Clinical Evaluation of Acute Exacerbation of Interstitial Lung Disease in a Single Tertiary Center: Perspectives before and after the Coronavirus Disease 2019 Pandemic
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Study Design
2.2. Samples and Clinical Data Collection
2.3. Definition of Stages
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Regional and Seasonal Distribution of Admitted Patients
3.3. Various Parameters on Admission between Survivor and Deceased Groups
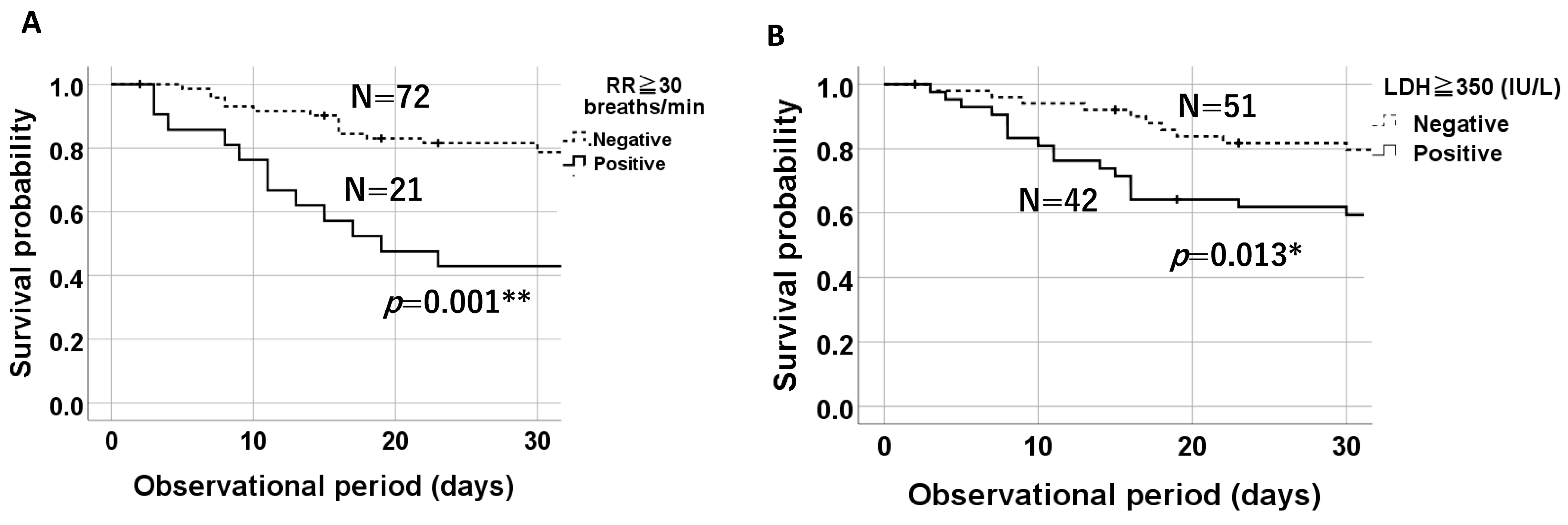
3.4. Comparison of 30-Day Survival Probabilities on a Kaplan–Meier Plot Based on the Presence of Tachypnea (RR ≥ 30 Breaths/Min) and Elevated LDH Levels (≥350 IU/L)
3.5. Prognostic Factors for an AE of IP
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jeganathan, N.; Sathananthan, M. The prevalence and burden of interstitial lung diseases in the USA. ERJ Open Res. 2022, 8, 00630-2021. [Google Scholar] [CrossRef] [PubMed]
- Kondoh, Y.; Suda, T.; Hongo, Y.; Yoshida, M.; Hiroi, S.; Iwasaki, K.; Takeshima, T.; Homma, S. Prevalence of idiopathic pulmonary fibrosis in Japan based on a claims database analysis. Respir. Res. 2022, 23, 24. [Google Scholar] [CrossRef] [PubMed]
- Collard, H.R.; Moore, B.B.; Flaherty, K.R.; Brown, K.K.; Kaner, R.J.; King, T.E., Jr.; Lasky, J.A.; Loyd, J.E.; Noth, I.; Olman, M.A.; et al. Acute exacerbations of idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2007, 176, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.W.; Johnson, J.E.; Browning, P.J.; Cruz-Gervis, R.A.; Davis, A.; Graham, B.S.; Brigham, K.L.; Oates, J.A., Jr.; Loyd, J.E.; Stecenko, A.A. Herpesvirus DNA is consistently detected in lungs of patients with idiopathic pulmonary fibrosis. J. Clin. Microbiol. 2003, 41, 2633–2640. [Google Scholar] [CrossRef]
- Ushiki, A.; Yamazaki, Y.; Hama, M.; Yasuo, M.; Hanaoka, M.; Kubo, K. Viral infections in patients with an acute exacerbation of idiopathic interstitial pneumonia. Respir. Investig. 2014, 52, 65–70. [Google Scholar] [CrossRef]
- Wootton, S.C.; Kim, D.S.; Kondoh, Y.; Chen, E.; Lee, J.S.; Song, J.W.; Huh, J.W.; Taniguchi, H.; Chiu, C.; Boushey, H.; et al. Viral infection in acute exacerbation of idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care. Med. 2011, 183, 1698–1702. [Google Scholar] [CrossRef]
- Saraya, T.; Kimura, H.; Kurai, D.; Tamura, M.; Ogawa, Y.; Mikura, S.; Sada, M.; Oda, M.; Watanabe, T.; Ohkuma, K.; et al. Clinical significance of respiratory virus detection in patients with acute exacerbation of interstitial lung diseases. Respir. Med. 2018, 136, 88–92. [Google Scholar] [CrossRef]
