Leukocyte Indices as Markers of Inflammation and Predictors of Outcome in Heart Failure with Preserved Ejection Fraction
Abstract
1. Introduction
2. Methods
2.1. Setting
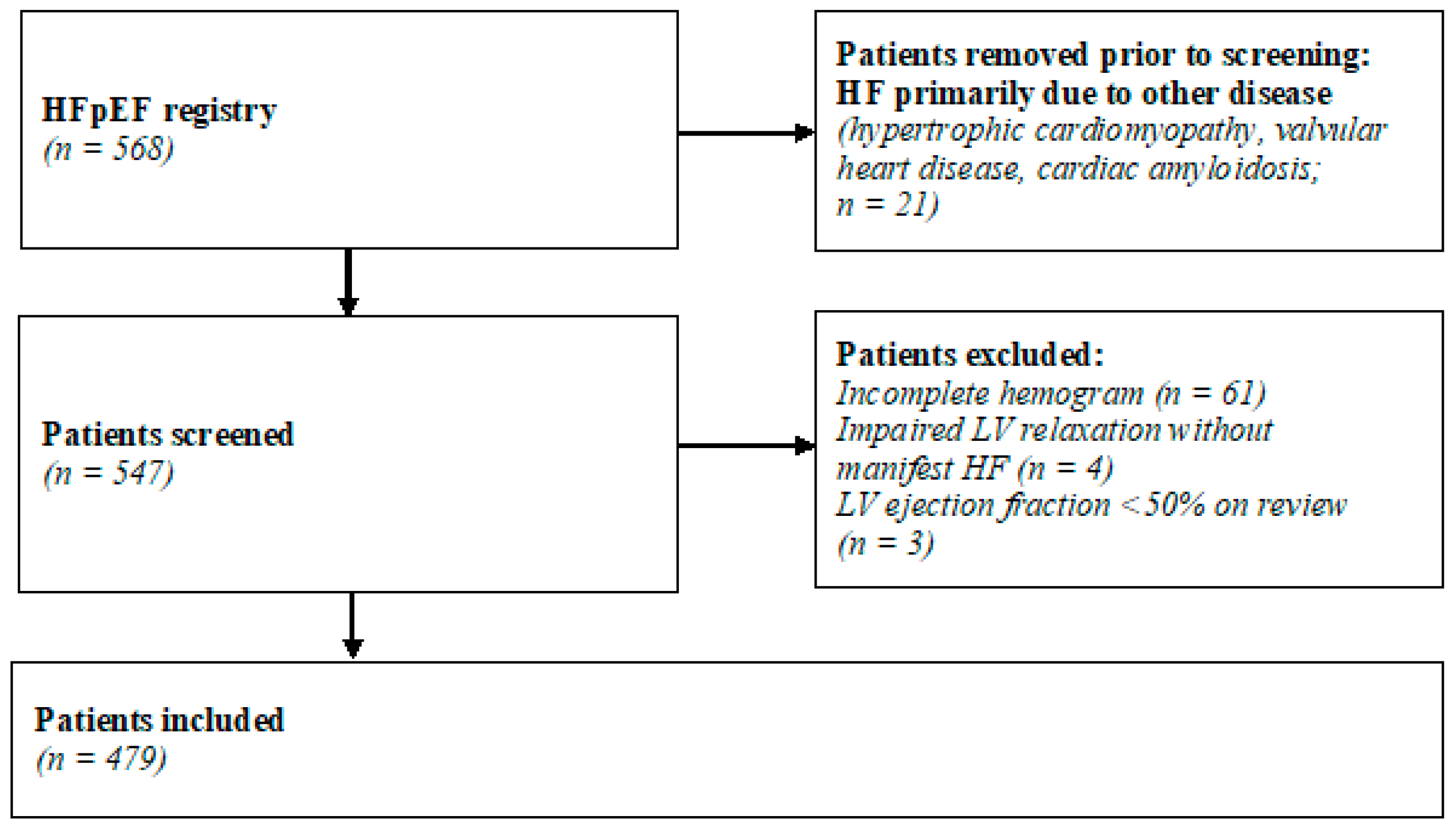
2.2. Subjects and Study Design
2.3. Diagnostic Criteria
2.4. Study Procedures
2.5. Parameters of Interest
2.6. Endpoints
2.7. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Association with Clinical Characteristics and Biomarkers of Heart Failure
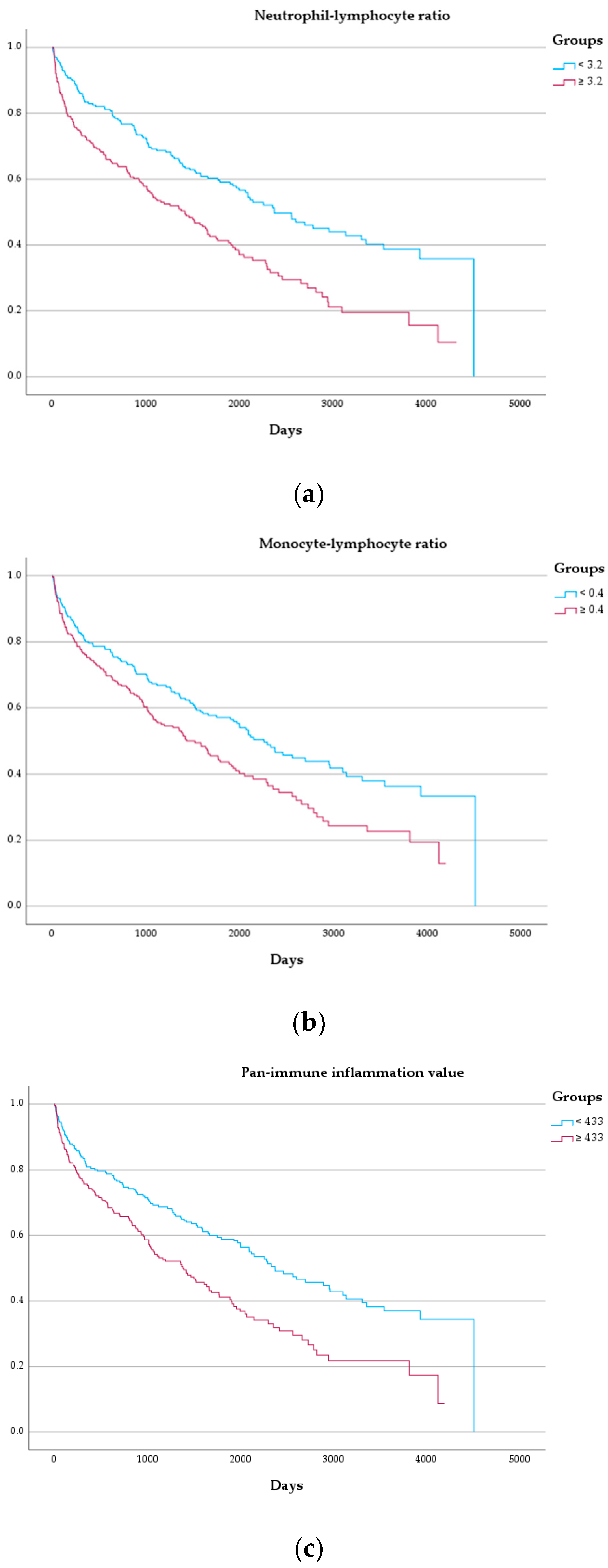
3.3. Outcome
4. Discussion
4.1. Clinical Implications
4.2. Pathophysiologic Considerations
4.3. Future Directions
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Borlaug, B.A.; Sharma, K.; Shah, S.J.; Ho, J.E. Heart Failure with Preserved Ejection Fraction: JACC Scientific Statement. J. Am. Coll. Cardiol. 2023, 81, 1810–1834. [Google Scholar] [CrossRef] [PubMed]
- Sanders-Van Wijk, S.; Tromp, J.; Beussink-Nelson, L.; Hage, C.; Svedlund, S.; Saraste, A.; Swat, S.A.; Sanchez, C.; Njoroge, J.; Tan, R.S.; et al. Proteomic Evaluation of the Comorbidity-Inflammation Paradigm in Heart Failure with Preserved Ejection Fraction: Results from the PROMIS-HFpEF Study. Circulation 2020, 142, 2029–2044. [Google Scholar] [CrossRef] [PubMed]
- Regan, J.A.; Truby, L.K.; Tahir, U.A.; Katz, D.H.; Nguyen, M.; Kwee, L.C.; Deng, S.; Wilson, J.G.; Mentz, R.J.; Kraus, W.E.; et al. Protein Biomarkers of Cardiac Remodeling and Inflammation Associated with HFpEF and Incident Events. Sci. Rep. 2022, 12, 20072. [Google Scholar] [CrossRef] [PubMed]
- Eltelbany, M.; Shah, P.; deFilippi, C. Biomarkers in HFpEF for Diagnosis, Prognosis, and Biological Phenotyping. Curr. Heart Fail. Rep. 2022, 19, 412–424. [Google Scholar] [CrossRef]
- Curran, F.M.; Bhalraam, U.; Mohan, M.; Singh, J.S.; Anker, S.D.; Dickstein, K.; Doney, A.S.; Filippatos, G.; George, J.; Metra, M.; et al. Neutrophil-to-lymphocyte Ratio and Outcomes in Patients with New-onset or Worsening Heart Failure with Reduced and Preserved Ejection Fraction. ESC Heart Fail. 2021, 8, 3168. