Unraveling of Molecular Mechanisms of Cognitive Frailty in Chronic Kidney Disease: How Exercise Makes a Difference
Abstract
:1. Introduction
2. Mechanisms Underlying the Pathogenesis of Cognitive Frailty in CKD
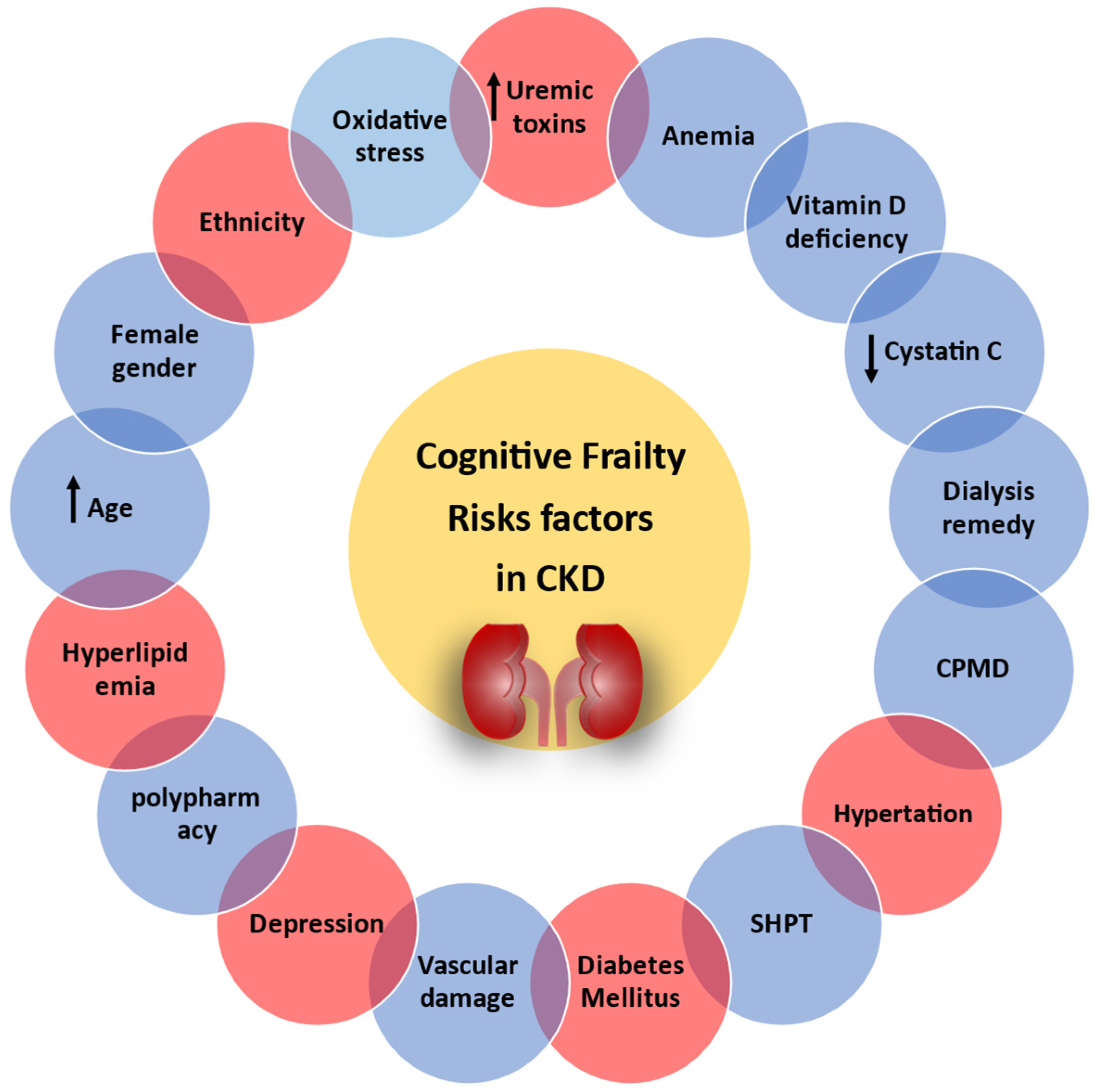
3. Risk Factors
3.1. Age, Oxidative Stress, and Inflammation
3.2. Female Gender
3.3. HD Remedy
3.4. Secondary Hyperparathyroidism
3.5. Anemia
3.6. Calcium and Phosphorus Metabolism Disorder
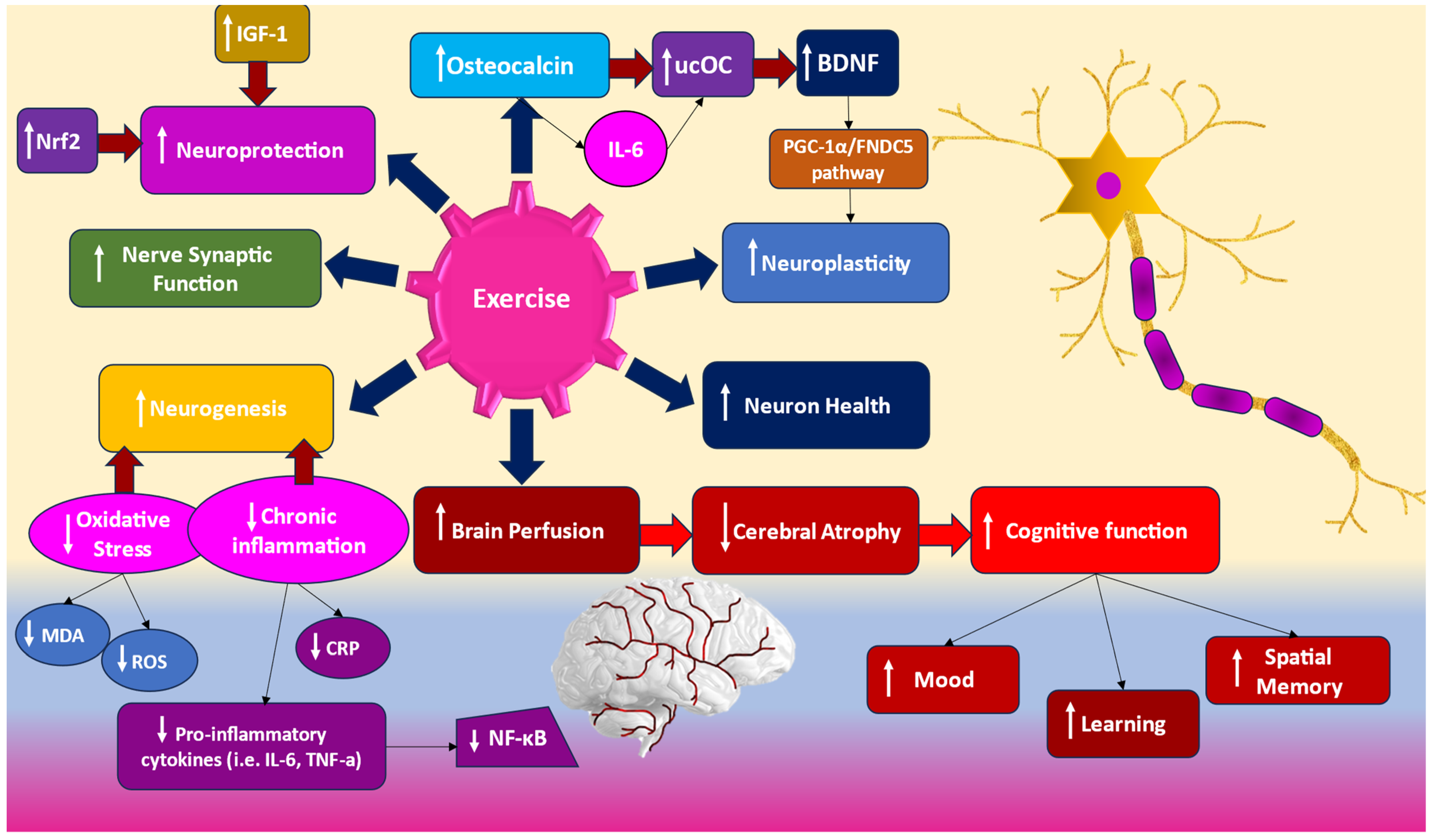
4. Therapeutic Interventions: How Does Exercise Makes a Difference?
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bello, A.K.; Okpechi, I.G.; Levin, A.; Ye, F.; Saad, S.; Zaidi, D. ISN–Global Kidney Health Atlas: A report by the International Society of Nephrology: An Assessment of Global Kidney Health Care Status Focussing on Capacity, Availability, Accessibility, Affordability and Outcomes of Kidney Disease; International Society of Nephrology: Brussels, Belgium, 2023; pp. 1–198. [Google Scholar]
- Xie, Y.; Bowe, B.; Mokdad, A.H.; Xian, H.; Yan, Y.; Li, T.; Maddukuri, G.; Tsai, C.-Y.; Floyd, T.; Al-Aly, Z. Analysis of the Global Burden of Disease study highlights the global, regional, and national trends of chronic kidney disease epidemiology from 1990 to 2016. Kidney Int. 2018, 94, 567–581. [Google Scholar] [CrossRef] [PubMed]
- Koushik, N.S.; McArthur, S.F.; Baird, A.D. Adult Chronic Kidney Disease: Neurocognition in Chronic Renal Failure. Neuropsychol. Rev. 2010, 20, 33–51. [Google Scholar] [CrossRef] [PubMed]
- Neumann, D.; Robinski, M.; Mau, W.; Girndt, M. Cognitive Testing in Patients with CKD: The Problem of Missing Cases. Clin. J. Am. Soc. Nephrol. 2017, 12, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Griva, K.; Thompson, D.; Jayasena, D.; Davenport, A.; Harrison, M.; Newman, S.P. Cognitive functioning pre-to post-kidney trans-plantation--a prospective study. Nephrol. Dial. Transpl. 2006, 21, 3275–3282. [Google Scholar] [CrossRef]
- Kurella, M.; Chertow, G.M.; Luan, J.; Yaffe, K. Cognitive Impairment in Chronic Kidney Disease. J. Am. Geriatr. Soc. 2004, 52, 1863–1869. [Google Scholar] [CrossRef]
- Nulsen, R.S.; Yaqoob, M.M.; Mahon, A.; Stoby-Fields, M.; Kelly, M.; Varagunam, M. Prevalence of Cognitive Impairment in Patients Attending Pre-Dialysis Clinic. J. Ren. Care 2008, 34, 121–126. [Google Scholar] [CrossRef]
- Griva, K.; Newman, S.P.; Harrison, M.J.; Hankins, M.; Davenport, A.; Hansraj, S.; Thompson, D. Acute Neuropsychological Changes in Hemodialysis and Peritoneal Dialysis Patients. Health Psychol. 2003, 22, 570–578. [Google Scholar] [CrossRef]
- Gokal, R. Quality of life in patients undergoing renal replacement therapy. Kidney Int. Suppl. 1993, 40, S23–S27. [Google Scholar]
- Kurella, M.; Mapes, D.L.; Port, F.K.; Chertow, G.M. Correlates and outcomes of dementia among dialysis patients: The Dialysis Outcomes and Practice Patterns Study. Nephrol. Dial. Transpl. 2006, 21, 2543–2548. [Google Scholar] [CrossRef]
- Sehgal, A.R.; Grey, S.F.; DeOreo, P.B.; Whitehouse, P.J. Prevalence, recognition, and implications of mental impairment among hemodialysis patients. Am. J. Kidney Dis. 1997, 30, 41–49. [Google Scholar] [CrossRef]
- Sarnak, M.J.; Tighiouart, H.; Scott, T.M.; Lou, K.V.; Sorensen, E.P.; Giang, L.M.; Drew, D.A.; Shaffi, K.; Strom, J.A.; Singh, A.K.; et al. Frequency of and risk factors for poor cognitive performance in hemodialysis patients. Neurology 2013, 80, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in Older Adults: Evidence for a Phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M157. [Google Scholar] [CrossRef]
- Bakas, D. Frailty syndrome in the elderly. Hell. J. Nurs. Sci. 2020, 13, 14–19. [Google Scholar] [CrossRef]
- Luo, B.; Luo, Z.; Zhang, X.; Xu, M.; Shi, C. Status of cognitive frailty in elderly patients with chronic kidney disease and construction of a risk prediction model: A cross-sectional study. BMJ Open 2022, 12, e060633. [Google Scholar] [CrossRef] [PubMed]
- Rockwood, K.; Mitnitski, A. Limits to deficit accumulation in elderly people. Mech. Ageing Dev. 2006, 127, 494–496. [Google Scholar] [CrossRef] [PubMed]
- Kelaiditi, E.; Cesari, M.; Canevelli, M.; Van Kan, G.A.; Ousset, P.J.; Gillette-Guyonnet, S.; Ritz, P.; Duveau, F.; Soto, M.E.; Provencher, V.; et al. Cognitive frailty: Rational and definition from an (I.A.N.A./I.A.G.G.) International Consensus Group. J. Nutr. Health Aging 2013, 17, 726–734. [Google Scholar] [CrossRef]
- Tamura, M.K.; Yaffe, K. Dementia and cognitive impairment in ESRD: Diagnostic and therapeutic strategies. Kidney Int. 2011, 79, 14–22. [Google Scholar] [CrossRef]
- Perazza, L.R.; Brown-Borg, H.M.; Thompson, L.V. Physiological Systems in Promoting Frailty. Compr. Physiol. 2022, 12, 3575–3620. [Google Scholar]
- Chu, N.M.; Gross, A.L.; Shaffer, A.A.; Haugen, C.E.; Norman, S.P.; Xue, Q.-L.; Sharrett, A.R.; Carlson, M.C.; Bandeen-Roche, K.; Segev, D.L.; et al. Frailty and Changes in Cognitive Function after Kidney Transplantation. J. Am. Soc. Nephrol. 2019, 30, 336–345. [Google Scholar] [CrossRef]
