The Prognostic Role of Advanced Lung Cancer Inflammation Index in Patients with Idiopathic Pulmonary Fibrosis
Abstract
1. Background
2. Materials and Methods
3. Statistical Analysis
4. Results
4.1. Baseline Characteristics of the Study Population
4.2. Association between ALI, GAP, NLR, BMI, FVC, DLCO, 6MWT, and Albumin
4.3. Cutoff Values of ALI Components for Predicting Mortality Risk
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zheng, Q.; Cox, I.A.; Campbell, J.A.; Xia, Q.; Otahal, P.; Graaff, B.; Corte, T.J.; Teoh, A.K.; Walters, E.H.; Palmer, A.J. Mortality and survival in idiopathic pulmonary fibrosis: A systematic review and meta-analysis. ERJ Open Res. 2022, 8, 00591–02021. [Google Scholar] [CrossRef] [PubMed]
- Nathan, S.D.; Reffett, T.; Brown, A.W.; Fischer, C.P.; Shlobin, O.A.; Ahmad, S.; Weir, N.; Sheridan, M.J. The Red Cell Distribution Width as a Prognostic Indicator in Idiopathic Pulmonary Fibrosis. Chest 2013, 143, 1692–1698. [Google Scholar] [CrossRef] [PubMed]
- Wells, A.U.; Desai, S.R.; Rubens, M.B.; Goh, N.S.; Cramer, D.; Nicholson, A.G.; Colby, T.V.; du Bois, R.M.; Hansell, D.M. Idiopathic pulmonary fibrosis: A composite physiologic index derived from disease extent observed by computed tomography. Am. J. Respir. Crit. Care Med. 2003, 167, 962–969. [Google Scholar] [CrossRef] [PubMed]
- Collard, H.R.; King, T.E., Jr.; Bartelson, B.B.; Vourlekis, J.S.; Schwarz, M.I.; Brown, K.K. Changes in clinical and physiologic variables predict survival in idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2003, 168, 538–542. [Google Scholar] [CrossRef]
- Desai, O.; Winkler, J.; Minasyan, M.; Herzog, E.L. The Role of Immune and Inflammatory Cells in Idiopathic Pulmonary Fibrosis. Front. Med. 2018, 5, 43. [Google Scholar] [CrossRef]
- Chen, X.; Hong, C.; Guo, Z.; Huang, H.; Ye, L. Association between advanced lung cancer inflammation index and all-cause and cardiovascular mortality among stroke patients: NHANES, 1999–2018. Front. Public Health 2024, 12, 1370322. [Google Scholar] [CrossRef]
- Maeda, D.; Kanzaki, Y.; Sakane, K.; Ito, T.; Sohmiya, K.; Hoshiga, M. Prognostic impact of a novel index of nutrition and inflammation for patients with acute decompensated heart failure. Heart Vessel. 2020, 35, 1201–1208. [Google Scholar] [CrossRef]
- Yuan, X.; Huang, B.; Wang, R.; Tie, H.; Luo, S. The prognostic value of advanced lung cancer inflammation index (ALI) in elderly patients with heart failure. Front. Cardiovasc. Med. 2022, 9, 934551. [Google Scholar] [CrossRef]
