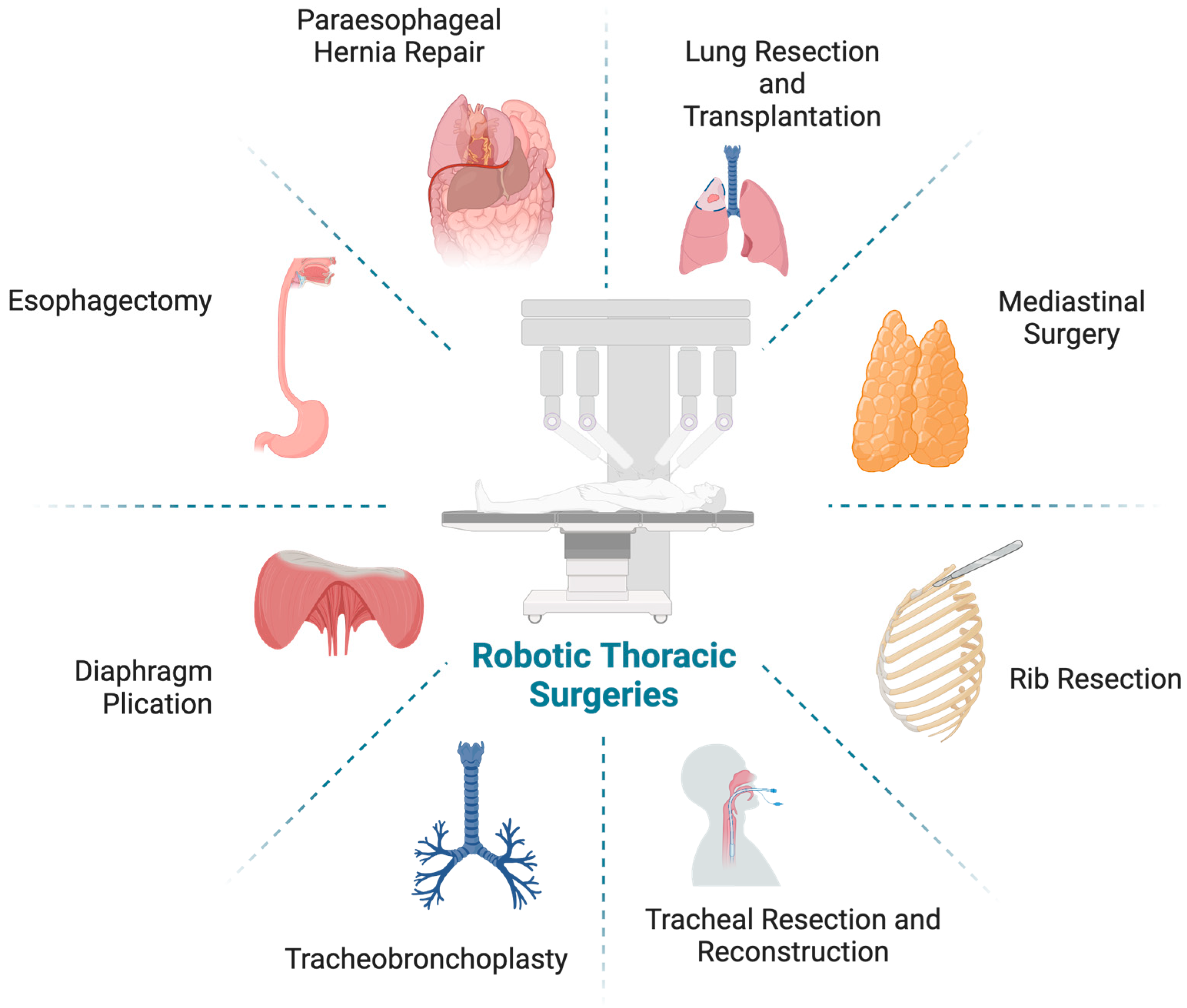
Adoption of the Robotic Platform across Thoracic Surgeries
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
3. Lung Resection
3.1. Wedge Resection
3.2. Segmentectomy
3.3. Sleeve Lobectomy
4. Lung Transplantation
5. Mediastinal Surgery
6. Rib Resection
7. Tracheal Resection and Reconstruction
8. Tracheobronchoplasty
9. Diaphragm Plication
10. Esophagectomy
11. Paraesophageal Hernia Repair
12. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Linsky, P.L.; Wei, B. Training in robotic thoracic surgery. J. Vis. Surg. 2018, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Raad, W.N.; Ayub, A.; Huang, C.-Y.; Guntman, L.; Rehmani, S.S.; Bhora, F.Y. Robotic Thoracic Surgery Training for Residency Programs: A Position Paper for an Educational Curriculum. Innov. Technol. Tech. Cardiothorac. Vasc. Surg. 2018, 13, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Altorki, N.; Wang, X.; Kozono, D.; Watt, C.; Landrenau, R.; Wigle, D.; Port, J.; Jones, D.R.; Conti, M.; Ashrafi, A.S.; et al. Lobar or Sublobar Resection for Peripheral Stage IA Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2023, 388, 489–498. [Google Scholar] [CrossRef]
- Martin, J.L.; Mack, S.J.; Rshaidat, H.; Collins, M.L.; Whitehorn, G.L.; Grenda, T.R.; Evans, N.R.; Okusanya, O.T. Wedge Resection Outcomes: A Comparison of Video-Assisted and Robot-Assisted Wedge Resections. Ann. Thorac. Surg. 2024, 118, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Demir, A.; Ayalp, K.; Ozkan, B.; Kaba, E.; Toker, A. Robotic and video-assisted thoracic surgery lung segmentectomy for malignant and benign lesions. Interact. Cardiovasc. Thorac. Surg. 2015, 20, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Baste, J.M.; Soldea, V.; Lachkar, S.; Rinieri, P.; Sarsam, M.; Bottet, B.; Peillon, C. Development of a precision multimodal surgical navigation system for lung robotic segmentectomy. J. Thorac. Dis. 2018, 10, S1195–S1204. [Google Scholar] [CrossRef]
- Liang, H.; Liang, W.; Zhao, L.; Chen, D.; Zhang, J.; Zhang, Y.; Tang, S.; He, J. Robotic versus Video-assisted Lobectomy/Segmentectomy for Lung Cancer: A Meta-analysis. Ann. Surg. 2018, 268, 254–259. [Google Scholar] [CrossRef]
- Ferrari-Light, D.; Cerfolio, R.J. Non-small cell lung cancer 2 cm or less: Robotic segmentectomy sets the gold standard against non-surgical therapy. Ann. Transl. Med. 2019, 7, S96. [Google Scholar] [CrossRef]
- Nguyen, D.; Gharagozloo, F.; Tempesta, B.; Meyer, M.; Gruessner, A. Long-term results of robotic anatomical segmentectomy for early-stage non-small-cell lung cancer. Eur. J. Cardio-Thorac. Surg. 2019, 55, 427–433. [Google Scholar] [CrossRef]
- Xie, B.; Sui, T.; Qin, Y.; Miao, S.; Jiao, W. [Comparison of Short-term Outcomes of Lung Segmentectomy by Robotic-assisted and Video-assisted Thoracoscopic Surgery]. Zhongguo Fei Ai Za Zhi 2019, 22, 767–771. [Google Scholar] [CrossRef]
- Kagimoto, A.; Tsutani, Y.; Izaki, Y.; Handa, Y.; Mimae, T.; Miyata, Y.; Okada, M. Initial experience of robotic anatomical segmentectomy for non-small cell lung cancer. Ultrasound Med. Biol. 2020, 50, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, C.; Hu, J.; Han, Y.; Huang, M.; Xiang, J.; Li, H. Early outcomes of robotic versus thoracoscopic segmentectomy for early-stage lung cancer: A multi-institutional propensity score-matched analysis. J. Thorac. Cardiovasc. Surg. 2020, 160, 1363–1372. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Huang, J.; Pan, F.; Li, J.; Liu, Y.; Hou, Y.; Song, W.; Luo, Q. Operative outcomes and long-term survival of robotic-assisted segmentectomy for stage IA lung cancer compared with video-assisted thoracoscopic segmentectomy. Transl. Lung Cancer Res. 2020, 9, 306–315. [Google Scholar] [CrossRef]
- Gergen, A.K.; White, A.M.; Mitchell, J.D.; Meguid, R.A.; Fullerton, D.A.; Scott, C.D.; Weyant, M.J. Introduction of robotic surgery leads to increased rate of segmentectomy in patients with lung cancer. J. Thorac. Dis. 2021, 13, 762–767. [Google Scholar] [CrossRef]
- Kodia, K.; Razi, S.S.; Alnajar, A.; Nguyen, D.M.; Villamizar, N. Comparative Analysis of Robotic Segmentectomy for Non-Small Cell Lung Cancer: A National Cancer Database Study. Innov. Technol. Tech. Cardiothorac. Vasc. Surg. 2021, 16, 280–287. [Google Scholar] [CrossRef]
- Mao, J.; Tang, Z.; Mi, Y.; Xu, H.; Li, K.; Liang, Y.; Wang, N.; Wang, L. Robotic and video-assisted lobectomy/segmentectomy for non-small cell lung cancer have similar perioperative outcomes: A systematic review and meta-analysis. Transl. Cancer Res. 2021, 10, 3883–3893. [Google Scholar] [CrossRef]