- Yi, C.; Lin, J.; Cao, P.; Chen, J.; Zhou, T.; Yang, R.; Lu, S.; Yu, X.; Yang, X. Prevalence and Prognosis of Coexisting Frailty and Cognitive Impairment in Patients on Continuous Ambulatory Peritoneal Dialysis. Sci. Rep. 2018, 8, 17305. [Google Scholar] [CrossRef]
- McAdams-DeMarco, M.A.; Tan, J.; Salter, M.L.; Gross, A.; Meoni, L.A.; Jaar, B.G.; Kao, W.-H.L.; Parekh, R.S.; Segev, D.L.; Sozio, S.M. Frailty and Cognitive Function in Incident Hemodialysis Patients. Clin. J. Am. Soc. Nephrol. 2015, 10, 2181–2189. [Google Scholar] [CrossRef] [PubMed]
- Coppolino, G.; Bolignano, D.; Gareri, P.; Ruberto, C.; Andreucci, M.; Ruotolo, G.; Rocca, M.; Castagna, A. Kidney function and cognitive decline in frail elderly: Two faces of the same coin? Int. Urol. Nephrol. 2018, 50, 1505–1510. [Google Scholar] [CrossRef] [PubMed]
- Otobe, Y.; Hiraki, K.; Hotta, C.; Nishizawa, H.; Izawa, K.P.; Taki, Y.; Imai, N.; Sakurada, T.; Shibagaki, Y. Mild cognitive impairment in older adults with pre-dialysis patients with chronic kidney disease: Prevalence and association with physical function. Nephrology 2018, 24, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Chang, J.; Sun, Q. Research Progress on Cognitive Frailty in Older Adults with Chronic Kidney Disease. Kidney Blood Press. Res. 2024, 49, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Williams, U.E.; Owolabi, M.O.; Ogunniyi, A.; Ezunu, E.O. Prevalence and Pattern of Neurocognitive Impairment in Nigerians with Stages 3 to 5 Chronic Kidney Disease. ISRN Neurol. 2013, 2013, 374890. [Google Scholar] [CrossRef]
- Gela, Y.Y.; Getu, A.A.; Adane, A.; Ayal, B.M.; Akalu, Y.; Ambelu, A.; Diress, M.; Yeshaw, Y. Cognitive Impairment and Associated Factors Among Chronic Kidney Disease Patients: A Comparative Cross-Sectional Study. Neuropsychiatr. Dis. Treat. 2021, 17, 1483–1492. [Google Scholar] [CrossRef]
- Viji, P.C.; Sk, S.M. Chronic kidney disease and cognitive status. Int. J. Pharm. Sci. Res. 2009, 8, 2184–2199. [Google Scholar]
- Leinau, L.; Murphy, T.E.; Bradley, E.; Fried, T. Relationship between Conditions Addressed by Hemodialysis Guidelines and Non-ESRD-Specific Conditions Affecting Quality of Life. Clin. J. Am. Soc. Nephrol. 2009, 4, 572–578. [Google Scholar] [CrossRef]
- Cook, W.L.; Jassal, S.V. Functional dependencies among the elderly on hemodialysis. Kidney Int. 2008, 73, 1289–1295. [Google Scholar] [CrossRef]
- Brady, C.B.; Gaziano, J.M.; Cxypoliski, R.A.; Guarino, P.D.; Kaufman, J.S.; Warren, S.R.; Hartigan, P.; Goldfarb, D.S.; Jamison, R.L. Homocysteine Lowering and Cognition in CKD: The Veterans Affairs Homocysteine Study. Am. J. Kidney Dis. 2009, 54, 440–449. [Google Scholar] [CrossRef]
- Murray, A.M.; Tupper, D.E.; Knopman, D.S.; Gilbertson, D.T.; Pederson, S.L.; Li, S.; Smith, G.E.; Hochhalter, A.K.; Collins, A.J.; Kane, R.L. Cognitive impairment in hemodialysis patients is common. Neurology 2006, 67, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Tamura, M.K.; Larive, B.; Unruh, M.L.; Stokes, J.B.; Nissenson, A.; Mehta, R.L.; Chertow, G.M. Prevalence and Correlates of Cognitive Impairment in Hemodialysis Patients. Clin. J. Am. Soc. Nephrol. 2010, 5, 1429–1438. [Google Scholar] [CrossRef] [PubMed]
- Nitta, K.; Hanafusa, N.; Kawaguchi, Y.; Tsuchiya, K. Physical function management for elderly dialysis patients: Prevention and improvement of frailty and disability. Ren. Replace. Ther. 2023, 9, 2. [Google Scholar] [CrossRef]
- Barcellos, F.C.; Santos, I.S.; Umpierre, D.; Bohlke, M.; Hallal, P.C. Effects of exercise in the whole spectrum of chronic kidney disease: A systematic review. Clin. Kidney J. 2015, 8, 753–765. [Google Scholar] [CrossRef] [PubMed]
- Cheung, A.K.; Sarnak, M.J.; Yan, G.; Dwyer, J.T.; Heyka, R.J.; Rocco, M.V.; Teehan, B.P.; Levey, A.S. Atherosclerotic cardiovascular disease risks in chronic hemodialysis patients. Kidney Int. 2000, 58, 353–362. [Google Scholar] [CrossRef]
- Seliger, S.L.; Gillen, D.L.; Longstreth, W.T.; Kestenbaum, B.; Stehman-Breen, C.O. Elevated risk of stroke among patients with end-stage renal disease. Kidney Int. 2003, 64, 603–609. [Google Scholar] [CrossRef]
- Weiner, D.E.; Scott, T.M.; Giang, L.M.; Agganis, B.T.; Sorensen, E.P.; Tighiouart, H.; Sarnak, M.J. Cardiovascular Disease and Cognitive Function in Maintenance Hemodialysis Patients. Am. J. Kidney Dis. 2011, 58, 773–781. [Google Scholar] [CrossRef]
- Weiner, D.E.; Bartolomei, K.; Scott, T.; Price, L.L.; Griffith, J.L.; Rosenberg, I.; Levey, A.S.; Folstein, M.F.; Sarnak, M.J. Albuminuria, Cognitive Functioning, and White Matter Hyperintensities in Homebound Elders. Am. J. Kidney Dis. 2009, 53, 438–447. [Google Scholar] [CrossRef]
- Harciarek, M.; Biedunkiewicz, B.; Lichodziejewska-Niemierko, M.; Dębska-Ślizień, A.; Rutkowski, B. Cognitive performance before and after kidney transplantation: A prospective controlled study of adequately dialyzed patients with end-stage renal disease. J. Int. Neuropsychol. Soc. 2009, 15, 684–694. [Google Scholar] [CrossRef]
- Drew, D.A.; Weiner, D.E.; Sarnak, M.J. Cognitive Impairment in CKD: Pathophysiology, Management, and Prevention. Am. J. Kidney Dis. 2019, 74, 782–790. [Google Scholar] [CrossRef]
- Guerreiro, R.; Bras, J. The age factor in Alzheimer’s disease. Genome Med. 2015, 7, 106. [Google Scholar] [CrossRef] [PubMed]