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Baumbach, A.; Böhm, M.; Burri, H.; Čelutkiene, J.; Chioncel, O.; Cleland, J.G.F.; Coats, A.J.S.; et al. 2021 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Reddy, Y.N.V.; Carter, R.E.; Obokata, M.; Redfield, M.M.; Borlaug, B.A. A Simple, Evidence-Based Approach to Help Guide Diagnosis of Heart Failure with Preserved Ejection Fraction. Circulation 2018, 138, 861–870. [Google Scholar] [CrossRef]
- Pieske, B.; Tschöpe, C.; De Boer, R.A.; Fraser, A.G.; Anker, S.D.; Donal, E.; Edelmann, F.; Fu, M.; Guazzi, M.; Lam, C.S.P.; et al. How to Diagnose Heart Failure with Preserved Ejection Fraction: The HFA-PEFF Diagnostic Algorithm: A Consensus Recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur. Heart J. 2019, 40, 3297–3317. [Google Scholar] [CrossRef]
- Rudski, L.G.; Lai, W.W.; Afilalo, J.; Hua, L.; Handschumacher, M.D.; Chandrasekaran, K.; Solomon, S.D.; Louie, E.K.; Schiller, N.B. Guidelines for the Echocardiographic Assessment of the Right Heart in Adults: A Report from the American Society of Echocardiography Endorsed by the European Association of Echocardiography, a Registered Branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J. Am. Soc. Echocardiogr. 2010, 23, 685–713. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Victor, M.A.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef]
- Hashemi Moghanjoughi, P.; Neshat, S.; Rezaei, A.; Heshmat-Ghahdarijani, K. Is the Neutrophil-to-Lymphocyte Ratio an Exceptional Indicator for Metabolic Syndrome Disease and Outcomes? Endocr. Pract. 2022, 28, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Sawant, A.C.; Adhikari, P.; Narra, S.R.; Srivatsa, S.V.; Mills, P.K.; Srivatsa, S.S. Neutrophil to Lymphocyte Ratio Predicts Short- and Long-Term Mortality Following Revascularization Therapy for ST Elevation Myocardial Infarction. Cardiol. J. 2014, 21, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Bhat, T.M.; Afari, M.E.; Garcia, L.A. Neutrophil Lymphocyte Ratio in Peripheral Vascular Disease: A Review. Expert. Rev. Cardiovasc. Ther. 2016, 14, 871–875. [Google Scholar] [CrossRef] [PubMed]