- Zhang, J.; Feng, Q.; Huang, Y.; Ouyang, L.; Luo, F. Updated Evaluation of Robotic- and Video-Assisted Thoracoscopic Lobectomy or Segmentectomy for Lung Cancer: A Systematic Review and Meta-Analysis. Front. Oncol. 2022, 12, 853530. [Google Scholar] [CrossRef] [PubMed]
- Yanagiya, M.; Nagano, M.; Nakajima, J. Fissureless technique of robotic left lingular segmentectomy for primary lung cancer with incomplete fissure: A case report. J. Cardiothorac. Surg. 2023, 18, 125. [Google Scholar] [CrossRef]
- Igai, H.; Nii, K.; Kamiyoshihara, M. Robotic upper division segmentectomy of the left upper lobe without turning the lung. Multimed. Man. Cardio-Thorac. Surg. 2024, 2024. [Google Scholar] [CrossRef]
- Igai, H.; Nii, K.; Kamiyoshihara, M. Two cases of lower lobe segmentectomy (left and right) using the lung-inverted approach in a robotic operation. Multimed. Man. Cardio-Thorac. Surg. 2024, 2024. [Google Scholar] [CrossRef]
- Schmid, T.; Augustin, F.; Kainz, G.; Pratschke, J.; Bodner, J. Hybrid Video-Assisted Thoracic Surgery-Robotic Minimally Invasive Right Upper Lobe Sleeve Lobectomy. Ann. Thorac. Surg. 2011, 91, 1961–1965. [Google Scholar] [CrossRef] [PubMed]
- Cerfolio, R.J. Robotic sleeve lobectomy: Technical details and early results. J. Thorac. Dis. 2016, 8, S223–S226. [Google Scholar] [CrossRef]
- Lin, M.-W.; Kuo, S.-W.; Yang, S.-M.; Lee, J.-M. Robotic-assisted thoracoscopic sleeve lobectomy for locally advanced lung cancer. J. Thorac. Dis. 2016, 8, 1747–1752. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chen, H.; Qiu, T.; Xuan, Y.; Luo, Y.; Shen, Y.; Jiao, W. Robotic-assisted sleeve lobectomy for right upper lobe combining with middle lobe resection of lung cancer. J. Vis. Surg. 2016, 2, 178. [Google Scholar] [CrossRef] [PubMed]
- Jo, M.S.; Kim, D.Y.; Jeong, J.Y.; Lee, G.D. Robotic sleeve lobectomy with four arms for lung cancer centrally located in the right lower lobe: A case report. J. Cardiothorac. Surg. 2017, 12, 108. [Google Scholar] [CrossRef]
- Qiu, T.; Zhao, Y.; Xuan, Y.; Jiao, W. Robotic-assisted double-sleeve lobectomy. J. Thorac. Dis. 2017, 9, E21–E25. [Google Scholar] [CrossRef]
- Tan, G.J.S.; Poon, J.S.; Khoo, P.L.Z.; Yoong, A.W.H.; Nardini, M.; Dunning, J. Robotic left lower sleeve lobectomy with bronchoplasty for the removal of a carcinoid tumour. J. Vis. Surg. 2018, 4, 84. [Google Scholar] [CrossRef]
- Durand, M. Four-arm robotic sleeve right upper lobectomy. Ann. Cardiothorac. Surg. 2019, 8, 286–287. [Google Scholar] [CrossRef]
- Egberts, J.H.; Moller, T.; Becker, T. Robotic-Assisted Sleeve Lobectomy Using the Four-Arm Technique in the DaVinci Si(R) and Xi(R) Systems. Thorac. Cardiovasc. Surg 2019, 67, 603–605. [Google Scholar] [CrossRef]
- Jiao, W.; Zhao, Y.; Qiu, T.; Xuan, Y.; Sun, X.; Qin, Y.; Liu, A.; Sui, T.; Cui, J. Robotic Bronchial Sleeve Lobectomy for Central Lung Tumors: Technique and Outcome. Ann. Thorac. Surg. 2019, 108, 211–218. [Google Scholar] [CrossRef]
- Qiu, T.; Zhao, Y.; Xuan, Y.; Qin, Y.; Niu, Z.; Shen, Y.; Jiao, W. Robotic sleeve lobectomy for centrally located non–small cell lung cancer: A propensity score–weighted comparison with thoracoscopic and open surgery. J. Thorac. Cardiovasc. Surg. 2020, 160, 838–846.e2. [Google Scholar] [CrossRef] [PubMed]
- Watkins, A.A.; Quadri, S.M.; Servais, E.L. Robotic-Assisted Complex Pulmonary Resection: Sleeve Lobectomy for Cancer. Innov. Technol. Tech. Cardiothorac. Vasc. Surg. 2021, 16, 132–135. [Google Scholar] [CrossRef]
- Paglialunga, P.L.; Molins, L.; Guzman, R.; Guirao, A.; Grando, L.; Sanchez-Lorente, D.; Guerrero, C.; Bello, I.; Quiroga, N.; Boada, M. Starting a robotic thoracic surgery program: From wedge resection to sleeve lobectomy in six months. Initial conclusions. Cir. Esp. (Engl. Ed.) 2023, 101, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Jacob, A.; Stamenkovic, S.A. Robotic-assisted thoracic surgery: Left upper lobe sleeve lobectomy for an endobronchial tumour. Multimed. Man. Cardio-Thorac. Surg. 2024, 2024. [Google Scholar] [CrossRef]
- Gonzalez-Rivas, D.; Koziej, P.-H.; Sediqi, S.; Ruprecht, B.; Jostmeyer, H.; Valdivia, D. Uniportal hybrid robotic-assisted right upper sleeve lobectomy in an 83-year-old patient with severe pulmonary hypertension. Ann. Cardiothorac. Surg. 2023, 12, 136–138. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Rivas, D.; Prado, R.F.; Garcia-Perez, A.; Bosinceanu, M.L.; Motas, N.; Manolache, V. Bilateral uniportal robotic-assisted thoracic surgery (RATS) sleeve lobectomy for a bilateral endobronchial lung cancer. Ann. Cardiothorac. Surg. 2023, 12, 64–66. [Google Scholar] [CrossRef]