- Saraya, T.; Kimura, H.; Kurai, D.; Ishii, H.; Takizawa, H. The molecular epidemiology of respiratory viruses associated with asthma attacks. A single-center observational study in Japan. Medicine 2017, 96, e8204. [Google Scholar] [CrossRef]
- Hamano, J.; Tokuda, Y. Changes in vital signs as predictors of bacterial infection in home care: A multi-center prospective cohort study. Postgrad. Med. 2017, 129, 283–287. [Google Scholar] [CrossRef]
- The Ministry of Health LaWoJ. Idiopathic Interstitial Pneumonia; 2024. Available online: https://www.nanbyou.or.jp/entry/302 (accessed on 18 September 2024).
- Mostafaei, S.; Sayad, B.; Azar, M.E.F.; Doroudian, M.; Hadifar, S.; Behrouzi, A.; Riahi, P.; Hussen, B.M.; Bayat, B.; Nahand, J.S.; et al. The role of viral and bacterial infections in the pathogenesis of IPF: A systematic review and meta-analysis. Respir. Res. 2021, 22, 53. [Google Scholar] [CrossRef]
- Sheng, G.; Chen, P.; Wei, Y.; Yue, H.; Chu, J.; Zhao, J.; Wang, Y.; Zhang, W.; Zhang, H.L. Viral Infection Increases the Risk of Idiopathic Pulmonary Fibrosis: A Meta-Analysis. Chest 2020, 157, 1175–1187. [Google Scholar] [CrossRef] [PubMed]
- Collard, H.R.; Yow, E.; Richeldi, L.; Anstrom, K.J.; Glazer, C. Suspected acute exacerbation of idiopathic pulmonary fibrosis as an outcome measure in clinical trials. Respir. Res. 2013, 14, 73. [Google Scholar] [CrossRef] [PubMed]
- Simon-Blancal, V.; Freynet, O.; Nunes, H.; Bouvry, D.; Naggara, N.; Brillet, P.Y.; Denis, D.; Cohen, Y.; Vincent, F.; Valeyre, D.; et al. Acute exacerbation of idiopathic pulmonary fibrosis: Outcome and prognostic factors. Respiration 2012, 83, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Yamazoe, M.; Tomioka, H. Acute exacerbation of idiopathic pulmonary fibrosis: A 10-year single-centre retrospective study. BMJ Open Respir. Res. 2018, 5, e000342. [Google Scholar] [CrossRef] [PubMed]
- Shindo, Y.; Ito, R.; Kobayashi, D.; Ando, M.; Ichikawa, M.; Goto, Y.; Fukui, Y.; Iwaki, M.; Okumura, J.; Yamaguchi, I.; et al. Risk factors for 30-day mortality in patients with pneumonia who receive appropriate initial antibiotics: An observational cohort study. Lancet Infect. Dis. 2015, 15, 1055–1065. [Google Scholar] [CrossRef]
- El-Beheidy, R.; Domouky, A.M.; Zidan, H.; Amer, Y.A. Serum KL-6 as predictive and prognostic marker of interstitial lung disease in childhood connective tissue diseases: A pilot study. Reumatismo 2021, 73, 147–155. [Google Scholar] [CrossRef]
- Zheng, P.; Zheng, X.; Takehiro, H.; Cheng, Z.J.; Wang, J.; Xue, M.; Lin, Q.; Huang, Z.; Huang, H.; Liao, C.; et al. The Prognostic Value of Krebs von den Lungen-6 and Surfactant Protein-A Levels in the Patients with Interstitial Lung Disease. J. Transl. Int. Med. 2021, 9, 212–222. [Google Scholar] [CrossRef]