- Jouneau, S.; Kerjouan, M.; Rousseau, C.; Lederlin, M.; Llamas-Guttierez, F.; De Latour, B.; Guillot, S.; Vernhet, L.; Desrues, B.; Thibault, R. What are the best indicators to assess malnutrition in idiopathic pulmonary fibrosis patients? A cross-sectional study in a referral center. Nutrition 2019, 62, 115–121. [Google Scholar] [CrossRef]
- Jouneau, S.; Crestani, B.; Thibault, R.; Lederlin, M.; Vernhet, L.; Valenzuela, C.; Wijsenbeek, M.; Kreuter, M.; Stansen, W.; Quaresma, M.; et al. Analysis of body mass index, weight loss and progression of idiopathic pulmonary fibrosis. Respir. Res. 2020, 21, 312. [Google Scholar] [CrossRef]
- Raghu, G.; Remy-Jardin, M.; Richeldi, L.; Thomson, C.C.; Inoue, Y.; Johkoh, T.; Kreuter, M.; Lynch, D.A.; Maher, T.M.; Martinez, F.J.; et al. Idiopathic Pulmonary Fibrosis (an Update) and Progressive Pulmonary Fibrosis in Adults: An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2022, 205, 18–47. [Google Scholar] [CrossRef] [PubMed]
- Ley, B.; Ryerson, C.J.; Vittinghoff, E.; Ryu, J.H.; Tomassetti, S.; Lee, J.S.; Poletti, V.; Buccioli, M.; Elicker, B.M.; Jones, K.D.; et al. A multidimensional index and staging system for idiopathic pulmonary fibrosis. Ann. Intern. Med. 2012, 156, 684–689. [Google Scholar] [CrossRef] [PubMed]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic. Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Wuyts, W.A.; Agostini, C.; Antoniou, K.M.; Bouros, D.; Chambers, R.C.; Cottin, V.; Egan, J.J.; Lambrecht, B.N.; Lories, R.; Parfrey, H.; et al. The pathogenesis of pulmonary fibrosis: A moving target. Eur. Respir. J. 2013, 41, 1207–1218. [Google Scholar] [CrossRef]
- Mantovani, A.; Cassatella, M.A.; Costantini, C.; Jaillon, S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat. Rev. Immunol. 2011, 11, 519–531. [Google Scholar] [CrossRef]
- Wynn, T.A. Integrating mechanisms of pulmonary fibrosis. J. Exp. Med. 2011, 208, 1339–1350. [Google Scholar] [CrossRef]
- Adane, T.; Melku, M.; Worku, Y.B.; Fasil, A.; Aynalem, M.; Kelem, A.; Geteva, S. The association between neutrophil-to-lymphocyte ratio and Glycemic control in type 2 diabetes mellitus: A systematic review and meta-analysis. J. Diabetes Res. 2023, 2023, 3117396. [Google Scholar] [CrossRef]
- Azab, B.; Camacho-Rivera, M.; Taioli, E. Average values and racial differences of neutrophil lymphocyte ratio among a nationally representative sample of United States subjects. PLoS ONE. 2014, 9, e112361. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, N.Y.; Na, S.H.; Youn, Y.H.; Shin, C.S. Reference values of neutrophil lymphocyte ratio, lymphocyte-monocyte ratio, platelet-lymphocyte ratio, and mean platelet volume in healthy adults in South Korea. Medicine 2018, 97, e11138. [Google Scholar] [CrossRef]
