moaddel, R.; Tanaka, T.; Egan, J.M.; Ferrucci, L. Relationship of Circulating Growth and Differentiation Factors 8 and 11 and Their Antagonists as Measured Using Liquid Chromatography–Tandem Mass Spectrometry With Age and Skeletal Muscle Strength in Healthy Adults. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Burch, P.M.; Pogoryelova, O.; Palandra, J.; Goldstein, R.; Bennett, D.; Fitz, L.; Guglieri, M.; Bettolo, C.M.; Straub, V.; Evangelista, T.; et al. Reduced Serum Myostatin Concentrations Associated with Genetic Muscle Disease Progression. J. Neurol. 2017, 264, 541–553. [Google Scholar] [CrossRef]
- Mariot, V.; Joubert, R.; Hourdé, C.; Féasson, L.; Hanna, M.; Muntoni, F.; Maisonobe, T.; Servais, L.; Bogni, C.; Le Panse, R.; et al. Downregulation of Myostatin Pathway in Neuromuscular Diseases May Explain Challenges of Anti-Myostatin Therapeutic Approaches. Nat. Commun. 2017, 8, 1859. [Google Scholar] [CrossRef]
- Baczek, J.; Silkiewicz, M.; Wojszel, Z.B. Myostatin as a Biomarker of Muscle Wasting and Other Pathologies-State of the Art and Knowledge Gaps. Nutrients 2020, 12, 2401. [Google Scholar] [CrossRef] [PubMed]
- Furihata, T.; Kinugawa, S.; Fukushima, A.; Takada, S.; Homma, T.; Masaki, Y.; Abe, T.; Yokota, T.; Oba, K.; Okita, K.; et al. Serum Myostatin Levels Are Independently Associated with Skeletal Muscle Wasting in Patients with Heart Failure. Int. J. Cardiol. 2016, 220, 483–487. [Google Scholar] [CrossRef]
- Wang, S.; Fang, L.; Cong, L.; Chung, J.P.W.; Li, T.C.; Chan, D.Y.L. Myostatin: A Multifunctional Role in Human Female Reproduction and Fertility—A Short Review. Reprod. Biol. Endocrinol. 2022, 20, 96. [Google Scholar] [CrossRef]
- Carvalho, L.P.; Basso-Vanelli, R.P.; Di Thommazo-Luporini, L.; Mendes, R.G.; Oliveira-Junior, M.C.; Vieira, R.d.P.; Bonjorno-Junior, J.C.; Oliveira, C.R.; Luporini, R.; Borghi-Silva, A. Myostatin and Adipokines: The Role of the Metabolically Unhealthy Obese Phenotype in Muscle Function and Aerobic Capacity in Young Adults. Cytokine 2018, 107, 118–124. [Google Scholar] [CrossRef]
- Milev, P.; Maurel, P.; Chiba, A.; Mevissen, M.; Popp, S.; Yamaguchi, Y.; Margolis, R.K.; Margolis, R.U. Differential Regulation of Expression of Hyaluronan-Binding Proteoglycans in Developing Brain: Aggrecan, Versican, Neurocan, and Brevican. Biochem. Biophys. Res. Commun. 1998, 247, 207–212. [Google Scholar] [CrossRef]
- Zhou, X.H.; Brakebusch, C.; Matthies, H.; Oohashi, T.; Hirsch, E.; Moser, M.; Krug, M.; Seidenbecher, C.I.; Boeckers, T.M.; Rauch, U.; et al. Neurocan Is Dispensable for Brain Development. Mol. Cell Biol. 2001, 21, 5970–5978. [Google Scholar] [CrossRef]