- Serra, R.; Ielapi, N.; Licastro, N.; Provenzano, M.; Andreucci, M.; Bracale, U.M.; Jiritano, F.; de Franciscis, S.; Mastroroberto, P.; Serraino, G.F. Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio as Biomarkers for Cardiovascular Surgery Procedures: A Literature Review. Rev. Recent. Clin. Trials 2021, 16, 173–179. [Google Scholar] [CrossRef]
- Jimeno, S.; Ventura, P.S.; Castellano, J.M.; García-Adasme, S.I.; Miranda, M.; Touza, P.; Lllana, I.; López-Escobar, A. Prognostic Implications of Neutrophil-Lymphocyte Ratio in COVID-19. Eur. J. Clin. Investig. 2021, 51, e13404. [Google Scholar] [CrossRef]
- García-Escobar, A.; Vera-Vera, S.; Tébar-Márquez, D.; Rivero-Santana, B.; Jurado-Román, A.; Jiménez-Valero, S.; Galeote, G.; Cabrera, J.Á.; Moreno, R. Neutrophil-to-Lymphocyte Ratio an Inflammatory Biomarker, and Prognostic Marker in Heart Failure, Cardiovascular Disease and Chronic Inflammatory Diseases: New Insights for a Potential Predictor of Anti-Cytokine Therapy Responsiveness. Microvasc. Res. 2023, 150, 104598. [Google Scholar] [CrossRef]
- Che, J.; Song, J.; Long, Y.; Wang, C.; Zheng, C.; Zhou, R.; Liu, Z. Association between the Neutrophil-Lymphocyte Ratio and Prognosis of Patients Admitted to the Intensive Care Unit With Chronic Heart Failure: A Retrospective Cohort Study. Angiology 2023, 75, 786–795. [Google Scholar] [CrossRef]
- Wu, C.C.; Wu, C.H.; Lee, C.H.; Cheng, C.I. Association between Neutrophil Percentage-to-Albumin Ratio (NPAR), Neutrophil-to-Lymphocyte Ratio (NLR), Platelet-to-Lymphocyte Ratio (PLR) and Long-Term Mortality in Community-Dwelling Adults with Heart Failure: Evidence from US NHANES 2005–2016. BMC Cardiovasc. Disord 2023, 23, 312. [Google Scholar] [CrossRef]
- Hua, Y.; Sun, J.Y.; Lou, Y.X.; Sun, W.; Kong, X.Q. Monocyte-to-Lymphocyte Ratio Predicts Mortality and Cardiovascular Mortality in the General Population. Int. J. Cardiol. 2023, 379, 118–126. [Google Scholar] [CrossRef]
- Tudurachi, B.S.; Anghel, L.; Tudurachi, A.; Sascău, R.A.; Stătescu, C. Assessment of Inflammatory Hematological Ratios (NLR, PLR, MLR, LMR and Monocyte/HDL-Cholesterol Ratio) in Acute Myocardial Infarction and Particularities in Young Patients. Int. J. Mol. Sci. 2023, 24, 14378. [Google Scholar] [CrossRef]