- Groseanu, L.; Nita, C. A Systematic Review of the Key Predictors of Progression and Mortality of Rheumatoid Arthritis-Associated Interstitial Lung Disease. Diagnostics 2024, 14, 1890. [Google Scholar] [CrossRef]
- Gono, T.; Kawaguchi, Y.; Satoh, T.; Kuwana, M.; Katsumata, Y.; Takagi, K.; Masuda, I.; Tochimoto, A.; Baba, S.; Okamoto, Y.; et al. Clinical manifestation and prognostic factor in anti-melanoma differentiation-associated gene 5 antibody-associated interstitial lung disease as a complication of dermatomyositis. Rheumatology 2010, 49, 1713–1719. [Google Scholar] [CrossRef]
- Zou, J.; Guo, Q.; Chi, J.; Wu, H.; Bao, C. HRCT score and serum ferritin level are factors associated to the 1-year mortality of acute interstitial lung disease in clinically amyopathic dermatomyositis patients. Clin. Rheumatol. 2015, 34, 707–714. [Google Scholar] [CrossRef]
- Kishaba, T.; Tamaki, H.; Shimaoka, Y.; Fukuyama, H.; Yamashiro, S. Staging of acute exacerbation in patients with idiopathic pulmonary fibrosis. Lung 2014, 192, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Murohashi, K.; Hara, Y.; Saigusa, Y.; Kobayashi, N.; Sato, T.; Yamamoto, M.; Kudo, M.; Kaneko, T. Clinical significance of Charlson comorbidity index as a prognostic parameter for patients with acute or subacute idiopathic interstitial pneumonias and acute exacerbation of collagen vascular diseases-related interstitial pneumonia. J. Thorac. Dis. 2019, 11, 2448–2457. [Google Scholar] [CrossRef] [PubMed]
- Kolb, M.; Bondue, B.; Pesci, A.; Miyazaki, Y.; Song, J.W.; Bhatt, N.Y.; Huggins, J.T.; Oldham, J.M.; Padilla, M.L.; Roman, J.; et al. Acute exacerbations of progressive-fibrosing interstitial lung diseases. Eur. Respir. Rev. 2018, 27, 180071. [Google Scholar] [CrossRef] [PubMed]



| Number of Patients (n = 93) | |
|---|---|
| Age | 80.0 (74.0–86.0) |
| Sex (Male) | 64.5% (n = 60) |
| Smoking | |
| Ex or Current | 66.7% (n = 62) |
| IIPs | |
| IPF | 44.1% (n = 42) |
| non-IPF (iNSIP) | 40.9% (n = 37) |
| CTD-ILD (N = 14) | |
| RA | 7.5% (n = 7) |
| DM | 4.3% (n = 4) |
| SLE | 1.1% (n = 1) |
| SSc | 2.2% (n = 2) |
| Stage I or II | 14.0% (n = 13) |
| Duration of illness (years) | 4.0 (2.0–5.0) |
| Comorbidities | |
| Asthma | 0% (n = 0) |
| COPD | 14.0% (n = 13) |
| Cardiac diseases | 23.7% (n = 22) |
| NIDDM type 2 | 14.0% (n = 13) |
| Maintenance hemodialysis | 1.1% (n = 1) |
| Malignant diseases | 19.3% (n = 18) |
| Respiratory viruses * | |
| SARS-CoV-2 | 2.5% (n = 2) |
| Bacteria | |
| MRSA | 1.1% (n = 1) |