- Toronto Lung Transplant, G. Unilateral lung transplantation for pulmonary fibrosis. N. Engl. J. Med. 1986, 314, 1140–1145. [Google Scholar] [CrossRef]
- DeVito Dabbs, A.D.; Dew, M.; Zaldonis, D.; Aubrecht, J.; Crespo, M.; Pilewski, J.; Bhama, J.; Gilbert, S.; Bermudez, C.; Toyoda, Y. 82: Patient-Reported Outcomes after the Minimally Invasive Approach to Lung Transplantation. J. Heart Lung Transplant. 2010, 29, S33. [Google Scholar] [CrossRef]
- Ahmed, H.; Zeschky, C.; Alayyar, M.; Husain, M.; Jothidasan, A.; Padukone, A.; Bello, S.; Marczin, N.; Smail, H.; Stock, U. Long Term Outcomes of Minimally Invasive Lung Transplantation Compared to Clamshell Approach. J. Heart Lung Transplant. 2022, 41, S265. [Google Scholar] [CrossRef]
- Thomas, J.; Chen, Q.; Malas, J.; Barnes, D.; Roach, A.; Peiris, A.; Premananthan, S.; Krishnan, A.; Rowe, G.; Gill, G.; et al. Impact of minimally invasive lung transplantation on early outcomes and analgesia use: A matched cohort study. J. Heart Lung Transplant. 2024, 43, 1358–1366. [Google Scholar] [CrossRef]
- Fischer, S.; Strüber, M.; Simon, A.R.; Anssar, M.; Wilhelmi, M.; Leyh, R.G.; Harringer, W.; Haverich, A. Video-assisted minimally invasive approach in clinical bilateral lung transplantation. J. Thorac. Cardiovasc. Surg. 2001, 122, 1196–1198. [Google Scholar] [CrossRef] [PubMed]
- Marczin, N.; Popov, A.F.; Zych, B.; Romano, R.; Kiss, R.; Sabashnikov, A.; Soresi, S.; De Robertis, F.; Bahrami, T.; Amrani, M.; et al. Outcomes of minimally invasive lung transplantation in a single centre: The routine approach for the future or do we still need clamshell incision? Interact. Cardiovasc. Thorac. Surg. 2016, 22, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Ascanio, F.; Royo-Crespo, I.; Rosado, J.; Sánchez, L.; Romero, L.; Durán-Rey, D.; Sánchez-Margallo, F.; Jauregui, A. Advances in robotic lung transplantation: Development and validation of a new surgical technique in animal models. Interdiscip. Cardiovasc. Thorac. Surg. 2023, 37, ivad179. [Google Scholar] [CrossRef]
- Ascanio, F.; Royo-Crespo, I.; Jauregui, A. Robotic Lung Transplantation: A Paradigm Shift in Surgical Strategy. Arch. Bronc. 2023, 60, 3–4. [Google Scholar] [CrossRef] [PubMed]
- Jiao, W.; Yang, R.; Zhao, Y.; Ge, N.; Qiu, T.; Sun, X.; Liu, Y.; Li, K.; Li, Z.; Yu, W.; et al. Robot-assisted single lung transplantation. Chin. Med. J. (Engl.) 2023, 136, 362–364. [Google Scholar] [CrossRef] [PubMed]
- Emerson, D.C.P.; Rampolla, R.; Chikwe, J.; Megna, D. Robotic-assisted lung transplantation: First in man. J. Heart Lung Transplant. 2024, 43, 158–161. [Google Scholar] [CrossRef]
- Burt, B.M.; Yao, X.; Shrager, J.; Antonicelli, A.; Padda, S.; Reiss, J.; Wakelee, H.; Su, S.; Huang, J.; Scott, W. Determinants of Complete Resection of Thymoma by Minimally Invasive and Open Thymectomy: Analysis of an International Registry. J. Thorac. Oncol. 2017, 12, 129–136. [Google Scholar] [CrossRef]
- Wu, W.-J.; Zhang, F.-Y.; Xiao, Q.; Li, X.-K. Does robotic-assisted thymectomy have advantages over video-assisted thymectomy in short-term outcomes? A systematic view and meta-analysis. Interact. Cardiovasc. Thorac. Surg. 2021, 33, 385–394. [Google Scholar] [CrossRef]
- Coco, D.; Leanza, S. Robotic thymectomy: A review of techniques and results. Pol. J. Cardio-Thorac. Surg. 2023, 20, 36–44. [Google Scholar] [CrossRef]
- Su, K.W.; Luketich, J.D.; Sarkaria, I.S. Robotic-assisted minimally invasive thymectomy for myasthenia gravis with thymoma. JTCVS Tech. 2022, 13, 270–274. [Google Scholar] [CrossRef]
- Grigoroiu, M.; Rheinwald, M.; Ryckembusch, L.; Kemper, J.; Brian, E.; Boddaert, G.; Seguin-Givelet, A.; Mariolo, A.V. Full subcostal subxiphoid robotic-assisted radical thymectomy: Preclinical cadaveric study for optimizing patient positioning, table settings, and port configuration. Updat. Surg. 2022, 74, 1733–1738. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, M.; Ishihara, S.; Okada, S.; Inoue, M. Robotic subxiphoid-optical thymectomy. Interact. Cardiovasc. Thorac. Surg. 2022, 35, ivac104. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, F.; Qiu, G.; Li, Z.; Chen, L.-Q.; Wang, Y. Surgical Tips to Improve Completeness of Transsubxiphoid Robotic Extended Thymectomy. Ann. Thorac. Surg. 2022, 114, e223–e225. [Google Scholar] [CrossRef] [PubMed]
- E, H.; Yang, C.; Zhang, L.; Xia, L.; Xu, L.; Song, N.; Hu, X.; Zhu, Y.; Chen, C.; Zhao, D. Perioperative outcomes comparison of robotic and video-assisted thoracoscopic thymectomy for thymic epithelial tumor: A single-center experience. Updat. Surg. 2023, 76, 1511–1519. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.F.; Cheng, C.; Suen, K.H.; Stein, H.; Chao, Y.K. A Preclinical Feasibility Study of Single-Port Robotic Subcostal Anatomical Lung Resection and Subxiphoid Thymectomy Using the da Vinci((R)) SP System. Diagnostics 2023, 13, 460. [Google Scholar] [CrossRef]