- Yeh, Y.-C.; Huang, M.-F.; Liang, S.-S.; Hwang, S.-J.; Tsai, J.-C.; Liu, T.-L.; Wu, P.-H.; Yang, Y.-H.; Kuo, K.-C.; Kuo, M.-C.; et al. Indoxyl sulfate, not p-cresyl sulfate, is associated with cognitive impairment in early-stage chronic kidney disease. Neurotoxicology 2016, 53, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wu, X.; Liu, S.; Zhao, Y.; Zhu, J.; Liu, K. Roles of Neuropeptide Y in Neurodegenerative and Neuroimmune Diseases. Front. Neurosci. 2019, 13, 869. [Google Scholar] [CrossRef]
- Zoccali, C.; D’arrigo, G.; Leonardis, D.; Pizzini, P.; Postorino, M.; Tripepi, G.; Mallamaci, F. Neuropeptide Y predicts cardiovascular events in chronic kidney disease patients. J. Hypertens. 2019, 37, 1359–1365. [Google Scholar] [CrossRef]
- Pépin, M.; Villain, C. Chronic kidney disease and cognitive impairment. Gériatrie Psychol. Neuropsychiatr. Viellisse Ment. 2020, 18, 429–435. [Google Scholar] [CrossRef]
- Kelly, D.M.; Rothwell, P.M. Disentangling the Relationship Between Chronic Kidney Disease and Cognitive Disorders. Front. Neurol. 2022, 13, 830064. [Google Scholar] [CrossRef] [PubMed]
- Murtaza, A.; Dasgupta, I. Chronic Kidney Disease and Cognitive Impairment. J. Stroke Cerebrovasc. Dis. 2021, 30, 105529. [Google Scholar] [CrossRef]
- Hullinger, R.; Puglielli, L. Molecular and cellular aspects of age-related cognitive decline and Alzheimers disease. Behav. Brain Res. 2017, 322, 191–205. [Google Scholar] [CrossRef]
- Jiang, T.T.; SYLD. Cognitive frailty in elderly patients with hemodialysis and its relationship with fear of falling. Chin. Nurs. Manag. 2020, 20, 1005–1009. [Google Scholar]
- Rapa, S.F.; Di Iorio, B.R.; Campiglia, P.; Heidland, A.; Marzocco, S. Inflammation and Oxidative Stress in Chronic Kidney Disease—Potential Therapeutic Role of Minerals, Vitamins and Plant-Derived Metabolites. Int. J. Mol. Sci. 2019, 21, 263. [Google Scholar] [CrossRef]
- Modlinger, P.S.; Wilcox, C.S.; Aslam, S. Nitric oxide, oxidative stress, and progression of chronic renal failure. Semin. Nephrol. 2004, 24, 354–365. [Google Scholar] [CrossRef] [PubMed]
- Schütt, A.; Bullock, T.H.; Başar, E. Dynamics of potentials from Invertebrate brains. In Brain Function and Oscillations: II In-tegrative Brain Function Neurophysiology and Cognitive Processes; Başar, E., Ed.; Springer: Heidelberg, Germany, 1999; pp. 91–108. [Google Scholar]
- Sajjad, I.; Grodstein, F.; Kang, J.H.; Curhan, G.C.; Lin, J. Kidney Dysfunction and Cognitive Decline in Women. Clin. J. Am. Soc. Nephrol. 2012, 7, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.Y.; Kim, J.S.; Kang, M.H.; Hwang, H.S.; Won, C.W.; Jeong, K.H. Renal dysfunction is associated with decline of cognitive function in community-dwelling older adults: Korean frailty and aging cohort study. BMC Geriatr. 2020, 20, 462. [Google Scholar] [CrossRef]
- Gurvich, C.; Thomas, N.; Kulkarni, J. Sex differences in cognition and aging and the influence of sex hormones. Handb. Clin. Neurol. 2020, 175, 103–115. [Google Scholar]
- Niu, H.; Álvarez-Álvarez, I.; Guillén-Grima, F.; Aguinaga-Ontoso, I. Prevalencia e incidencia de la enfermedad de Alzheimer en Europa: Metaanlisis. Neurología 2017, 32, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Mizumasa, T.; Hirakata, H.; Yoshimitsu, T.; Hirakata, E.; Kubo, M.; Kashiwagi, M.; Tanaka, H.; Kanai, H.; Fujimi, S.; Iida, M. Dialysis-Related Hypotension as a Cause of Progressive Frontal Lobe Atrophy in Chronic Hemodialysis Patients: A 3-Year Prospective Study. Nephron Clin. Pr. 2004, 97, c23–c30. [Google Scholar] [CrossRef]
- Kamata, T.; Hishida, A.; Takita, T.; Sawada, K.; Ikegaya, N.; Maruyama, Y.; Miyajima, H.; Kaneko, E. Morphologic Abnormalities in the Brain of Chronically Hemodialyzed Patients without Cerebrovascular Disease. Am. J. Nephrol. 2000, 20, 27–31. [Google Scholar] [CrossRef]
- Savazzi, G.M.; Cusmano, F.; Bergamaschi, E.; Vinci, S.; Allegri, L.; Garini, G. Hypertension as an Etiopathological Factor in the Development of Cerebral Atrophy in Hemodialyzed Patients. Nephron 1999, 81, 17–24. [Google Scholar] [CrossRef]
- Eldehni, M.T.; Odudu, A.; McIntyre, C.W. Randomized Clinical Trial of Dialysate Cooling and Effects on Brain White Matter. J. Am. Soc. Nephrol. 2015, 26, 957–965. [Google Scholar] [CrossRef]
- Giang, L.M.; Weiner, D.E.; Agganis, B.T.; Scott, T.; Sorensen, E.P.; Tighiouart, H.; Sarnak, M.J. Cognitive Function and Dialysis Adequacy: No Clear Relationship. Am. J. Nephrol. 2011, 33, 33–38. [Google Scholar] [CrossRef]
- Usdin, T.B.; Gruber, C.; Bonner, T.I. Identification and Functional Expression of a Receptor Selectively Recognizing Parathyroid Hormone, the PTH2 Receptor. J. Biol. Chem. 1995, 270, 15455–15458. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Zou, L.; Wu, H.; Shan, Y.; Liu, G.; Zheng, S.; Wang, L. Altered brain structural and cognitive impairment in end-stage renal disease patients with secondary hyperparathyroidism. Acta Radiol. 2019, 61, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Tentori, F.; Wang, M.; Bieber, B.A.; Karaboyas, A.; Li, Y.; Jacobson, S.H.; Andreucci, V.E.; Fukagawa, M.; Frimat, L.; Mendelssohn, D.C.; et al. Recent Changes in Therapeutic Approaches and Association with Outcomes among Patients with Secondary Hyperparathyroidism on Chronic Hemodialysis. Clin. J. Am. Soc. Nephrol. 2015, 10, 98–109. [Google Scholar] [CrossRef] [PubMed]