| Pseudomonas aeruginosa | 1.1% (n = 1) |
| Vital signs | |
| BT (°C) | 37.0 ± 0.76 |
| RR (breaths/min) | 24.0 (18.0–28.0) |
| ΔHR/ΔBT | 27.8 (15.2–56.0) |
| SpO2 (%) | 82.1 (78.6–85.3) |
| BMI | 21.5 (18.7–24.1) |
| Symptoms | |
| nasal discharge | 5.4% (n = 5) |
| sore throat | 1.1% (n = 1) |
| dyspnea | 73.1% (n = 68) |
| Antifibrotic agents | 9.7% (n = 9) |
| Previous episodes of AEs | 14.0% (n = 13) |
| Before the Pandemic (n = 73) | After the Pandemic (n = 20) | p Value | |
|---|---|---|---|
| Age | 80 (70–83) | 79 (70–87) | 0.918 |
| Sex (Male) | 68.5% (n = 50) | 50.0% (n = 10) | 0.186 |
| Smoking | |||
| Ex or Current | 72.6% (n = 53) | 50.0%(n = 10) | 0.065 |
| IIPs | 84.9% (n = 62) | 85.0% (n = 17) | 1 |
| IPF | 46.6% (n = 34) | 35.0% (n = 7) | 0.411 |
| non-IPF (iNSIP) | 38.4% (n = 28) | 50.0%(n = 10) | 0.414 |
| CTD-ILD | 15.1% (n = 11) | 15.0% (n = 3) | 1 |
| RA | 8.2% (n = 6) | 5.0% (n = 1) | 1 |
| DM PM | 6.8% (n = 5) | 0% (n = 0) | 0.581 |
| SLE | 1.4% (n = 1) | 0% (n = 0) | 1 |
| SSc | 0% (n = 0) | 10.0% (n = 2) | 0.044 |
| Stage I or II | 17.8% (n = 13) | 0% (n = 0) | 0.052 |
| Duration of illness (years) | 4.0 (3.0–5.0) | 4.0 (1.0–6.0) | 0.501 |
| Comorbidities | |||
| Asthma | 0% (n = 0) | 0% (n = 0) | N.D |
| COPD | 8.2%(n = 6) | 35.0%(n = 7) | 0.006 |
| Cardiac diseases | 19.2% (n = 14) | 40.0% (n = 8) | 0.074 |
| NIDDM type 2 | 8.2% (n = 6) | 35.0% (n = 7) | 0.006 |
| Maintenance hemodialysis | 1.4% (n = 1) | 0% (n = 0) | 1 |
| Malignant diseases | 17.8% (n = 13) | 25.0% (n = 5) | 0.526 |
| Respiratory viruses | |||
| SARS-CoV-2 | 0% (n = 0) | 10.0% (n = 2) | 0.044 |
| Bacteria | 1.4% (n = 1) | 5.0% (n = 1) | 0.386 |
| MRSA | 1.4% (n = 1) | 0% (n = 0) | 1 |
| Pseudomonas aeruginosa | 0% (n = 0) | 5.0% (n = 1) | 0.215 |
| Vital signs | |||
| BT (°C) | 36.6 (36.6–37.2) | 36.7 (36.6–37.1) | 0.191 |
| RR (breaths/min) | 22 (18–28) | 24 (20–32) | 0.209 |
| ΔHR/ΔBT | 36.3 (15.9–190.3) | 16.5 (7.5–32.0) | 0.109 |
| SpO2 (%) | 88.0 (82.0–95.0) | 86.0 (81.0–91.0) | 0.784 |
| BMI | 22.4 (19.4–25.3) | 21.5 (16.0–26.0) | 0.726 |
| Symptoms | |||
| nasal discharge | 5.5% (n = 4) | 5.0% (n = 1) | 1 |
| sore throat | 1.4% (n = 1) | 0% (n = 0) | 1 |
| dyspnea | 1.4% (n = 1) | 0% (n = 0) | 0.085 |
| Antifibrotic agents | 12.3% (n = 9) | 0% (n = 0) | 0.197 |
| Previous episodes of AEs | 16.4% (n = 12) | 5.0% (n = 1) | 0.285 |
| All Patients (n = 93) | Survivors (n = 48) | Deceased (n = 45) | p Value |
|---|---|---|---|
| Age | 79 (70–84) | 81 (72–85) | 0.142 |
| Sex | 62.5% (n = 30) | 66.7% (n = 30) | 0.829 |
| Duration of illness (years) | 4.0 (2.8–5.3) | 4.0 (2.5–5.0) | 0.634 |