- Azenha, L.F.; Deckarm, R.; Minervini, F.; Dorn, P.; Lutz, J.; Kocher, G.J. Robotic vs. Transsternal Thymectomy: A Single Center Experience over 10 Years. J. Clin. Med. 2021, 10, 4991. [Google Scholar] [CrossRef]
- Geraci, T.C.; Ferrari-Light, D.; Pozzi, N.; Cerfolio, R.J. Midterm Results for Robotic Thymectomy for Malignant Disease. Ann. Thorac. Surg. 2021, 111, 1675–1681. [Google Scholar] [CrossRef]
- Tamagawa, S.; Hashimoto, K.; Ichinose, J.; Matsuura, Y.; Nakao, M.; Okumura, S.; Satoh, Y.; Mun, M. Phrenic nerve interposition in a completely portal robotic thymectomy. JTCVS Tech. 2023, 20, 182–185. [Google Scholar] [CrossRef]
- McCormack, A.J.; El Zaeedi, M.; Dorsey, M.; Cerfolio, R.J. A chest tube after robotic thymectomy is unnecessary. JTCVS Open 2023, 16, 1004–1007. [Google Scholar] [CrossRef] [PubMed]
- Gharagozloo, F.; Meyer, M.; Tempesta, B.J.; Margolis, M.; Strother, E.T.; Tummala, S. Robotic en bloc first-rib resection for Paget-Schroetter disease, a form of thoracic outlet syndrome: Technique and initial results. Innovations 2012, 7, 39–44. [Google Scholar] [CrossRef]
- Kocher, G.J.; Zehnder, A.; Lutz, J.A.; Schmidli, J.; Schmid, R.A. First Rib Resection for Thoracic Outlet Syndrome: The Robotic Approach. World J. Surg. 2018, 42, 3250–3255. [Google Scholar] [CrossRef] [PubMed]
- Gharagozloo, F.; Meyer, M.; Tempesta, B.; Gruessner, S. Robotic transthoracic first-rib resection for Paget–Schroetter syndrome. Eur. J. Cardio-Thorac. Surg. 2019, 55, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Gharagozloo, F.; Meyer, M.; Tempesta, B.; Werden, S. Robotic First Rib Resection for Thoracic Outlet Syndrome. Surg. Technol. Int. 2020, 36, 239–244. [Google Scholar]
- Pupovac, S.S.; Lee, P.C.; Zeltsman, D.; Jurado, J.; Hyman, K.; Singh, V. Robotic-Assisted First Rib Resection: Our Experience and Review of the Literature. Semin. Thorac. Cardiovasc. Surg. 2020, 32, 1115–1120. [Google Scholar] [CrossRef]
- Gharagozloo, F.; Atiquzzaman, N.; Meyer, M.; Tempesta, B.; Werden, S. Robotic first rib resection for thoracic outlet syndrome. J. Thorac. Dis. 2021, 13, 6141–6154. [Google Scholar] [CrossRef]
- Zehnder, A.; Lutz, J.; Dorn, P.; Minervini, F.; Kestenholz, P.; Gelpke, H.; Schmid, R.A.; Kocher, G.J. Robotic-Assisted Thoracoscopic Resection of the First Rib for Vascular Thoracic Outlet Syndrome: The New Gold Standard of Treatment? J. Clin. Med. 2021, 10, 3952. [Google Scholar] [CrossRef]
- Gkikas, A.; Lampridis, S.; Patrini, D.; Kestenholz, P.B.; Azenha, L.F.; Kocher, G.J.; Scarci, M.; Minervini, F. Thoracic Outlet Syndrome: Single Center Experience on Robotic Assisted First Rib Resection and Literature Review. Front. Surg. 2022, 9, 848972. [Google Scholar] [CrossRef] [PubMed]
- Coyan, G.; Daon, E. Resection of supernumerary intrathoracic rib using robotic-assisted video-assisted thoracoscopic surgery. Surg. Radiol. Anat. 2016, 38, 415–417. [Google Scholar] [CrossRef]
- Liu, B.; Gao, S.; Wu, Q.; Li, H.; Zhang, G.; Fu, J. A case report of robotic-assisted resection of large fibrous benign tumor of second rib. J. Cardiothorac. Surg. 2022, 17, 329. [Google Scholar] [CrossRef] [PubMed]
- Rojo, M.; Abdelsattar, Z. Robotic resection of a second rib osteochondroma. Multimed. Man. Cardiothorac. Surg. 2023, 2023. [Google Scholar] [CrossRef]
- Zehnder, A.; Dorn, P.; Lutz, J.; Minervini, F.; Kestenholz, P.; Gelpke, H.; Schmid, R.A.; Kocher, G.J. Completely Thoracoscopic 3-Port Robotic First Rib Resection for Thoracic Outlet Syndrome. Ann. Thorac. Surg. 2022, 114, 1238–1244. [Google Scholar] [CrossRef] [PubMed]
- Ureña, A.; Déniz, C.; Muñoz, A.; Macía, I.; Rivas, F.; Ramos, R. Uniportal robotic-assisted thoracoscopic surgery: Resection of the first rib. Ann. Cardiothorac. Surg. 2023, 12, 62–63. [Google Scholar] [CrossRef] [PubMed]
- Reyes, M.; Alaparthi, S.; Roedl, J.B.; Moreta, M.C.; Evans, N.R.; Grenda, T.; Okusanya, O.T. Robotic First Rib Resection in Thoracic Outlet Syndrome: A Systematic Review of Current Literature. J. Clin. Med. 2023, 12, 6689. [Google Scholar] [CrossRef]
- Burt, B.M.; Palivela, N.; Cekmecelioglu, D.; Paily, P.; Najafi, B.; Lee, H.-S.; Montero, M. Safety of robotic first rib resection for thoracic outlet syndrome. J. Thorac. Cardiovasc. Surg. 2020, 162, 1297–1305.e1. [Google Scholar] [CrossRef]
- Lazzaro, R.; Patton, B. Commentary: Robotic first rib resection-Building the next pillar. JTCVS Tech. 2020, 1, 110–111. [Google Scholar] [CrossRef]