- Lourida, I.; Thompson-Coon, J.; Dickens, C.M.; Soni, M.; Kuźma, E.; Kos, K.; Llewellyn, D.J. Parathyroid Hormone, Cognitive Function and Dementia: A Systematic Review. PLoS ONE 2015, 10, e0127574. [Google Scholar] [CrossRef] [PubMed]
- Chou, F.-F.; Chen, J.-B.; Hsieh, K.-C.; Liou, C.-W. Cognitive changes after parathyroidectomy in patients with secondary hyperparathyroidism. Surgery 2008, 143, 526–532. [Google Scholar] [CrossRef]
- Wallace, H.J.; Wallace, I.R.; McCaffrey, P. Cognitive decline reversed by cinacalcet. QJM 2015, 108, 59–61. [Google Scholar] [CrossRef]
- Miglinas, M.; Cesniene, U.; Janusaite, M.M.; Vinikovas, A. Cerebrovascular Disease and Cognition in Chronic Kidney Disease Patients. Front. Cardiovasc. Med. 2020, 7, 96. [Google Scholar] [CrossRef]
- Palmer, S.C.; McGregor, D.O.; Craig, J.C.; Elder, G.; Macaskill, P.; Strippoli, G.F. Vitamin D compounds for people with chronic kidney disease requiring dialysis. Cochrane Database Syst. Rev. 2009, CD005633. [Google Scholar] [CrossRef]
- Ketteler, M.; Block, G.A.; Evenepoel, P.; Fukagawa, M.; Herzog, C.A.; McCann, L.; Moe, S.M.; Shroff, R.; Tonelli, M.A.; Toussaint, N.D.; et al. Executive summary of the 2017 KDIGO Chronic Kidney Disease–Mineral and Bone Disorder (CKD–MBD) Guideline Update: Whats changed and why it matters. Kidney Int. 2017, 92, 26–36. [Google Scholar] [CrossRef]
- Hou, W.; Chang, J.; Wang, Y.; Qi, Z.; Sun, Q. Research progress on cognitive frailty in elderly patients with chronic kidney disease. Chin. J. Geriatr. 2020, 39, 1108–1112. [Google Scholar]
- Tamura, M.K.; Vittinghoff, E.; Yang, J.; Go, A.S.; Seliger, S.L.; Kusek, J.W.; Lash, J.; Cohen, D.L.; Simon, J.; Batuman, V.; et al. Anemia and risk for cognitive decline in chronic kidney disease. BMC Nephrol. 2016, 17, 13. [Google Scholar]
- Koyama, A.K.; Nee, R.; Yu, W.; Choudhury, D.; Heng, F.; Cheung, A.K.; Norris, K.C.; Cho, M.E.; Yan, G. Role of Anemia in Dementia Risk Among Veterans With Incident CKD. Am. J. Kidney Dis. 2023, 82, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Blasco-Colmenares, E.; Farag, Y.M.K.; Zhao, D.; Guallar, E.; Finkelstein, F.O. Anemia, CKD, and Cognitive Function: The National Health and Nutrition Examination Survey. Kidney360 2024, 5, 895–899. [Google Scholar] [CrossRef]
- Shaker, A.M.; Mohamed, O.M.; Mohamed, M.F.; El-Khashaba, S.O. Impact of correction of anemia in end-stage renal disease patients on cerebral circulation and cognitive functions. Saudi J. Kidney Dis. Transpl. 2018, 29, 1333–1341. [Google Scholar] [CrossRef]
- Rroji, M.; Figurek, A.; Viggiano, D.; Capasso, G.; Spasovski, G. Phosphate in the Context of Cognitive Impairment and Other Neurological Disorders Occurrence in Chronic Kidney Disease. Int. J. Mol. Sci. 2022, 23, 7362. [Google Scholar] [CrossRef]
- Yamada, S.; Tsuruya, K.; Taniguchi, M.; Tokumoto, M.; Fujisaki, K.; Hirakata, H.; Fujimi, S.; Kitazono, T. Association Between Serum Phosphate Levels and Stroke Risk in Patients Undergoing Hemodialysis. Stroke 2016, 47, 2189–2196. [Google Scholar] [CrossRef]
- Shah, B.; Jagtap, P.; Sarmah, D.; Datta, A.; Raut, S.; Sarkar, A.; Bohra, M.; Singh, U.; Baidya, F.; Kalia, K.; et al. Cerebro-renal interaction and stroke. Eur. J. Roscience 2021, 53, 1279–1299. [Google Scholar] [CrossRef]
- Hamed, S.A.; Hamed, S.A. Neurologic conditions and disorders of uremic syndrome of chronic kidney disease: Presentations, causes, and treatment strategies. Expert. Rev. Clin. Pharmacol. 2018, 12, 61–90. [Google Scholar] [CrossRef] [PubMed]
- Hanna, R.M.; Ferrey, A.; Rhee, C.M.; Kalantar-Zadeh, K. Renal-Cerebral Pathophysiology: The Interplay between Chronic Kidney Disease and Cerebrovascular Disease. J. Stroke Cerebrovasc. Dis. 2021, 30, 105461. [Google Scholar] [CrossRef]
- Drew, D.A.; Tighiouart, H.; Scott, T.M.; Lou, K.V.; Fan, L.; Shaffi, K.; Weiner, D.E.; Sarnak, M.J. FGF-23 and cognitive performance in hemodialysis patients. Hemodial. Int. 2014, 18, 78–86. [Google Scholar] [CrossRef]
- Kuriyama, N.; Ozaki, E.; Mizuno, T.; Ihara, M.; Mizuno, S.; Koyama, T.; Matsui, D.; Watanabe, I.; Akazawa, K.; Takeda, K.; et al. Association between α-Klotho and Deep White Matter Lesions in the Brain: A Pilot Case Control Study Using Brain MRI. J. Alzheimer’s Dis. 2017, 61, 145–155. [Google Scholar] [CrossRef]
- Mengel-From, J.; Soerensen, M.; Nygaard, M.; McGue, M.; Christensen, K.; Christiansen, L. Genetic Variants in KLOTHO Associate With Cognitive Function in the Oldest Old Group. J. Gerontol. Ser. A 2016, 71, 1151–1159. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Nakashima, A.; Ohkido, I.; Kasai, K.; Yokoo, T. Association between serum phosphate levels and anemia in non-dialysis patients with chronic kidney disease: A retrospective cross-sectional study from the Fuji City CKD Network. BMC Nephrol. 2023, 24, 244. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.-R.; Park, S.K.; Jung, J.Y.; Kim, Y.H.; Oh, Y.K.; Yoo, T.H.; Sung, S. The Prevalence and Management of Anemia in Chronic Kidney Disease Patients: Result from the KoreaN Cohort Study for Outcomes in Patients With Chronic Kidney Disease (KNOW-CKD). J. Korean Med. Sci. 2017, 32, 249–256. [Google Scholar] [CrossRef]