- Minta, K.; Brinkmalm, G.; Thelin, E.P.; Al Nimer, F.; Piehl, F.; Tullberg, M.; Jeppsson, A.; Portelius, E.; Zetterberg, H.; Blennow, K.; et al. Cerebrospinal Fluid Brevican and Neurocan Fragment Patterns in Human Traumatic Brain Injury. Clin. Chim. Acta 2021, 512, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Minta, K.; Brinkmalm, G.; Portelius, E.; Johansson, P.; Svensson, J.; Kettunen, P.; Wallin, A.; Zetterberg, H.; Blennow, K.; Andreasson, U. Brevican and Neurocan Peptides as Potential Cerebrospinal Fluid Biomarkers for Differentiation Between Vascular Dementia and Alzheimer’s Disease. J. Alzheimers Dis. 2021, 79, 729–741. [Google Scholar] [CrossRef] [PubMed]
- Hußler, W.; Höhn, L.; Stolz, C.; Vielhaber, S.; Garz, C.; Schmitt, F.C.; Gundelfinger, E.D.; Schreiber, S.; Seidenbecher, C.I. Brevican and Neurocan Cleavage Products in the Cerebrospinal Fluid—Differential Occurrence in ALS, Epilepsy and Small Vessel Disease. Front. Cell Neurosci. 2022, 16, 838432. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Zeng, X.; Li, H.; Ju, L.; Feng, J.; Yang, J. Repulsive Guidance Molecule-a and Central Nervous System Diseases. Biomed. Res. Int. 2021, 2021, 5532116. [Google Scholar] [CrossRef] [PubMed]
- Courage, C.; Houge, G.; Gallati, S.; Schjelderup, J.; Rieubland, C. 15q26.1 Microdeletion Encompassing Only CHD2 and RGMA in Two Adults with Moderate Intellectual Disability, Epilepsy and Truncal Obesity. Eur. J. Med. Genet. 2014, 57, 520–523. [Google Scholar] [CrossRef]
- Jin, D.; Liu, H.-X.; Hirai, H.; Torashima, T.; Nagai, T.; Lopatina, O.; Shnayder, N.A.; Yamada, K.; Noda, M.; Seike, T.; et al. CD38 Is Critical for Social Behaviour by Regulating Oxytocin Secretion. Nature 2007, 446, 41–45. [Google Scholar] [CrossRef]
- Salmina, A.B.; Lopatina, O.; Ekimova, M.V.; Mikhutkina, S.V.; Higashida, H. CD38/Cyclic ADP-Ribose System: A New Player for Oxytocin Secretion and Regulation of Social Behaviour. J. Neuroendocrinol. 2010, 22, 380–392. [Google Scholar] [CrossRef] [PubMed]
- Alberati-Giani, D.; Buchli, R.; Malherbe, P.; Broger, C.; Lang, G.; Köhler, C.; Lahm, H.W.; Cesura, A.M. Isolation and Expression of a cDNA Clone Encoding Human Kynureninase. Eur. J. Biochem. 1996, 239, 460–468. [Google Scholar] [CrossRef]
- Shi, H.; Enriquez, A.; Rapadas, M.; Martin, E.M.M.A.; Wang, R.; Moreau, J.; Lim, C.K.; Szot, J.O.; Ip, E.; Hughes, J.N.; et al. NAD Deficiency, Congenital Malformations, and Niacin Supplementation. N. Engl. J. Med. 2017, 377, 544–552. [Google Scholar] [CrossRef]
- Schweiger, M.; Hennig, K.; Lerner, F.; Niere, M.; Hirsch-Kauffmann, M.; Specht, T.; Weise, C.; Oei, S.L.; Ziegler, M. Characterization of Recombinant Human Nicotinamide Mononucleotide Adenylyl Transferase (NMNAT), a Nuclear Enzyme Essential for NAD Synthesis. FEBS Lett. 2001, 492, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Reichova, A.; Schaller, F.; Bukatova, S.; Bacova, Z.; Muscatelli, F.; Bakos, J. The Impact of Oxytocin on Neurite Outgrowth and Synaptic Proteins in Magel2-Deficient Mice. Dev. Neurobiol. 2021, 81, 366–388. [Google Scholar] [CrossRef] [PubMed]