- Burt, B.M.; Palivela, N.; Karimian, A.; Goodman, M.B. Transthoracic robotic first rib resection: Twelve steps. JTCVS Tech. 2020, 1, 104–109. [Google Scholar] [CrossRef]
- Palivela, N.B.; Burt, B.M. Transthoracic Robotic First and Cervical Rib Resection for Thoracic Outlet Syndrome. Ann. Surg. 2023, 277, E1215–E1216. [Google Scholar] [CrossRef] [PubMed]
- Egyud, M.R.; Holmes, S.; Burt, B.M. Technical Aspects of Robotic First Rib Resection. Thorac. Surg. Clin. 2023, 33, 265–271. [Google Scholar] [CrossRef]
- Jiao, W.; Zhao, Y.; Luo, Y.; Wang, H.; Yang, X.; Ren, X.; Zhang, L.; Luo, Y. Totally robotic-assisted non-circumferential tracheal resection and anastomosis for leiomyoma in an elderly female. J. Thorac. Dis. 2015, 7, 1857–1860. [Google Scholar] [CrossRef]
- Qiu, T.; Zhao, Y.; Song, J.; Jiao, W. Robotic circumferential tracheal resection and reconstruction via a completely portal approach. Thorac. Cancer 2019, 10, 378–380. [Google Scholar] [CrossRef]
- Li, S.; Ai, Q.; Liang, H.; Liu, H.; Yang, C.; Deng, H.; Zhong, Y.; Zhang, J.; He, J. Nonintubated Robotic-assisted Thoracic Surgery for Tracheal/Airway Resection and Reconstruction: Technique Description and Preliminary Results. Ann. Surg. 2022, 275, e534–e536. [Google Scholar] [CrossRef] [PubMed]
- Zalepugas, D.; Schnorr, P.; Schmidt, J.; Bedetti, B. Non-intubated robotic-assisted thoracic surgery for tracheal/airway resection and reconstruction safe: Editorial commentary. Ann. Transl. Med. 2021, 9, 1707. [Google Scholar] [CrossRef]
- Spaggiari, L.; Galetta, D.; Iacono, G.L.; Cara, A.; Bertolaccini, L.; Casiraghi, M.; Mohamed, S. Robotic-assisted tracheal resection for adenoid cystic carcinoma with extracorporeal membrane oxygenation support. JTCVS Tech. 2023, 21, 244–246. [Google Scholar] [CrossRef]
- Dal Negro, R.W.; Tognella, S.; Guerriero, M.; Micheletto, C. Prevalence of tracheobronchomalacia and excessive dynamic airway collapse in bronchial asthma of different severity. Multidiscip. Respir. Med. 2013, 8, 32. [Google Scholar] [CrossRef]
- Boiselle, P.M.; O’Donnell, C.R.; Bankier, A.A.; Ernst, A.; Millet, M.E.; Potemkin, A.; Loring, S.H. Tracheal collapsibility in healthy volunteers during forced expiration: Assessment with multidetector CT. Radiology 2009, 252, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Bakhos, C.T.; Abbas, A.E. The evolution of tracheobronchoplasty. J. Vis. Surg. 2022, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- Tse, D.G.; Han, S.M.; Charuworn, B.; Kaufer, E.S. Video-assisted thoracoscopic surgical tracheobronchoplasty for tracheobronchomalacia. J. Thorac. Cardiovasc. Surg. 2011, 142, 714–716. [Google Scholar] [CrossRef]
- Lazar, J.F.; Posner, D.H.; Palka, W.; Spier, L.N.; Lazzaro, R.S. Robotically Assisted Bilateral Bronchoplasty for Tracheobronchomalacia. Innovations 2015, 10, 428–430. [Google Scholar] [CrossRef]
- Lazzaro, R.; Kontopidis, I.; Medina, B.D. Just breathe: 12-step robotic tracheobronchoplasty. JTCVS Tech. 2023, 21, 239–243. [Google Scholar] [CrossRef]
- Milman, S.; Ng, T. Robotic tracheobronchoplasty is feasible, but which patients truly benefit? J. Thorac. Cardiovasc. Surg. 2019, 157, 801–802. [Google Scholar] [CrossRef]
- Lazzaro, R.S.; Patton, B.D.; Wasserman, G.A.; Karp, J.; Cohen, S.; Inra, M.L.; Scheinerman, S.J. Robotic-assisted tracheobronchoplasty: Quality of life and pulmonary function assessment on intermediate follow-up. J. Thorac. Cardiovasc. Surg. 2022, 164, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Seastedt, K.P.; Wilson, J.L.; Gangadharan, S.P. Robotic Surgery for Tracheobronchomalacia. Thorac. Surg. Clin. 2023, 33, 61–69. [Google Scholar] [CrossRef]
- Inra, M.L.; Wasserman, G.A.; Karp, J.; Cohen, S.; Scheinerman, S.J.; Lazzaro, R.S. Improvement in postoperative lung function in patients with moderate to severe airway obstruction after robotic-assisted thoracoscopic tracheobronchoplasty. J. Thorac. Cardiovasc. Surg. 2023, 165, 876–885. [Google Scholar] [CrossRef]
- Kadiyala, M.; Maxfield, M.W.; Uy, K.F.; Blankenship, D.; Adler, A.C. Successful Use of an EZ-blocker for Lung Isolation and Visualization of Sutures During Minimally Invasive Robotic Tracheobronchoplasty in a Patient With Difficult Airway. J. Cardiothorac. Vasc. Anesth. 2022, 36, 2522–2525. [Google Scholar] [CrossRef]
- Groth, S.S.M.D.; Andrade, R.S.M.D. Diaphragmatic Eventration. Thorac. Surg. Clin. 2009, 19, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Taberham, R.J.; Raza, A.; Alzetani, A.; Woo, E.B.; Chamberlain, M.H.; Koulaxouzidis, G.; Amer, K.M. VATS Plication of the Diaphragm: A Descriptive Observational 10-Year Southampton Experience. Innovations 2017, 12, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Nardini, M.; Jayakumar, S.; Migliore, M.; Nosotti, M.; Paul, I.; Dunning, J. Minimally Invasive Plication of the Diaphragm: A Single-Center Prospective Study. Innov. Technol. Tech. Cardiothorac. Vasc. Surg. 2021, 16, 343–349. [Google Scholar] [CrossRef]