- Gijsberts, C.M.; Ellenbroek, G.H.J.M.; ten Berg, M.J.; Huisman, A.; van Solinge, W.W.; Lam, C.S.; Asselbergs, F.W.; den Ruijter, H.M.; Pasterkamp, G.; Hoefer, I.E.; et al. Effect of Monocyte-to-Lymphocyte Ratio on Heart Failure Characteristics and Hospitalizations in a Coronary Angiography Cohort. Am. J. Cardiol. 2017, 120, 911–916. [Google Scholar] [CrossRef] [PubMed]
- Magoon, R.; Shri, I.; Kashav, R.C.; Dey, S.; Kohli, J.K.; Grover, V.; Gupta, V. Atrial Fibrillation and Perioperative Inflammation (FIBRILLAMMED Study): A Retrospective Analysis of the Predictive Role of Preoperative Albumin-Adjusted Platelet-Leukocytic Indices in OPCABG. Turk. J. Anaesthesiol. Reanim. 2023, 51, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Murat, B.; Murat, S.; Ozgeyik, M.; Bilgin, M. Comparison of Pan-Immune-Inflammation Value with Other Inflammation Markers of Long-Term Survival after ST-Segment Elevation Myocardial Infarction. Eur. J. Clin. Investig. 2023, 53, e13872. [Google Scholar] [CrossRef]
- Inan, D.; Erdogan, A.; Pay, L.; Genc, D.; Demırtola, A.I.; Yıldız, U.; Guler, A.; Tekkesin, A.I.; Karagoz, A. The Prognostic Impact of Inflammation in Patients with Decompensated Acute Heart Failure, as Assessed Using the Pan-Immune Inflammation Value (PIV). Scand. J. Clin. Lab. Investig. 2023, 83, 371–378. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Liu, L.; Cao, S.; Jin, T.; Chen, L.; Wu, G.; Zong, G. Association of Systemic Inflammatory Response Index and Pan-Immune-Inflammation-Value with Long-Term Adverse Cardiovascular Events in ST-Segment Elevation Myocardial Infarction Patients After Primary Percutaneous Coronary Intervention. J. Inflamm. Res. 2023, 16, 3437–3454. [Google Scholar] [CrossRef]
- Cetinkaya, Z.; Kelesoglu, S.; Tuncay, A.; Yilmaz, Y.; Karaca, Y.; Karasu, M.; Secen, O.; Cinar, A.; Harman, M.; Sahin, S.; et al. The Role of Pan-Immune-Inflammation Value in Determining the Severity of Coronary Artery Disease in NSTEMI Patients. J. Clin. Med. 2024, 13, 1295. [Google Scholar] [CrossRef]