- Zinellu, A.; Paliogiannis, P.; Sotgiu, E.; Mellino, S.; Mangoni, A.A.; Zinellu, E.; Negri, S.; Collu, C.; Pintus, G.; Serra, A.; et al. Blood Cell Count Derived Inflammation Indexes in Patients With Idiopathic Pulmonary Fibrosis. Lung 2020, 198, 821–827. [Google Scholar] [CrossRef]
- D’Alessandro, M.; Bergantini, L.; Carleo, A.; Cameli, P.; Perrone, A.; Fossi, A.; Sestini, P.; Bargagli, B. Neutrophil-To-Lymphocyte Ratio in Bronchoalveolar Lavage From IPF Patients: A Novel Prognostic Biomarker? Minerva Med. 2022, 113, 526–531. [Google Scholar] [CrossRef] [PubMed]
- Paliogiannis, P.; Satta, R.; Deligia, G.; Farina, G.; Bassu, S.; Mangoni, A.A.; Carru, C.; Zinellu, A. Associations between the neutrophil-to-lymphocyte and the platelet-to-lymphocyte ratios and the presence and severity of psoriasis: A systematic review and meta-analysis. Clin. Exp. Med. 2019, 19, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Paliogiannis, P.; Fois, A.G.; Sotgia, S.; Mangoni, A.A.; Zinellu, E.; Pirina, P.; Carru, C.; Zinellu, A. The neutrophil-to-lymphocyte ratio as a marker of chronic obstructive pulmonary disease and its exacerbations: A systematic review and meta-analysis. Eur. J. Clin. Investig. 2018, 48, e12984. [Google Scholar] [CrossRef] [PubMed]
- Paliogiannis, P.; Fois, A.G.; Sotgia, S.; Mangoni, A.A.; Zinellu, E.; Pirina, P.; Negri, S.; Carru, C.; Zinellu, A. Neutrophil to lymphocyte ratio and clinical outcomes in COPD: Recent evidence and future perspectives. Eur. Respir. Rev. 2018, 27, 170113. [Google Scholar] [CrossRef] [PubMed]
- Mochimaru, T.; Ueda, S.; Suzuki, Y.; Asano, K.; Fukunaga, K. Neutrophil-to-lymphocyte ratio as a novel independent predictor of severe exacerbation in patients with asthma. Ann. Allergy Asthma Immunol. 2019, 122, 337–339. [Google Scholar] [CrossRef]
- Mikolasch, T.A.; George, P.M.; Sahota, J.; Nancarrow, T.; Barratt, S.L.; Woodhead, F.A.; Kouranos, V.; Cope, V.S.A.; Creamer, A.W.; Fidan, S.; et al. Multi-center evaluation of baseline neutrophil-to-lymphocyte (NLR) ratio as an independent predictor of mortality and clinical risk stratifier in idiopathic pulmonary fibrosis. EClinicalMedicine 2023, 55, 101758. [Google Scholar] [CrossRef]