- Hunt, A.R.B.; Stuart, C.M.; Gergen, A.K.; Bang, T.J.; Reihman, A.E.; Helmkamp, L.J.; Lin, Y.; Mitchell, J.D.; Meguid, R.A.; Scott, C.D.; et al. Long-Term Patient-Reported Symptom Improvement and Quality of Life after Transthoracic Diaphragm Plication in Adults. J. Am. Coll. Surg. 2023, 237, 533–544. [Google Scholar] [CrossRef]
- Kwak, T.; Lazzaro, R.; Pournik, H.; Ciaburri, D.; Tortolani, A.; Gulkarov, I. Robotic thoracoscopic plication for symptomatic diaphragm paralysis. J. Robot. Surg. 2011, 6, 345–348. [Google Scholar] [CrossRef]
- Ahn, J.; Suh, J.; Jeong, J. Robot-assisted thoracoscopic surgery with simple laparoscopy for diaphragm eventration. Thorac. Cardiovasc. Surg. 2013, 61, 499–501. [Google Scholar] [CrossRef]
- Counts, S.J.; Saffarzadeh, A.G.; Blasberg, J.D.; Kim, A.W. Robotic Transthoracic Primary Repair of a Diaphragmatic Hernia and Reduction of an Intrathoracic Liver. Innovations 2018, 13, 54–55. [Google Scholar] [CrossRef] [PubMed]
- Daniels, A.; Danau, T.; Chierchia, G.B.; de Asmundis, C.; Lamote, J.; Smets, D. Robot-assisted thoracoscopic diaphragm plication for symptomatic diaphragm paralysis after cryoballoon ablation. Hear. Case Rep. 2019, 5, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, L.; Zhao, D. Outcomes and technique of robotic diaphragm plication. J. Thorac. Dis. 2021, 13, 6113–6115. [Google Scholar] [CrossRef] [PubMed]
- Hurley, P.; Djouani, A.; Lampridis, S.; Bille, A. Diaphragmatic paralysis post COVID-19 treated with robot-assisted plication reinforced with acellular dermal matrix: A report of two cases. Monaldi Arch. Chest Dis. 2022, 93, 2367. [Google Scholar] [CrossRef]
- Gergen, A.K.; Stuart, C.M.; Wojcik, B.M.; Meguid, R.A.; Scott, C.D. Robotic-Assisted Transthoracic Diaphragm Plication. Oper. Tech. Thorac. Cardiovasc. Surg. 2023, 29, 216–227. [Google Scholar] [CrossRef]
- Xu, P.P.; Chang, X.P.; Tang, S.T.; Li, S.; Cao, G.Q.; Zhang, X.; Chi, S.Q.; Fang, M.J.; Yang, D.H.; Li, X.Y. Robot-assisted thoracoscopic plication for diaphragmatic eventration. J. Pediatr. Surg. 2020, 55, 2787–2790. [Google Scholar] [CrossRef]
- Biswas Roy, S.; Haworth, C.; Ipsen, T.; Kang, P.; Hill, D.; Do, A.; Kuo, E. Transabdominal robot-assisted diaphragmatic plication: A 3.5-year experience. Eur. J. Cardio-Thorac. Surg. 2018, 53, 247–253. [Google Scholar] [CrossRef]
- Bin Asaf, B.; Kodaganur Gopinath, S.; Kumar, A.; Puri, H.V.; Pulle, M.V.; Bishnoi, S. Robotic diaphragmatic plication for eventration: A retrospective analysis of efficacy, safety, and feasibility. Asian J. Endosc. Surg. 2021, 14, 70–76. [Google Scholar] [CrossRef]
- Zwischenberger, B.A.; Kister, N.; Zwischenberger, J.B.; Martin, J.T. Laparoscopic Robot-Assisted Diaphragm Plication. Ann. Thorac. Surg. 2016, 101, 369–371. [Google Scholar] [CrossRef]
- Du, V.X.; Groth, S.S. Robot-Assisted Laparoscopic Diaphragm Plication. Oper. Tech. Thorac. Cardiovasc. Surg. 2022, 27, 449–460. [Google Scholar] [CrossRef]
- Gritsiuta, A.I.; Gordon, M.; Bakhos, C.T.; Abbas, A.E.; Petrov, R.V. Minimally Invasive Diaphragm Plication for Acquired Unilateral Diaphragm Paralysis: A Systematic Review. Innov. Technol. Tech. Cardiothorac. Vasc. Surg. 2022, 17, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Melvin, W.S.; Needleman, B.J.; Krause, K.R.; Schneider, C.; Wolf, R.K.; Michler, R.E.; Ellison, E.C. Computer-enhanced robotic telesurgery. Initial experience in foregut surgery. Surg. Endosc. 2002, 16, 1790–1792. [Google Scholar] [CrossRef] [PubMed]
- Kernstine, K.H.; DeArmond, D.T.; Karimi, M.; Van Natta, T.L.; Campos, J.H.; Yoder, M.R.; Everett, J.E. The robotic, 2-stage, 3-field esophagolymphadenectomy. J. Thorac. Cardiovasc. Surg. 2004, 127, 1847–1849. [Google Scholar] [CrossRef] [PubMed]
- Hoelzen, J.P.; Frankauer, B.E.; Szardenings, C.; Roy, D.; Pollmann, L.; Fortmann, L.; Merten, J.; Rijcken, E.; Juratli, M.A.; Pascher, A. Reducing the Risks of Esophagectomies: A Retrospective Comparison of Hybrid versus Full-Robotic-Assisted Minimally Invasive Esophagectomy (RAMIE) Approaches. J. Clin. Med. 2023, 12, 5823. [Google Scholar] [CrossRef]