- Badura, K.; Janc, J.; Wąsik, J.; Gnitecki, S.; Skwira, S.; Młynarska, E.; Rysz, J.; Franczyk, B.; Badura, K.; Janc, J.; et al. Anemia of Chronic Kidney DiseaseA Narrative Review of Its Pathophysiology, Diagnosis, and Management. Biomedicines 2024, 12, 1191. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.-H.; Leu, J.-G.; Fang, Y.-W.; Liou, H.-H. High Fibroblast Growth Factor 23 Levels Associated With Low Hemoglobin Levels in Patients with Chronic Kidney Disease Stages 3 and 4. Medicine 2016, 95, e3049. [Google Scholar] [CrossRef]
- Nam, K.H.; Kim, H.; An, S.Y.; Lee, M.; Cha, M.-U.; Park, J.T.; Yoo, T.-H.; Lee, K.-B.; Kim, Y.-H.; Sung, S.-A.; et al. Circulating Fibroblast Growth Factor-23 Levels are Associated with an Increased Risk of Anemia Development in Patients with Nondialysis Chronic Kidney Disease. Sci. Rep. 2018, 8, 7294. [Google Scholar] [CrossRef]
- Mehta, R.; Cai, X.; Hodakowski, A.; Lee, J.; Leonard, M.; Ricardo, A.; Chen, J.; Hamm, L.; Sondheimer, J.; Dobre, M.; et al. Fibroblast Growth Factor 23 and Anemia in the Chronic Renal Insufficiency Cohort Study. Clin. J. Am. Soc. Nephrol. 2017, 12, 1795–1803. [Google Scholar] [CrossRef]
- Yiannopoulou, K.G.; Anastasiou, A.I.; Kyrozis, A.; Anastasiou, I.P. Donepezil Treatment for Alzheimer’s Disease in Chronic Dialysis Patients. Case Rep. Nephrol. Dial. 2019, 9, 126–136. [Google Scholar] [CrossRef]
- Xie, Z.; Tong, S.; Chu, X.; Feng, T.; Geng, M. Chronic Kidney Disease and Cognitive Impairment: The Kidney-Brain Axis. Kidney Dis. 2022, 8, 275–285. [Google Scholar] [CrossRef]
- Cai, H.; Li, G.; Hua, S.; Liu, Y.; Chen, L. Effect of exercise on cognitive function in chronic disease patients: A meta-analysis and systematic review of randomized controlled trials. Clin. Interv. Aging 2017, 12, 773–783. [Google Scholar] [CrossRef] [PubMed]
- Kren, A.; Bogataj, P. The Impact of Intradialytic Cognitive and Physical Training Program on the Physical and Cognitive Abilities in End-Stage Kidney Disease Patients: A Randomized Clinical Controlled Trial. Brain Sci. 2023, 13, 1228. [Google Scholar] [CrossRef]
- McAdams-DeMarco, M.A.; Konel, J.; Warsame, F.; Ying, H.; Fernández, M.G.; Carlson, M.C.; Fine, D.M.; Appel, L.J.; Segev, D.L. Intradialytic Cognitive and Exercise Training May Preserve Cognitive Function. Kidney Int. Rep. 2018, 3, 81–88. [Google Scholar] [CrossRef]
- Manfredini, F.; Mallamaci, F.; D’Arrigo, G.; Baggetta, R.; Bolignano, D.; Torino, C.; Lamberti, N.; Bertoli, S.; Ciurlino, D.; Rocca-Rey, L.; et al. Exercise in Patients on Dialysis: A Multicenter, Randomized Clinical Trial. J. Am. Soc. Nephrol. 2016, 28, 1259–1268. [Google Scholar] [CrossRef]
- Nakamura-Taira, N.; Horikawa, N.; Oka, F.; Igarashi, Y.; Kobayashi, S.; Kato, S.; Enomoto, T.; Kimura, H.; Watanabe, Y.; Kumada, T.; et al. Quasi-cluster randomized trial of a six-month low-intensity group-based resistance exercise for hemodialysis patients on depression and cognitive function: A 12-month follow-up. Health Psychol. Behav. Med. 2021, 9, 741–760. [Google Scholar] [CrossRef] [PubMed]
- Belik, F.S.; Silva, V.R.O.E.; Braga, G.P.; Bazan, R.; Vogt, B.P.; Caramori, J.C.T.; Barretti, P.; de Souza Gonçalves, R.; Bôas, P.J.F.V.; Hueb, J.C.; et al. Influence of Intradialytic Aerobic Training in Cerebral Blood Flow and Cognitive Function in Patients with Chronic Kidney Disease: A Pilot Randomized Controlled Trial. Nephron 2018, 140, 9–17. [Google Scholar]
- Otobe, Y.; Yamada, M.; Hiraki, K.; Onari, S.; Taki, Y.; Sumi, H.; Hachisuka, R.; Han, W.; Takahashi, M.; Suzuki, M.; et al. Physical Exercise Improves Cognitive Function in Older Adults with Stage 3–4 Chronic Kidney Disease: A Randomized Controlled Trial. Am. J. Nephrol. 2021, 52, 929–939. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.T.B.; Ramos, G.S.M.; Guaraldo, S.A.; Uezima, C.B.B.; Martins, J.P.L.B.; Junior, E.R. Comparison of cognitive function between patients on chronic hemodialysis who carry out assisted physical activity and inactive ones. J. Bras. Nefrol. 2011, 33, 27–30. [Google Scholar] [CrossRef]
- Zhao, M.; Xiao, M.; Tan, Q.; Lyu, J.; Lu, F. The effect of aerobic exercise on oxidative stress in patients with chronic kidney disease: A systematic review and meta-analysis with trial sequential analysis. Ren. Fail. 2023, 45, 2252093. [Google Scholar] [CrossRef]
- Bradshaw, E.; Alejmi, A.; Rossetti, G.; D’avossa, G.; Macdonald, J.H. Exercise and Cognitive Function Chronic Kidney Disease: A Systematic Review and Meta-Analysis of Efficacy and Harms. Clin. J. Am. Soc. Nephrol. 2024, 10-2215. [Google Scholar] [CrossRef]
- Gleeson, M.; Bishop, N.C.; Stensel, D.J.; Lindley, M.R.; Mastana, S.S.; Nimmo, M.A. The anti-inflammatory effects of exercise: Mechanisms and implications for the prevention and treatment of disease. Nat. Rev. Immunol. 2011, 11, 607–615. [Google Scholar] [CrossRef]