- Ates, T.; Oncul, M.; Dilsiz, P.; Topcu, I.C.; Civas, C.C.; Alp, M.I.; Aklan, I.; Ates Oz, E.; Yavuz, Y.; Yilmaz, B.; et al. Inactivation of Magel2 Suppresses Oxytocin Neurons through Synaptic Excitation-Inhibition Imbalance. Neurobiol. Dis. 2019, 121, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Camerino, C. Oxytocin’s Regulation of Thermogenesis May Be the Link to Prader-Willi Syndrome. Curr. Issues Mol. Biol. 2023, 45, 4923–4935. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.L.; Tamura, R.; Butler, M.G.; Kimonis, V.; Sulsona, C.; Gold, J.-A.; Driscoll, D.J. Oxytocin Treatment in Children with Prader-Willi Syndrome: A Double-Blind, Placebo-Controlled, Crossover Study. Am. J. Med. Genet. A 2017, 173, 1243–1250. [Google Scholar] [CrossRef]
- Badawy, A.A.-B. Kynurenine Pathway of Tryptophan Metabolism: Regulatory and Functional Aspects. Int. J. Tryptophan Res. 2017, 10, 1178646917691938. [Google Scholar] [CrossRef]
- Jayaram, H.N.; Kusumanchi, P.; Yalowitz, J.A. NMNAT Expression and Its Relation to NAD Metabolism. Curr. Med. Chem. 2011, 18, 1962–1972. [Google Scholar] [CrossRef]
- Park, J.; van Koeverden, P.; Singh, B.; Gupta, R.S. Identification and Characterization of Human Ribokinase and Comparison of Its Properties with E. Coli Ribokinase and Human Adenosine Kinase. FEBS Lett. 2007, 581, 3211–3216. [Google Scholar] [CrossRef]
- Gudgeon, J.; Marín-Rubio, J.L.; Trost, M. The Role of Macrophage Scavenger Receptor 1 (MSR1) in Inflammatory Disorders and Cancer. Front. Immunol. 2022, 13, 1012002. [Google Scholar] [CrossRef]
- Krefft, M.; Frydecka, D.; Zalsman, G.; Krzystek-Korpacka, M.; Śmigiel, R.; Gębura, K.; Bogunia-Kubik, K.; Misiak, B. A Pro-Inflammatory Phenotype Is Associated with Behavioural Traits in Children with Prader-Willi Syndrome. Eur. Child. Adolesc. Psychiatry 2021, 30, 899–908. [Google Scholar] [CrossRef] [PubMed]
- Theodoro, M.F.; Talebizadeh, Z.; Butler, M.G. Body Composition and Fatness Patterns in Prader-Willi Syndrome: Comparison with Simple Obesity. Obesity 2006, 14, 1685–1690. [Google Scholar] [CrossRef]


| Variable | OB (n = 34) | PWS (n = 53) | p-Value |
|---|---|---|---|
| Age (years) | 35.2 ± 9.2 | 33.51 ± 10.9 | 0.45 |
| Gender Female (n, %) | 47% | 55% | 0.58 |
| BMI (kg/m2) | 41.1 ± 4.4 | 38.58 ± 8.94 | 0.13 |
| Waist circumference (cm) | 119.5 ± 14.3 | 120.87 ± 16.72 | 0.71 |
| FFM (%) | 54 ± 6.32 | 51.6 ± 7.2 | 0.12 |
| FM (kg) | 53.35 ± 8.73 | 44.62 ± 15.4 | 0.004 |