- Zhang, X.; Ren, Y.; Xie, B.; Ye, O.; Ban, C.; Zhang, S.; Zhu, M.; Liu, Y.; Wang, S.; Geng, J.; et al. Blood monocyte counts as a prognostic biomarker andpredictor in Chinese patients with idiopathic pulmonary fibrosis. Front. Med. 2022, 9, 955125. [Google Scholar] [CrossRef]
- Takuma, S.; Suzuki, Y.; Kono, M.; Hasegawa, H.; Hashimoto, D.; Yokomura, K.; Mori, K.; Shimizu, M.; Inoue, Y.; Yasui, H.; et al. Neutrophil-lymphocyte ratio being associated with mortality risk in patients receiving antifibrotic therapy. Respir. Med. 2024, 223, 107542. [Google Scholar] [CrossRef]
- Achaiah, A.; Rathnapala, A.; Pereira, A.; Bothwell, H.; Dwivedi, K.; Barker, R.; Iotchkova, V.; Benamore, R.; Hoyles, R.K.; Ho, L.P. Neutrophil lymphocyte ratio as an indicator for disease progression in Idiopathic Pulmonary Fibrosis. BMJ Open Respir. Res. 2022, 9, e001202. [Google Scholar] [CrossRef]
- Suzuki, Y.; Kono, M.; Hasegawa, H.; Hashimoto, D.; Yokomura, K.; Imokawa, S.; Inoue, Y.; Hozumi, H.; Karayama, M.; Furuhashi, K.; et al. Neutrophil-lymphocyte ratio in patients with idiopathic pleuroparenchymal fibroelastosis. BMJ Open Respir. Res. 2023, 10, e001763. [Google Scholar] [CrossRef]
- Li, B.; Zhang, X.; Xu, G.; Zhang, S.; Song, H.; Yang, K.; Dai, H.; Wang, C. Serum prealbumin is a prognostic indicator in idiopathic pulmonary fibrosis. Clin. Respir. J. 2019, 13, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Mori, K.; Aono, Y.; Kono, M.; Hasegawa, H.; Yokomura, K.; Hozumi, H.; Karayama, M.; Furuhashi, K.; Enomoto, N.; et al. Cause of mortality and sarcopenia in patients with idiopathic pulmonary fibrosis receiving antifibrotic therapy. Respirology 2021, 26, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Mori, K.; Aono, Y.; Kono, M.; Hasegawa, H.; Yokomura, K.; Naoi, H.; Hozumi, H.; Karayama, M.; Furuhashi, K.; et al. Combined assessment of the GAP index and body mass index at antifibrotic therapy initiation for prognosis of idiopathic pulmonary fibrosis. Sci. Rep. 2021, 11, 18579. [Google Scholar] [CrossRef] [PubMed]
- Jouneau, S.; Rousseau, C.; Lederlin, M.; Lescoat, A.; Kerjouan, M.; Chauvin, P.; Luque-Paz, D.; Guillot, S.; Oger, E.; Vernhet, L.; et al. Malnutrition and decreased food intake at diagnosis are associated with hospitalization and mortality of idiopathic pulmonary fibrosis patients. Clin. Nutr. 2022, 41, 1335–1342. [Google Scholar] [CrossRef]
- Jouneau, S.; Crestani, B.; Thibault, R.; Lederlin, M.; Vernhet, L.; Yang, M.; Morgenthien, E.; Kirchgaessler, K.; Cottin, V. Post hoc analysis of clinical outcomes in placebo- and Pirfenidone-treated patients with iPF stratified by BMi and weight loss. Respiration 2022, 101, 142–154. [Google Scholar] [CrossRef]