- Hamer, M.; Sabia, S.; Batty, G.D.; Shipley, M.J.; Tabák, A.G.; Singh-Manoux, A.; Kivimaki, M. Physical Activity and Inflammatory Markers Over 10 Years. Circulation 2012, 126, 928–933. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, H.-L.; Yang, C.-M. Role of Redox Signaling in Neuroinflammation and Neurodegenerative Diseases. Biomed. Res. Int. 2013, 2013, 484613. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhang, H.; Liang, J.; Huang, J.; Chen, N. Exercise suppresses neuroinflammation for alleviating Alzheimers disease. J. Neuroinflammation 2023, 20, 76. [Google Scholar] [CrossRef] [PubMed]
- Gray, N.E.; Farina, M.; Tucci, P.; Saso, L. The Role of the NRF2 Pathway in Maintaining and Improving Cognitive Function. Biomedicines 2022, 10, 2043. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Buttari, B.; Profumo, E.; Tucci, P.; Saso, L. A Perspective on Nrf2 Signaling Pathway for Neuroinflammation: A Potential Therapeutic Target in Alzheimer’s and Parkinson’s Diseases. Front. Cell. Neurosci. 2022, 15, 787258. [Google Scholar] [CrossRef]
- Deus, C.M.; Teixeira, J.; Raimundo, N.; Tucci, P.; Borges, F.; Saso, L.; Oliveira, P.J. Modulation of cellular redox environment as a novel therapeutic strategy for Parkinson’s disease. Eur. J. Clin. Investig. 2022, 52, e13820. [Google Scholar] [CrossRef]
- Abreu, C.C.; Cardozo, L.F.; Stockler-Pinto, M.B.; Esgalhado, M.; Barboza, J.E.; Frauches, R.; Mafra, D. Does resistance exercise performed during dialysis modulate Nrf2 and NF-κB in patients with chronic kidney disease? Life Sci. 2017, 188, 192–197. [Google Scholar] [CrossRef] [PubMed]
- De Brito, J.S.; Borges, N.A.; De Reis, D.C.M.D.; Da Silva, G.S.; Dos Fonseca, L.D.; Ribeiro, M.M.F.; Chermut, T.R.; Moura, M.C.; Oliveira, L.C.; De Paiva, B.R.; et al. Effects of intradialytic bicycle ergometer exercise on transcription factors NF-B and Nrf2 in patients with chronic kidney disease: A randomized crossover clinical trial. J. Bodyw. Mov. Ther. 2024, 40, 1492–1501. [Google Scholar] [CrossRef]
- Jaberi, S.; Fahnestock, M. Mechanisms of the Beneficial Effects of Exercise on Brain-Derived Neurotrophic Factor Expression in Alzheimer’s Disease. Biomolecules 2023, 13, 1577. [Google Scholar] [CrossRef]
- Bradburn, S.; McPhee, J.S.; Bagley, L.; Sipila, S.; Stenroth, L.; Narici, M.V.; Pääsuke, M.; Gapeyeva, H.; Osborne, G.; Sassano, L.; et al. Association between osteocalcin and cognitive performance in healthy older adults. Age Ageing 2016, 45, 844–849. [Google Scholar] [CrossRef] [PubMed]
- Nicolini, C.; Michalski, B.; Toepp, S.L.; Turco, C.V.; D‘Hoine, T.; Harasym, D.; Gibala, M.J.; Fahnestock, M.; Nelson, A.J. A Single Bout of High-intensity Interval Exercise Increases Corticospinal Excitability, Brain-derived Neurotrophic Factor, and Uncarboxylated Osteolcalcin in Sedentary, Healthy Males. Neuroscience 2020, 437, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Wrann, C.D.; White, J.P.; Salogiannnis, J.; Laznik-Bogoslavski, D.; Wu, J.; Ma, D.; Lin, J.D.; Greenberg, M.E.; Spiegelman, B.M. Exercise Induces Hippocampal BDNF through a PGC-1/FNDC5 Pathway. Cell Metab. 2013, 18, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Wu, P.-H.; Tarr, P.T.; Lindenberg, K.S.; St-Pierre, J.; Zhang, C.-Y.; Mootha, V.K.; Jäger, S.; Vianna, C.R.; Reznick, R.M.; et al. Defects in Adaptive Energy Metabolism with CNS-Linked Hyperactivity in PGC-1α Null Mice. Cell 2004, 119, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Li, S.; Lucas, E.K.; Cowell, R.M.; Lin, J.D. Neuronal Inactivation of Peroxisome Proliferator-activated Receptor Coactivator 1α (PGC-1α) Protects Mice from Diet-induced Obesity and Leads to Degenerative Lesions. J. Biol. Chem. 2010, 285, 39087–39095. [Google Scholar] [CrossRef]
- Lucas, E.K.; Markwardt, S.J.; Gupta, S.; Meador-Woodruff, J.H.; Lin, J.D.; Overstreet-Wadiche, L.; Cowell, R.M. Parvalbumin Deficiency and GABAergic Dysfunction in Mice Lacking PGC-1α. J. Neurosci. 2010, 30, 7227–7235. [Google Scholar] [CrossRef]
- Steiner, J.L.; Murphy, E.A.; McClellan, J.L.; Carmichael, M.D.; Davis, J.M. Exercise training increases mitochondrial biogenesis in the brain. J. Appl. Physiol. 2011, 111, 1066–1071. [Google Scholar] [CrossRef]
- Leick, L.; Lyngby, S.S.; Wojtasewski, J.F.; Pilegaard, H. PGC-1 is required for training-induced prevention of age-associated decline in mitochondrial enzymes in mouse skeletal muscle. Exp. Gerontol. 2010, 45, 336–342. [Google Scholar] [CrossRef]
- Moreira, O.C.; Estébanez, B.; Martínez-Florez, S.; de Paz, J.A.; Cuevas, M.J.; González-Gallego, J. Mitochondrial Function and Mitophagy in the Elderly: Effects of Exercise. Oxidative Med. Cell. Longev. 2017, 2017, 2012798. [Google Scholar] [CrossRef]
- Peng, H.; Wang, Q.; Lou, T.; Qin, J.; Jung, S.; Shetty, V.; Li, F.; Wang, Y.; Feng, X.-H.; Mitch, W.E.; et al. Myokine mediated muscle-kidney crosstalk suppresses metabolic reprogramming and fibrosis in damaged kidneys. Nat. Commun. 2017, 8, 1493. [Google Scholar] [CrossRef]
- Chi, P.-J.; Hung, S.-Y.; Hsiao, F.-T.; Liou, H.-H.; Tsai, J.-P. Serum osteocalcin concentration as an independent biomarker of osteoporosis in patients with chronic kidney disease. Clin. Nephrol. 2022, 98, 1. [Google Scholar] [CrossRef] [PubMed]