- Watanabe, M.; Kuriyama, K.; Terayama, M.; Okamura, A.; Kanamori, J.; Imamura, Y. Robotic-Assisted Esophagectomy: Current Situation and Future Perspectives. Ann. Thorac. Cardiovasc. Surg. 2023, 29, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Weindelmayer, J.; De Pasqual, C.A.; Turolo, C.; Gervasi, M.C.; Sacco, M.; Bencivenga, M.; Giacopuzzi, S. Robotic versus open Ivor-Lewis esophagectomy: A more accurate lymph node dissection without burdening the leak rate. J. Surg. Oncol. 2023, 127, 1109–1115. [Google Scholar] [CrossRef]
- Ekeke, C.N.; Kuiper, G.M.; Luketich, J.D.; Ruppert, K.M.; Copelli, S.J.; Baker, N.; Levy, R.M.; Awais, O.; Christie, N.A.; Dhupar, R.; et al. Comparison of robotic-assisted minimally invasive esophagectomy versus minimally invasive esophagectomy: A propensity-matched study from a single high-volume institution. J. Thorac. Cardiovasc. Surg. 2023, 166, 374–382.e1. [Google Scholar] [CrossRef]
- van der Sluis, P.C.; van der Horst, S.; May, A.M.; Schippers, C.; Brosens, L.A.A.; Joore, H.C.A.; Kroese, C.C.; Haj Mohammad, N.; Mook, S.; Vleggaar, F.P.; et al. Robot-assisted Minimally Invasive Thoracolaparoscopic Esophagectomy Versus Open Transthoracic Esophagectomy for Resectable Esophageal Cancer: A Randomized Controlled Trial. Ann. Surg 2019, 269, 621–630. [Google Scholar] [CrossRef]
- Khaitan, P.G.; Vekstein, A.M.; Thibault, D.; Kosinski, A.; Hartwig, M.G.; Block, M.; Gaissert, H.; Wolf, A.S. Robotic Esophagectomy Trends and Early Surgical Outcomes: The US Experience. Ann. Thorac. Surg. 2023, 115, 710–717. [Google Scholar] [CrossRef]
- Till, B.M.; Grenda, T.R.; Okusanya, O.T.; Evans Iii, N.R. Robotic Minimally Invasive Esophagectomy. Thorac. Surg. Clin. 2023, 33, 81–88. [Google Scholar] [CrossRef]
- Knitter, S.; Maurer, M.M.; Winter, A.; Dobrindt, E.M.; Seika, P.; Ritschl, P.V.; Raakow, J.; Pratschke, J.; Denecke, C. Robotic-Assisted Ivor Lewis Esophagectomy Is Safe and Cost Equivalent Compared to Minimally Invasive Esophagectomy in a Tertiary Referral Center. Cancers 2023, 16, 112. [Google Scholar] [CrossRef] [PubMed]
- Dunnican, W.J.; Singh, T.P.; Guptill, G.G.; Doorly, M.G.; Ata, A. Early robotic experience with paraesophageal hernia repair and Nissen fundoplication: Short-term outcomes. J. Robot. Surg. 2008, 2, 41–44. [Google Scholar] [CrossRef] [PubMed]
- DeUgarte, D.A.; Hirschl, R.B.; Geiger, J.D. Robotic Repair of Congenital Paraesophageal Hiatal Hernia. J. Laparoendosc. Adv. Surg. Tech. 2009, 19 (Suppl. 1), S187–S189. [Google Scholar] [CrossRef]
- Fu, S.S.; Carton, M.M.; Ghaderi, I.; Galvani, C.A. Robotic-Assisted Simultaneous Repair of Paraesophageal Hernia and Morgagni Hernia: Technical Report. J. Laparoendosc. Adv. Surg. Tech. 2018, 28, 745–750. [Google Scholar] [CrossRef]
- Gehrig, T.; Mehrabi, A.; Fischer, L.; Kenngott, H.; Hinz, U.; Gutt, C.N.; Muller-Stich, B.P. Robotic-assisted paraesophageal hernia repair—A case-control study. Langenbeck’s Arch. Surg. 2013, 398, 691–696. [Google Scholar] [CrossRef]
- Galvani, C.A.; Loebl, H.; Osuchukwu, O.; Samamé, J.; Apel, M.E.; Ghaderi, I. Robotic-Assisted Paraesophageal Hernia Repair: Initial Experience at a Single Institution. J. Laparoendosc. Adv. Surg. Tech. 2016, 26, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Vasudevan, V.; Reusche, R.; Nelson, E.; Kaza, S. Robotic paraesophageal hernia repair: A single-center experience and systematic review. J. Robot. Surg. 2018, 12, 81–86. [Google Scholar] [CrossRef]
- Gerull, W.D.; Cho, D.; Kuo, I.; Arefanian, S.; Kushner, B.S.; Awad, M.M. Robotic Approach to Paraesophageal Hernia Repair Results in Low Long-Term Recurrence Rate and Beneficial Patient-Centered Outcomes. J. Am. Coll. Surg. 2020, 231, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Sarkaria, I.S.; Latif, M.J.; Bianco, V.J.; Bains, M.S.; Rusch, V.W.; Jones, D.R.; Rizk, N.P. Early operative outcomes and learning curve of robotic assisted giant paraesophageal hernia repair. Int. J. Med. Robot. Comput. Assist. Surg. 2017, 13, e1730. [Google Scholar] [CrossRef]
- Bhatt, H.; Wei, B. Comparison of laparoscopic vs. robotic paraesophageal hernia repair: A systematic review. J. Thorac. Dis. 2023, 15, 1494–1502. [Google Scholar] [CrossRef]
- Lekarczyk, A.; Sinha, H.; Dvir, D.; Goyert, J.; Airhart, A.; Reddy, R.M. Similar hospital profits with robotic-assisted paraesophageal hiatal hernia repair, despite higher or supply costs. Surg. Endosc. 2023, 37, 3952–3955. [Google Scholar] [CrossRef] [PubMed]
- Panse, N.S.; Prasath, V.; Quinn, P.L.; Chokshi, R.J. Economic evaluation of robotic and laparoscopic paraesophageal hernia repair. Surg. Endosc. 2023, 37, 6806–6817. [Google Scholar] [CrossRef] [PubMed]
- Sowards, K.J.; Holton, N.F.; Elliott, E.G.; Hall, J.; Bajwa, K.S.; Snyder, B.E.; Wilson, T.D.; Mehta, S.S.; Walker, P.A.; Chandwani, K.D.; et al. Safety of robotic assisted laparoscopic recurrent paraesophageal hernia repair: Insights from a large single institution experience. Surg. Endosc. 2020, 34, 2560–2566. [Google Scholar] [CrossRef] [PubMed]