- Mochizuka, Y.; Suzuki, Y.; Kono, M.; Hasegawa, H.; Hashimoto, D.; Yokomura, K.; Inoue, Y.; Yasui, H.; Hozumi, H.; Karayama, M.; et al. Geriatric Nutritional Risk Index is a predictor of tolerability of antifibrotic therapy and mortality risk in patients with idiopathic pulmonary fibrosis. Respirology 2023, 28, 775–783. [Google Scholar] [CrossRef]
| Variables | |
| Gender, M/F (n, %) | 80 (78.4)/22 (21.6) |
| Age, years | 70.4 ± 7.39 |
| Smokers, never/current/ex (n, %) | 13 (12.7)/16 (15.7)/73 (71.6) |
| BMI (kg/m2) | 24.1 ± 1.79 |
| Disease duration (months) | 33.4 ± 8.61 |
| AE (n, %) | 15 (14.7) |
| Charlson comorbidity index | 1.4 ± 0.62 |
| GAP Stages | |
| I | 44 (43.1) |
| II | 32 (31.4) |
| III | 26 (25.5) |
| GAP index (1/2/3/4/5/6/7) | 2 (2.0)/12 (11.8)/30 (29.4)/16 (15.7)/16 (15.7)/19 (18.6)/7 (6.9) |
| Pulmonary function test | |
| FVC, %-pred | 70.2 ± 7.54 |
| FEV1, %-pred | 74.7 ± 7.43 |
| DLCO, % | 49.7 ± 11.82 |
| 6MWT (meters) | 359.2 ± 49.42 |
| Laboratory variables | |
| Neutrophils (109/L) | 5.50 ± 1.26 |
| Lymphocytes (109/L) | 1.7 ± 0.35 |
| Monocytes (109/L) | 1.7 ± 0.35 |
| Albumin, g/dL | 3.5 ± 0.69 |
| LDH, U/L | 212.9 ± 65.90 |
| ALT (U/L) | 22.8 ± 28.70 |
| AST (U/L) | 20.8 ± 7.11 |
| NLR | 3.3 ± 1.05 |
| ALI | 29.6 ± 15.32 |
| Survivor/non-survivor (n/%) | 91 (89.2)/11 (10.8) |
| Nintedanib/pirfenidone (n/%) | 53 (52.0)/49 (48.0) |
| ALI | ||||
|---|---|---|---|---|
| n | Median (IQR) | p | ||
| GAP stages | 1 (0–3) | 44 | 38.5 (18.60) a | 0.000 |
| 2 (4–5) | 32 | 21.6 (7.35) b | ||
| 3 (6–8) | 26 | 17.5 (10.72) c | ||
| FVC (median split) | <70 | 44 | 21.1 (9.58) | 0.000 |
| ≥70 | 58 | 31.3 (20.05 | ||
| DLCO | <51 | 49 | 20.3 (10.75) | 0.000 |
| ≥51 | 53 | 32.0 (20.04) | ||
| 6MWT (meters) | <350 | 36 | 19.6 (11.63) | 0.001 |
| ≥350 | 66 | 29.7 (17.65) | ||
| Charlson comorbidity index | ≤1 | 65 | 27.5 (19.96) | 0.233 |
| >1 | 37 | 22.1 (12.59) | ||
| Nintedanib (n/%) | 53 | 24.7 (12.29) | 0.150 | |
| Pirfenidone (n/%) | 49 | 28.7 (23.31) | ||
| GAP stage 1 (n = 44) | GAP stage 1 (n = 44) | GAP stage 3 (n = 26) | ||
| BMI | 25.2 ± 1.30 a | 23.7 ± 1.50 b | 22.5 ± 1.42 c | 0.000 |
| NLR | 2.5 ± 0.71 a | 3.6 ± 0.70 b | 4.1 ± 1.15 b, c | 0.000 |
| Albumin | 4.0 ± 0.53 a | 3.36 ± 0.45 b | 2.93 ± 0.11 c | 0.000 |
| Neutrophils (109/L) | 4.5 ± 0.84 a | 6.1 ± 0.76 b | 6.5 ± 1.08 b, c | 0.000 |
| Lymphocytes (109/L) | 1,6 (0) | 1.7 (0) | 1.6 (0) | 0.070 |