| Systolic pressure (mm Hg) | 129.4 ± 17.3 | 127.3 ± 9.6 | 0.46 |
| Diastolic pressure (mm Hg) | 80.6 ± 8.3 | 80 ± 6 | 0.69 |
| Fasting glucose (mg/dL) | 91.1 ± 14.8 | 101.7 ± 44.4 | 0.19 |
| Insulin (mU/L) | 19.8 ± 10.5 | 12.1 ± 7.31 | 0.00013 |
| HOMA-IR | 4.5 ± 2.7 | 3.1 ± 2.1 | 0.005 |
| HbA1c (%) | 5.5 ± 0.6 | 6 ± 1.6 | 0. 09 |
| Total cholesterol (mg/dL) | 184.8 ± 29.6 | 182.3 ± 36.5 | 0.74 |
| HDL cholesterol (mg/dL) | 43.9 ± 8.9 | 49.92 ± 14.2 | 0.03 |
| LDL cholesterol (mg/dL) | 124.3 ± 27.1 | 118 ± 29.5 | 0.32 |
| Triglycerides (mg/dL) | 140.6 ± 49.6 | 131.6.6 ± 100.72 | 0.63 |
| AST (U.I./L) | 23 ± 8.9 | 21.45 ± 11.2 | 0.51 |
| ALT (U.I./L) | 30.5 ± 16.5 | 28.7 ± 30 | 0.76 |
| GGT (U.I./L) | 42.8 ± 41.4 | 34.4 ± 58.7 | 0.47 |
| Acronym | Protein | UNIPROT | M (n = 24) | F (n = 29) | Difference | FC | Adj. p-Value |
|---|---|---|---|---|---|---|---|
| DDR1 | Epithelial discoidin domain-containing receptor 1 | Q08345 | 8.59 | 8.35 | −0.238 | 0.73 | 0.01 |
| WFIKKN1 | WAP, Kazal, immunoglobulin, Kunitz, and NTR domain-containing protein 1 | Q96NZ8 | 4.70 | 4.19 | −0.516 | 0.73 | 0.03 |
| GDF-8 | Growth/Differentiation Factor 8/Myostatin | O14793 | 3.93 | 3.21 | −0.723 | 0.58 | 0.04 |
| Acronym | Protein | UNIPROT | OB | PWS | Difference | FC | Adj. p-Val |
|---|---|---|---|---|---|---|---|
| RGMA | Repulsive Guidance Molecule BMP Co-Receptor A | Q96B86 | 11.5 | 11.1 | −0.463 | 0.73 | 3.306 × 10−5 |
| TNFRSF12A | TNF Receptor Superfamily Member 12A | Q9NP84 | 6.45 | 5.99 | −0.461 | 0.73 | 0.0003438 |
| GDF-8 | Growth/Differentiation Factor 8/Myostatin | O14793 | 4.33 | 3.55 | −0.788 | 0.58 | 0.0003438 |
| MANF | Mesencephalic Astrocyte-Derived Neurotrophic Factor | P55145 | 9.22 | 8.8 | −0.418 | 0.75 | 0.0003676 |
| IFI30 | Gamma-interferon-inducible lysosomal thiol reductase | P13284 | 5.67 | 5.08 | −0.593 | 0.66 | 0.0007137 |
| CLEC1B | C-Type Lectin Domain Family 1 Member B | Q9P126 | 12.5 | 12.1 | −0.36 | 0.78 | 0.001707 |
| BCAN | Brevican | Q96GW7 | 5.68 | 6.08 | 0.397 | 1.32 | 0.001707 |
| HMOX2 | Heme Oxygenase 2 | P30519 | 2.36 | 1.97 | −0.395 | 0.76 | 0.003391 |
| TBCB | Tubulin Folding Cofactor B | Q99426 | 5.45 | 4.58 | −0.872 | 0.55 | 0.003391 |
| PMVK 0 | Phosphomevalonate Kinase | Q15126 | 5.46 | 4.59 | −0.869 | 0.55 | 0.003391 |
| WWP2 | WW Domain Containing E3 Ubiquitin Protein Ligase 2 | O00308 | 5.48 | 5.11 | −0.373 | 0.77 | 0.003391 |
| EREG | Proepiregulin | O14944 | 5.19 | 4.34 | −0.843 | 0.56 | 0.003391 |
| NAA10 | N-Alpha-Acetyltransferase 10, NatA Catalytic Subunit | P41227 | 4.02 | 3.51 | −0.511 | 0.70 | 0.003391 |
| PTPN1 | Protein Tyrosine Phosphatase Non-Receptor Type 1 | P18031 | 4.87 | 4.25 | −0.627 | 0.65 | 0.005352 |