| BMI | 21.6 (18.7–24.6) | 21.5 (19.0–24.0) | 0.860 |
| Smoker (ex or current) | 66.7% (n = 32) | 68.9% (n = 31) | 0.829 |
| SpO2 (%) | 90.0 (83.0–94.0) | 84.0 (73–91.0) | 0.008 ** |
| Hypoxemia (<SpO2 94%) | 79.2% (n = 48) | 88.9% (n = 45) | 0.264 |
| RR (breaths/min) | 20 (18–22) | 30 (24–36) | 0.013 * |
| HR (beats/min) | 91(80–106) | 85 (78–102) | 0.997 |
| RR ≥ 20 (breaths/min) | 58.3% (n = 28) | 73.3% (n = 33) | 0.190 |
| RR ≥ 30 (breaths/min) | 12.5%(n = 6) | 33.3% (n = 15) | 0.025 * |
| ΔHR/ΔBT | 27.8 (19.5–117.5) | 35.0(9.6–188) | 0.694 |
| Body temperature (°C) | 36.7 (36.6–37.1) | 36.6 (36.6–36.7) | 0.375 |
| IIPs | 85.4% (n = 41) | 84.4% (n = 38) | 1.0 |
| IPF | 43.8% (n = 21) | 44.4% (n = 20) | 0.824 |
| non-IPF (iNSIP) | 41.7% (n = 20) | 40.0% (n = 18) | 1.0 |
| CTD-ILD | |||
| RA | 6.3% (n = 3) | 11.1% (n = 5) | 0.205 |
| DM | 2.1% (n = 1) | 0% (n = 0) | 0.330 |
| SLE | 2.1% (n = 1) | 0% (n = 0) | 0.330 |
| Stage I or II * | 10.4% (n = 5) | 17.8% (n = 8) | 0.755 |
| Comorbidities | |||
| COPD | 12.5% (n = 6) | 15.6% (n = 7) | 0.769 |
| Cardiac diseases | 16.7% (n = 8) | 31.1% (n = 14) | 0.149 |
| NIDDM type 2 | 16.7% (n = 8) | 11.1% (n = 5) | 0.440 |
| Maintenance hemodialysis | 2.1% (n = 1) | 0% (n = 0) | 0.330 |
| Malignant diseases | 14.5% (n = 7) | 22.9% (n = 11) | 0.871 |
| Previous episodes of AE | 12.5% (n = 6) | 15.6% (n = 7) | 0.769 |
| HOT on admission | 22.9% (n = 11) | 17.8% (n = 8) | 0.612 |
| Hospital days | 28.0 (21.0–39.0) | 18.0 (13.0–33.0) | 0.003 ** |
| Duration of hypoxemia or needs more O2 supply than usual (days) | 9.5 (3.0–13.0) | 10.0(5.0–16.0) | 0.106 |
| Respiratory viruses | 4.2% (n = 2) | 0% (n = 0) | 0.495 |
| Bacteria | 0% (n = 0) | 4.4% (n = 2) | 0.231 |
| Symptoms | |||
| Nasal discharge | 6.3% (n = 3) | 4.4% (n = 2) | 1 |
| Sore throat | 2.1% (n = 1) | 0% (n = 0) | 1 |
| Dyspnea on efforts | 68.8% (n = 33) | 77.8% (n = 35) | 0.358 |
| Antifibrotic agents | 8.3% (n = 4) | 11.1% (n = 5) | 0.735 |
| Treatments | |||
| IVCY | 18.8% (n = 9) | 42.2% (n = 19) | 0.023 * |
| mPSL pulse | 81.3% (n = 39) | 91.1% (n = 41) | 0.235 |
| Laboratory data | |||
| WBC | 9400 (7275–10925) | 11,000 (9100–13,650) | 0.004 ** |
| Monocyte | 7.1 (6.1–10.9) | 6.2 (5.0–7.5) | 0.010 * |
| Albumin | 3.1 (2.7–3.4) | 2.9 (2.5–3.2) | 0.101 |
| Plt | 24.9 (18.1–32.2) | 20.4 (15.2–30.2) | 0.089 |
| CRP | 7.51 (3.60–12.8) | 10.2(5.5–14.4) | 0.114 |
| LDH | 317 (251–363) | 384 (295–517) | 0.004 ** |
| LDH ≥ 350 | 39.6% (n = 19) | 51.1% (n = 23) | 0.005 ** |
| KL-6 | 989 (691–1707) | 988 (547–2145) | 0.913 |
| SP-D | 252 (127–434) | 401 (270–719) | 0.007 ** |
| SP-D ≥ 314 | 29.2% (n = 14) | 40.4% (n = 18) | 0.046 * |