| Monocytes (109/L) | 0.8 (0) | 0.7 (0) | 0.9 (0) | 0.114 |
| ALI | 38 (16.59) a | 25.1 (7.43) b | 17.6 (4.27) c | 0.000 |
| ALI Quantile 1 [<21.2] (n = 36) | ALI Quantile 2 [3–31] (n = 33) | ALI Quantile 3 [>31.5] (n = 33) | ||
| BMI | 22.9 ± 1.38 a | 24.1 ± 1.73 b | 25.2 ± 1.43 c | 0.000 |
| NLR | 4.2 ± 0.96 a | 3.3 ± 0.45 b | 2.3 ± 0.56 c | 0.000 |
| Albumin | 2.8 ± 0.48 a | 3.7 ± 0.41 b | 4.0 ± 0.55 c | 0.000 |
| Neutrophils (109/L) | 6.4 ± 0,96 a | 5.7 ± 0.86 b | 4.2 ± 0.76 c | 0.000 |
| Lymphocytes (109/L) | 1.6 (0) a | 1.7 (0) b | 1.9 (1) b, c | 0.000 |
| Monocytes (109/L) | 0.9 (0) | 0.8 (0) | 0.8 (0) | 0.534 |
| n (%) | n (%) | n (%) | X2; p | |
| Nintedanib (n/%) | 20 (27.7) | 21 (39.6) | 12 (22.6) | 5.204; 0.074 |
| Pirfenidone (n/%) | 16 (32.7) | 12 (24.5) | 21 (42.9) | |
| Gap Stage | FVC | DLCO | 6MWT | ||
|---|---|---|---|---|---|
| ALI | r | −0.815 | 0.498 | 0.637 | 0.445 |
| p | 0.000 | 0.000 | 0.000 | 0.000 | |
| BMI | r | −0.634 | 0.406 | 0.493 | 0.499 |
| p | 0.000 | 0.000 | 0.000 | 0.000 | |
| NLR | r | 0.638 | −0.348 | −0.525 | −0.257 |
| p | 0.000 | 0.000 | 0.000 | 0.009 | |
| Albumin | r | −0.636 | 0.410 | 0.431 | 0.412 |
| p | 0.000 | 0.000 | 0.000 | 0.000 |
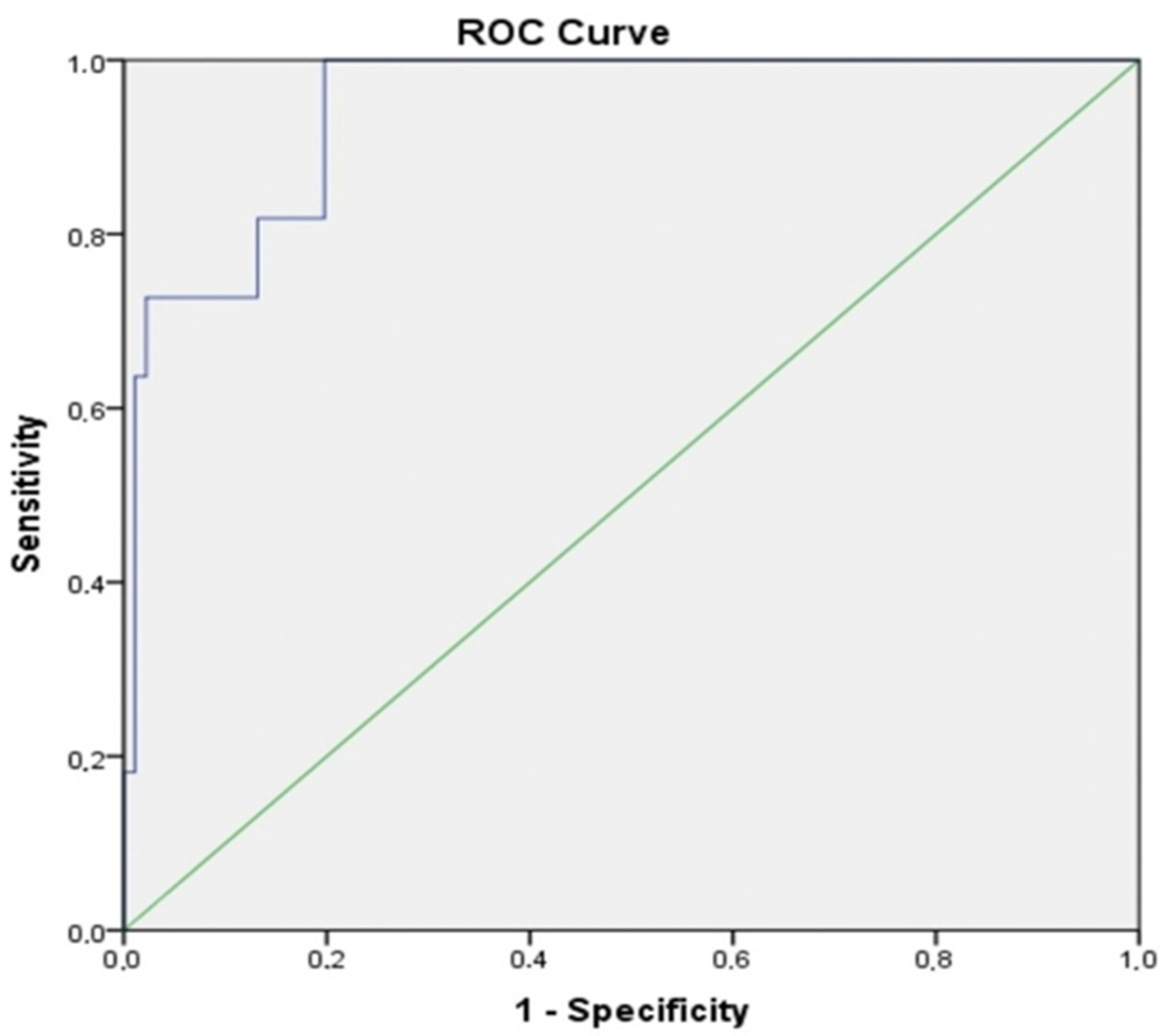
| AUC (%95) | Cut Off | p | Sensitivity (%) | Specificity (%) | |
|---|---|---|---|---|---|
| Albumin | 0.952 (0.904–1.000) | 2.45 | 0.000 | 63.6 | 97.8 |
| BMI | 0.885 (0.811–0.959) | 21.84 | 0.000 | 54.5 | 94.5 |
| ALI | 0.945 (0.892–0.998) | 11.20 | 0.000 | 63.6 | 98.9 |
| NLR | 0.768 (0.600–0.936) | 5.25 | 0.000 | 36.4 | 98.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bozkuş, F.; Keskin, O. The Prognostic Role of Advanced Lung Cancer Inflammation Index in Patients with Idiopathic Pulmonary Fibrosis. J. Clin. Med. 2024, 13, 5874. https://doi.org/10.3390/jcm13195874
Bozkuş F, Keskin O. The Prognostic Role of Advanced Lung Cancer Inflammation Index in Patients with Idiopathic Pulmonary Fibrosis. Journal of Clinical Medicine. 2024; 13(19):5874. https://doi.org/10.3390/jcm13195874
Chicago/Turabian StyleBozkuş, Fulsen, and Olgun Keskin. 2024. "The Prognostic Role of Advanced Lung Cancer Inflammation Index in Patients with Idiopathic Pulmonary Fibrosis" Journal of Clinical Medicine 13, no. 19: 5874. https://doi.org/10.3390/jcm13195874
APA StyleBozkuş, F., & Keskin, O. (2024). The Prognostic Role of Advanced Lung Cancer Inflammation Index in Patients with Idiopathic Pulmonary Fibrosis. Journal of Clinical Medicine, 13(19), 5874. https://doi.org/10.3390/jcm13195874