| FKBP5 | Peptidyl-prolyl cis-trans isomerase FKBP5 | Q13451 | 6.53 | 5.69 | −0.839 | 0.56 | 0.005503 |
| MSR1 | Macrophage Scavenger Receptor 1 | P21757 | 7.46 | 7.09 | −0.368 | 0.77 | 0.00734 |
| NCAN | Neurocan | O14594 | 8.59 | 8.87 | 0.28 | 1.21 | 0.007448 |
| KYNU | Kynureninase | Q16719 | 6.78 | 6.25 | −0.529 | 0.69 | 0.01025 |
| SCARA5 | Scavenger Receptor Class A Member 5 | Q6ZMJ2 | 8.02 | 7.84 | −0.178 | 0.88 | 0.01025 |
| CD38 | Cyclic ADP-Ribose Hydrolase 1 | P28907 | 5.36 | 5.07 | −0.296 | 0.81 | 0.01025 |
| EPHB6 | Ephrin type-B receptor 6 | O15197 | 7.46 | 7.73 | 0.266 | 1.20 | 0.01678 |
| CD63 | Tetraspanin-30/Ocular Melanoma-Associated Antigen | P08962 | 8.17 | 7.74 | −0.424 | 0.75 | 0.01686 |
| NMNAT1 | Nicotinamide Nucleotide Adenylyltransferase 1 | Q9HAN9 | 5.92 | 5.3 | −0.626 | 0.65 | 0.01796 |
| SMPD1 | Sphingomyelin Phosphodiesterase 1 | P17405 | 5.6 | 5.91 | 0.314 | 1.24 | 0.01796 |
| SMOC2 | SPARC Related Modular Calcium Binding 2 | Q9H3U7 | 10.3 | 10 | −0.256 | 0.84 | 0.02182 |
| DSG3 | Desmoglein 3 | P32926 | 4.7 | 4.99 | 0.288 | 1.22 | 0.02272 |
| NPM1 | Nucleophosmin 1 | P06748 | 4.45 | 4.02 | −0.432 | 0.74 | 0.03768 |
| SRP14 | Signal Recognition Particle 14 | P37108 | 5.78 | 5.27 | −0.504 | 0.71 | 0.04092 |
| RBKS | Ribokinase | Q9H477 | 7.84 | 7.44 | −0.403 | 0.76 | 0.04723 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pascut, D.; Giraudi, P.J.; Banfi, C.; Ghilardi, S.; Tiribelli, C.; Bondesan, A.; Caroli, D.; Grugni, G.; Sartorio, A. Characterization of Circulating Protein Profiles in Individuals with Prader–Willi Syndrome and Individuals with Non-Syndromic Obesity. J. Clin. Med. 2024, 13, 5697. https://doi.org/10.3390/jcm13195697
Pascut D, Giraudi PJ, Banfi C, Ghilardi S, Tiribelli C, Bondesan A, Caroli D, Grugni G, Sartorio A. Characterization of Circulating Protein Profiles in Individuals with Prader–Willi Syndrome and Individuals with Non-Syndromic Obesity. Journal of Clinical Medicine. 2024; 13(19):5697. https://doi.org/10.3390/jcm13195697
Chicago/Turabian StylePascut, Devis, Pablo José Giraudi, Cristina Banfi, Stefania Ghilardi, Claudio Tiribelli, Adele Bondesan, Diana Caroli, Graziano Grugni, and Alessandro Sartorio. 2024. "Characterization of Circulating Protein Profiles in Individuals with Prader–Willi Syndrome and Individuals with Non-Syndromic Obesity" Journal of Clinical Medicine 13, no. 19: 5697. https://doi.org/10.3390/jcm13195697
APA StylePascut, D., Giraudi, P. J., Banfi, C., Ghilardi, S., Tiribelli, C., Bondesan, A., Caroli, D., Grugni, G., & Sartorio, A. (2024). Characterization of Circulating Protein Profiles in Individuals with Prader–Willi Syndrome and Individuals with Non-Syndromic Obesity. Journal of Clinical Medicine, 13(19), 5697. https://doi.org/10.3390/jcm13195697