- Tartaglia, N.; Pavone, G.; Di Lascia, A.; Vovola, F.; Maddalena, F.; Fersini, A.; Pacilli, M.; Ambrosi, A. Robotic voluminous paraesophageal hernia repair: A case report and review of the literature. J. Med. Case Rep. 2020, 14, 25. [Google Scholar] [CrossRef]
- Gerull, W.D.; Cho, D.; Arefanian, S.; Kushner, B.S.; Awad, M.M. Favorable peri-operative outcomes observed in paraesophageal hernia repair with robotic approach. Surg. Endosc. 2021, 35, 3085–3089. [Google Scholar] [CrossRef]
- Tonelli, C.M.; Baker, M.S.; Luchette, F.A.; Cohn, T. Laparoscopic and robotic paraesophageal hernia repair in United States veterans: Clinical outcomes and risk factors associated with reoperation recurrence. Am. J. Surg. 2023, 225, 519–522. [Google Scholar] [CrossRef]
- Reza, J.A.; Bakhos, C.; Su, S.; Petrov, R.; Abbas, A.E. Robotic Belsey Mark IV Repair of the Paraesophageal Hernia. Innov. Technol. Tech. Cardiothorac. Vasc. Surg. 2023, 18, 84–89. [Google Scholar] [CrossRef]
- Manfredini, B.; Zirafa, C.C.; Romano, G.; Bagalà, E.; Cariello, C.; Davini, F.; Melfi, F. Intraoperative Catastrophes during Robotic Lung Resection: A Single-Center Experience and Review of the Literature. Life 2023, 13, 215. [Google Scholar] [CrossRef]
- Sakakura, N.; Nakada, T.; Shirai, S.; Takahara, H.; Suzuki, A.; Takahashi, Y.; Kuroda, H. Emergency rollout and conversion procedures during the three-arm robotic open-thoracotomy-view approach. Interact. Cardiovasc. Thorac. Surg. 2022, 34, 1045–1051. [Google Scholar] [CrossRef]
- Nelson, D.B.; Mehran, R.J.; Mitchell, K.G.; Rajaram, R.; Correa, A.M.; Bassett, R.L.; Antonoff, M.B.; Hofstetter, W.L.; Roth, J.A.; Sepesi, B.; et al. Robotic-Assisted Lobectomy for Non-Small Cell Lung Cancer: A Comprehensive Institutional Experience. Ann. Thorac. Surg. 2019, 108, 370–376. [Google Scholar] [CrossRef]
- Rogers, M.P.; Janjua, H.; Eguia, E.; Lozonschi, L.; Toloza, E.M.; Kuo, P.C. Adopting robotic thoracic surgery impacts hospital overall lung resection case volume. Am. J. Surg. 2022, 223, 571–575. [Google Scholar] [CrossRef] [PubMed]
- Harrison, O.J.; Maraschi, A.; Routledge, T.; Lampridis, S.; LeReun, C.; Bille, A. A cost analysis of robotic vs. video-assisted thoracic surgery: The impact of the learning curve and the COVID-19 pandemic. Front. Surg. 2023, 10, 1123329. [Google Scholar] [CrossRef] [PubMed]
- Shanahan, B.; Kreaden, U.S.; Sorensen, J.; Stamenkovic, S.; Redmond, K.C. Is robotic lobectomy cheaper? A micro-cost analysis. J. Robot. Surg. 2022, 16, 1441–1450. [Google Scholar] [CrossRef]
- Bellini, V.; Valente, M.; Del Rio, P.; Bignami, E. Artificial intelligence in thoracic surgery: A narrative review. J. Thorac. Dis. 2021, 13, 6963–6975. [Google Scholar] [CrossRef] [PubMed]
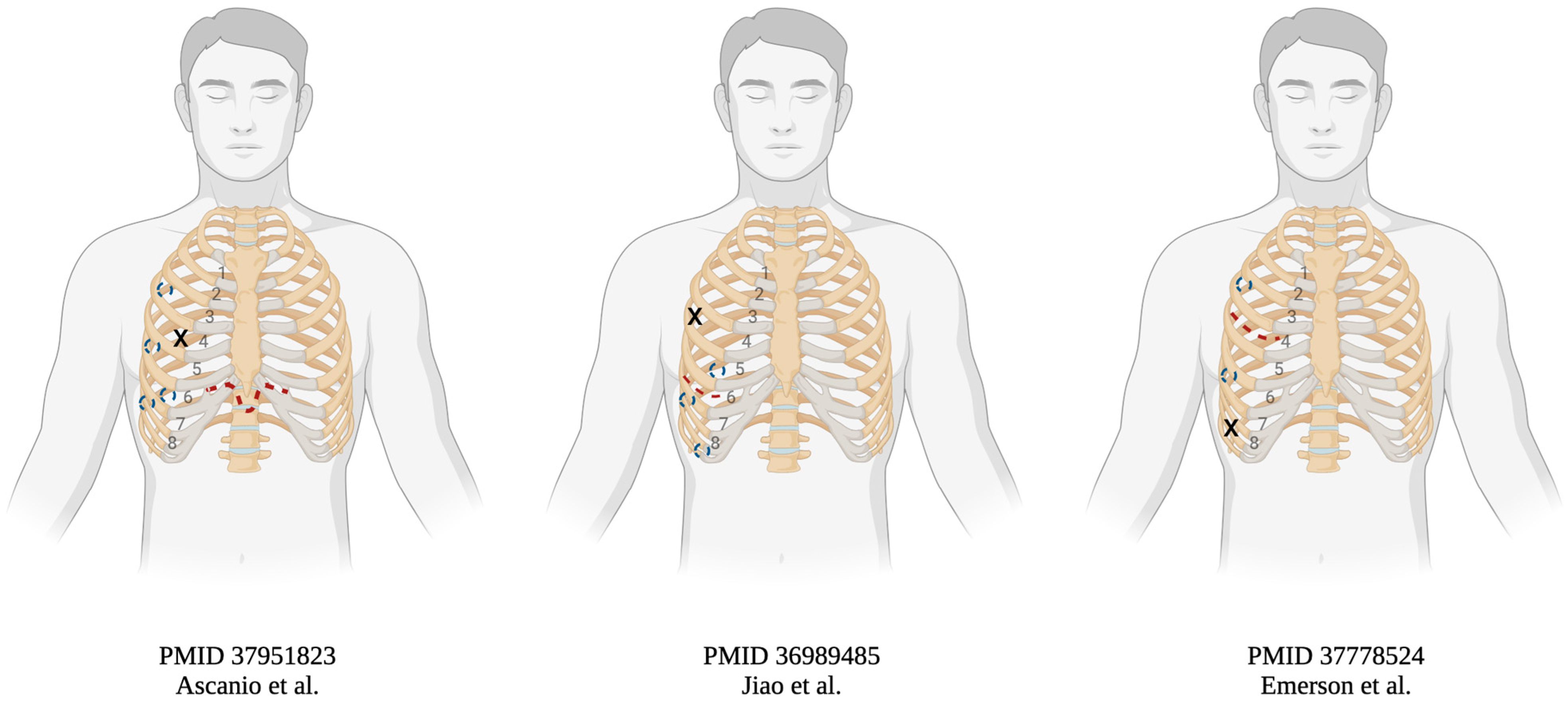
| Authors (Years) | Demographic (Age/Gender) | Indication | Transplanted Side | Approach | Implant Time (min) | On Pump (Y/N) | Postoperative Course |
|---|---|---|---|---|---|---|---|
| Ascanio et al. (2023) | 65 M | Interstitial pneumonia | Not specified | 8 cm subxiphoid and 4 ports | N/A | N | Regular analgesia, no opioids |
| 71 M | IPF | Left | as above | N/A | N | Regular analgesia, no opioids | |
| Jiao et al. (2023) | 59 M | COPD | Right | 8 cm 6th intercostal incision and 4 ports | WIT 72 | N | Discharged POD19 |
| Emerson et al. (2024) | 69 M | COPD | Right | 6 cm 6th intercostal incision, 8th intercostal incision, 2 ports | WIT 88 a | N | Discharged POD11, no opioids |
| 63 M | IPF | Right | as above | WIT 111 | Y | N/A | |
| 73 F | COPD | Right | as above | WIT 85 a | N | N/A | |
| 67 M | COPD | Bilateral | as above | WIT 68/86 | N/N | N/A | |
| 71 F | COPD | Bilateral b | as above | WIT 81/NA | N/Y | N/A | |
| 66 M | COPD | Right | as above | WIT 58 | N | N/A | |
| 64 F | IPF | Bilateral | as above | WIT 67/78 a | N/N | N/A | |
| 66 M | IPF | Right | as above | WIT 66 | Y | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tung, K.H.; Yendamuri, S.; Seastedt, K.P. Adoption of the Robotic Platform across Thoracic Surgeries. J. Clin. Med. 2024, 13, 5764. https://doi.org/10.3390/jcm13195764
Tung KH, Yendamuri S, Seastedt KP. Adoption of the Robotic Platform across Thoracic Surgeries. Journal of Clinical Medicine. 2024; 13(19):5764. https://doi.org/10.3390/jcm13195764
Chicago/Turabian StyleTung, Kaity H., Sai Yendamuri, and Kenneth P. Seastedt. 2024. "Adoption of the Robotic Platform across Thoracic Surgeries" Journal of Clinical Medicine 13, no. 19: 5764. https://doi.org/10.3390/jcm13195764