- Deus, L.A.; De Corrêa, H.D.; Neves, R.V.P.; Reis, A.L.; Honorato, F.S.; Silva, V.L.; Souza, M.K.; De Araújo, T.B.; De Alves, L.S.D.; Sousa, C.V.; et al. Are Resistance Training–Induced BDNF in Hemodialysis Patients Associated with Depressive Symptoms, Quality of Life, Antioxidant Capacity, and Muscle Strength? An Insight for the Muscle–Brain–Renal Axis. Int. J. Environ. Res. Public Health 2021, 18, 11299. [Google Scholar] [CrossRef] [PubMed]
- Arjunan, A.; Sah, D.K.; Woo, M.; Song, J. Identification of the molecular mechanism of insulin-like growth factor-1 (IGF-1): A promising therapeutic target for neurodegenerative diseases associated with metabolic syndrome. Cell Biosci. 2023, 13, 16. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, A.J.; Brasel, J.A.; Hintz, R.L.; Mohan, S.U.; Cooper, D.M. Acute effect of brief low-and high-intensity exercise on circulating insulin-like growth factor (IGF) I, II, and IGF-binding protein-3 and its proteolysis in young healthy men. J. Clin. Endocrinol. Metab. 1996, 81, 3492–3497. [Google Scholar]
- Foster, P.P.; Rosenblatt, K.P.; Kuljiš, R.O. Exercise-Induced Cognitive Plasticity, Implications for Mild Cognitive Impairment and Alzheimers Disease. Front. Neurol. 2011, 2, 28. [Google Scholar] [CrossRef]
- Cotman, C.W.; Berchtold, N.C.; Christie, L.-A. Exercise builds brain health: Key roles of growth factor cascades and inflammation. Trends Neurosci. 2007, 30, 464–472. [Google Scholar] [CrossRef]
- Trejo, J.L.; Carro, E.; Torres-Alemán, I. Circulating Insulin-Like Growth Factor I Mediates Exercise-Induced Increases in the Number of New Neurons in the Adult Hippocampus. J. Neurosci. 2001, 21, 1628–1634. [Google Scholar] [CrossRef]
- Ding, Q.; Vaynman, S.; Akhavan, M.; Ying, Z.; Gomez-Pinilla, F. Insulin-like growth factor I interfaces with brain-derived neurotrophic factor-mediated synaptic plasticity to modulate aspects of exercise-induced cognitive function. Neuroscience 2006, 140, 823–833. [Google Scholar] [CrossRef]
- Miller, N.J.; Rice-Evans, C.; Davies, M.J.; Gopinathan, V.; Milner, A. A Novel Method for Measuring Antioxidant Capacity and its Application to Monitoring the Antioxidant Status in Premature Neonates. Clin. Sci. 1993, 84, 407–412. [Google Scholar] [CrossRef]
- Prelević, V.; Radunović, D.; Antunović, T.; Ratković, M.; Gligorovic-Bahranovic, N.; Gledović, B.; Vujošević, S.; Nedovic-Vukovic, M.; Bašić-Jukić, N. Increased Serum Level of IGF-1 Correlates with Better Cognitive Status in End-Stage Renal Disease Patients Undergoing Hemodialysis. Ther. Apher. Dial. 2018, 22, 118–123. [Google Scholar] [CrossRef]
- Gould, D.W.; Graham-Brown, M.P.; Watson, E.L.; Viana, J.L.; Smith, A.C. Physiological benefits of exercise in pre-dialysis chronic kidney disease. Nephrology 2014, 19, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.A.F.; Ding, W.; Manfredi, T.J.; Solares, G.S.; O’neill, E.F.; Clements, K.M.; Ryan, N.D.; Kehayias, J.J.; Fielding, R.A.; Evans, W.J.; et al. Insulin-like growth factor I in skeletal muscle after weight-lifting exercise in frail elders. Am. J. Physiol. Metab. 1999, 277, E135–E143. [Google Scholar] [CrossRef] [PubMed]
- Moinuddin, I.; Leehey, D.J. A Comparison of Aerobic Exercise and Resistance Training in Patients With and Without Chronic Kidney Disease. Adv. Chronic Kidney Dis. 2008, 15, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Nindl, B.C.; Headley, S.A.; Tuckow, A.P.; Pandorf, C.E.; Diamandi, A.; Khosravi, M.J.; Welles, R.; Jones, M.; Germain, M. IGF-I system responses during 12 weeks of resistance training in end-stage renal disease patients. Growth Horm. IGF Res. 2004, 14, 245–250. [Google Scholar] [CrossRef]
| Authors, Year | Design | Sample Size | Cognitive Function Tests | Prevalence of Cognitive Impairment |
|---|---|---|---|---|
| Kurella et al., 2004 [6] | Cross-sectional study | N = 160 N1 = 80 non-dialysis, CKD (stage III to IV) patients N2 = 80 ESRD undergoing HD | 3MS, trails B, CVLT | A total of 27% in ESRD, 15% in patients with no advanced CKD and non in subjects with mild to moderate CKD. |
| Murray et al., 2006 [32] | Cross-sectional study | N = 338 ESRD patients un-dergoing HD | 3MS, HVLT-R, 9 color trails 1 and 210 (a test similar to Halsted–Reitan trails A and B, but using alternating colors instead of numbers for trail B), Stroop interference test, BVMT-R, COWAT, clock-drawing test, Wechsler digit span, and the geriatric depression scale (short form) | A total of 13.9% were classified with mild impairment, 36.1% with moderate impairment, 37.3% with severe impairment, and 12.7% with normal cognition. Only 2.9% had a documented history of cognitive impairment. |
| Sehgal et al., 1997 [11] | Cross-sectional study | N = 336 ESRD patients undergoing HD | MMSE | A total of 22% had mild mental impairment and 8% moderate to severe mental impairment. |
| Cook et al., 2008 [30] | Cross-sectional study | N = 162 ESRD patients undergoing HD | MMSE | A total of 27% had cognitive impairment. |
| Tamura et al., 2010 [33] | Cross-sectional study | N = 383 ESRD patients undergoing HD | 3MS impairment in executive function (trails B) | By using the 3MS, the cognitive impairment prevalence was 26% among HD patients. |