| Pulmonary function test | |||
| VC | 70.8 (55.2–86.6) | 82.6 (60.9–90.2) | 0.639 |
| FVC | 72.5 (56.4–92.6) | 80.9 (63.1–84.7) | 0.736 |
| FEV1.0% | 84.2 (80.1–86.9) | 79.9 (73.1–86.3) | 0.691 |
| %FEV1.0 | 74.8 (59.9–92.8) | 74.2 (70.0–87.0) | 0.791 |
| %DLCO | 41.6 (33.3–54.3) | 57.4 (30.0–70.6) | 0.336 |
| %DLCO/VA | 50.0(40.0–75.3) | 59.4 (49.8–74.3) | 0.428 |
| Parameter | Hazard Ratio (95%CI) | p Value |
|---|---|---|
| Age, yr | 1.036 (0.974–1.103) | 0.262 |
| Male sex | 1.538 (0.493–4.801) | 0.459 |
| LDH ≥ 350 (IU/L) | 3.997(1.452–11.0) | 0.007 ** |
| RR ≥ 30 (breaths/min) | 4.854 (1.613–14.609) | 0.005 ** |
| Parameter | Hazard Ratio (95%CI) | p Value |
|---|---|---|
| Age, yr | 1.036 (0.997–1.076) | 0.069 |
| Male sex | 2.022 (0.961–4.254) | 0.064 |
| LDH ≥ 350 (IU/L) | 2.783 (1.480–5.235) | 0.001 ** |
| RR ≥ 30 (breaths/min) | 3.332 (1.710–6.492) | <0.001 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takagi, R.; Saraya, T.; Yamada, S.; Nakajima, K.; Doi, K.; Akizawa, T.; Ishikawa, N.; Kurokawa, N.; Kobayashi, F.; Nunokawa, H.; et al. Clinical Evaluation of Acute Exacerbation of Interstitial Lung Disease in a Single Tertiary Center: Perspectives before and after the Coronavirus Disease 2019 Pandemic. J. Clin. Med. 2024, 13, 5733. https://doi.org/10.3390/jcm13195733
Takagi R, Saraya T, Yamada S, Nakajima K, Doi K, Akizawa T, Ishikawa N, Kurokawa N, Kobayashi F, Nunokawa H, et al. Clinical Evaluation of Acute Exacerbation of Interstitial Lung Disease in a Single Tertiary Center: Perspectives before and after the Coronavirus Disease 2019 Pandemic. Journal of Clinical Medicine. 2024; 13(19):5733. https://doi.org/10.3390/jcm13195733
Chicago/Turabian StyleTakagi, Ryo, Takeshi Saraya, Sho Yamada, Kei Nakajima, Kazuyuki Doi, Takatora Akizawa, Narishige Ishikawa, Nozomi Kurokawa, Fumi Kobayashi, Hiroki Nunokawa, and et al. 2024. "Clinical Evaluation of Acute Exacerbation of Interstitial Lung Disease in a Single Tertiary Center: Perspectives before and after the Coronavirus Disease 2019 Pandemic" Journal of Clinical Medicine 13, no. 19: 5733. https://doi.org/10.3390/jcm13195733
APA StyleTakagi, R., Saraya, T., Yamada, S., Nakajima, K., Doi, K., Akizawa, T., Ishikawa, N., Kurokawa, N., Kobayashi, F., Nunokawa, H., Aso, J., Nakamoto, Y., Ishida, M., Sada, M., Honda, K., Nakamoto, K., Takata, S., & Ishii, H. (2024). Clinical Evaluation of Acute Exacerbation of Interstitial Lung Disease in a Single Tertiary Center: Perspectives before and after the Coronavirus Disease 2019 Pandemic. Journal of Clinical Medicine, 13(19), 5733. https://doi.org/10.3390/jcm13195733