| Brady et al., 2009 [31] | Randomized controlled trial | N = 236 Veterans with ESRD | TICSm, digit span forward—span, digit span backward—span, verbal fluency, global cognition z-score composite, memory z-score composite, geriatric depression scale | The cognitive prevalence rate was 20% among participants. |
| Leinau et al., 2009 [29] | Observational cohort study | N = 109 ESRD patients undergoing HD | MMSE, EXIT25 | Of the participants younger than 60 years old, 30% had cognitive impairment. In contrast, those older than 60 years had a higher cognitive impairment prevalence (48%). |
| Gela et al., 2021 [27] | Comparative Cross-Sectional Study | N = 116 CKD patients | SMMSE | The prevalence of cognitive impairment among CKD patients was 49.1%. |
| Viji et al., 2009 [28] | Observational and cross-sectional study | N = 210 CKD patients | MoCA | A total of 53.8% had mild cognitive impairment and 46.2% had normal cognitive status. |
| Williams et al., 2013 [26] | Observational and cross-sectional study | N = 79 CKD patients (Stage III to V) | CSI’D, TMTA, TMTB | More CKD patients had cognitive impairment compared with controls using CSI’D (51.9% versus 2.5%, p < 0.001), TMTA (53.2% versus 0%, p < 0.001), and TMTB (40% versus 0%, p < 0.001). |
| Authors, Year | Design | Sample Size | Duration, Type of Exercise, and Groups | Cognitive Measurements | Functional Capacity and Other Measurements | Main Results after Exercise Training Programs |
|---|---|---|---|---|---|---|
| Kren et al., 2023 [94] | RCT | Ν = 44 HD patients | Intradialytic cycling and cognitive training 3 days per week for 12 weeks (exercise group) vs. standard care (control group). | MoCA, SDMT | HGS, 10-STS, stork balance test | Significant time * group interaction effect for SDMT (p < 0.001) and MoCA (p < 0.001). No significant interaction was observed for 10-STS, HGS, and the stork balance test (p > 0.05). |
| McAdams et al., 2018 [95] | RCT | N = 20 HD patients | Three months of intradialytic training (exercise group) vs. cognitive training (cognitive group) vs. standard care (control group). | 3MS, TMTA, TMTB | - | Cognitive decline in psychomotor speed and executive function seen with standard care was possibly prevented by cognitive and exercise training, but not in all domains. |
| Manfredini et al. [96] | RCT | N = 227 HD patients | Six months normal physical activity (control; n = 145) vs. personalized walking exercise program at home (n = 151); 227 patients (exercise n = 104; control n = 123). | Cognitive function domain was assessed through KDQOL-SF | 6MWT, 5xSTS, KDQOL-SF | After 6 months of training, HD patients improved their 6MWT and 5xSTS scores. The cognitive function score (p = 0.04) and quality of social interaction score (p = 0.01) in the kidney disease component of the KDQOL-SF improved significantly in the exercise group. |
| Nakamura-Taira et al., 2021 [97] | Quasi-cluster RCT | N = 42 HD patients | Six months, three times per week, intradialytic resistance intervention (exercise group) vs. stretching (control group) and 12-month follow-up. | MoCA-J | PHQ-9, AIS, NPI-Q, exercise self-efficacy | No significant effects on depression, cognitive function, and NPI-Q. |
| Belik et al., 2018 [98] | RCT | N = 30 HD patients | Four months intradialytic aerobic training (exercise group) vs. standard care (control group). | MMSE | IPAQ, Transcranial Doppler for cerebral blood flow assessment | Significant improvements of cognitive impairment and basilar maximum blood flow velocity in the exercise group. |
| Otobe et al., 2022 [99] | RCT | N = 44 Non-dialysis CKD patients | Group exercise training at the hospital once a week and independent exercise at home twice a week or more for 24 weeks (exercise group) vs. general care (control group). | Wechsler Memory Scale-Revised | - | Patients in the exercise group showed significantly greater changes in the Wechsler memory scale-revised logical memory delayed recall (p = 0.03) and in the immediate and delayed recall (p = 0.02) scores compared to patients in the control group. |
| Martins et al., 2011 [100] | RCT | N = 86 HD patients | Six months exercise (exercise group) vs. usual care (control group). | 3MS | - | Better cognitive function was observed in active patients compared to the inactive ones (p < 0.05). Moreover, active patients over 60 years of age had better cognitive results than untrained ones (p < 0.05). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michou, V.; Tsamos, G.; Vasdeki, D.; Deligiannis, A.; Kouidi, E. Unraveling of Molecular Mechanisms of Cognitive Frailty in Chronic Kidney Disease: How Exercise Makes a Difference. J. Clin. Med. 2024, 13, 5698. https://doi.org/10.3390/jcm13195698
Michou V, Tsamos G, Vasdeki D, Deligiannis A, Kouidi E. Unraveling of Molecular Mechanisms of Cognitive Frailty in Chronic Kidney Disease: How Exercise Makes a Difference. Journal of Clinical Medicine. 2024; 13(19):5698. https://doi.org/10.3390/jcm13195698
Chicago/Turabian StyleMichou, Vasiliki, Georgios Tsamos, Dimitra Vasdeki, Asterios Deligiannis, and Evangelia Kouidi. 2024. "Unraveling of Molecular Mechanisms of Cognitive Frailty in Chronic Kidney Disease: How Exercise Makes a Difference" Journal of Clinical Medicine 13, no. 19: 5698. https://doi.org/10.3390/jcm13195698