- Lund, L.H.; Lam, C.S.P.; Pizzato, P.E.; Gabrielsen, A.; Michaëlsson, E.; Nelander, K.; Ericsson, H.; Holden, J.; Folkvaljon, F.; Mattsson, A.; et al. Rationale and Design of ENDEAVOR: A Sequential Phase 2b-3 Randomized Clinical Trial to Evaluate the Effect of Myeloperoxidase Inhibition on Symptoms and Exercise Capacity in Heart Failure with Preserved or Mildly Reduced Ejection Fraction. Eur. J. Heart Fail. 2023, 25, 1696–1707. [Google Scholar] [CrossRef] [PubMed]
- Shchendrygina, A.; Rachina, S.; Cherkasova, N.; Suvorov, A.; Komarova, I.; Mukhina, N.; Ananicheva, N.; Gasanova, D.; Sitnikova, V.; Koposova, A.; et al. Colchicine in Patients with Heart Failure and Preserved Left Ventricular Ejection Fraction: Rationale and Design of a Prospective, Randomised, Open-Label, Crossover Clinical Trial. Open Heart 2023, 10, e002360. [Google Scholar] [CrossRef]
- Bai, B.; Cheng, M.; Jiang, L.; Xu, J.; Chen, H.; Xu, Y. High Neutrophil to Lymphocyte Ratio and Its Gene Signatures Correlate with Diastolic Dysfunction in Heart Failure with Preserved Ejection Fraction. Front. Cardiovasc. Med. 2021, 8, 614757. [Google Scholar] [CrossRef]
- Poledniczek, M.; Neumayer, C.; Kopp, C.W.; Schlager, O.; Gremmel, T.; Jozkowicz, A.; Gschwandtner, M.E.; Koppensteiner, R.; Wadowski, P.P. Micro- and Macrovascular Effects of Inflammation in Peripheral Artery Disease—Pathophysiology and Translational Therapeutic Approaches. Biomedicines 2023, 11, 2284. [Google Scholar] [CrossRef]
- Michaëlsson, E.; Lund, L.H.; Hage, C.; Shah, S.J.; Voors, A.A.; Saraste, A.; Redfors, B.; Grove, E.L.; Barasa, A.; Richards, A.M.; et al. Myeloperoxidase Inhibition Reverses Biomarker Profiles Associated with Clinical Outcomes in HFpEF. JACC Heart Fail. 2023, 11, 775–787. [Google Scholar] [CrossRef] [PubMed]
- Metzler, K.D.; Goosmann, C.; Lubojemska, A.; Zychlinsky, A.; Papayannopoulos, V. A Myeloperoxidase-Containing Complex Regulates Neutrophil Elastase Release and Actin Dynamics during NETosis. Cell Rep. 2014, 8, 883–896. [Google Scholar] [CrossRef] [PubMed]
- Alfaidi, M.; Wilson, H.; Daigneault, M.; Burnett, A.; Ridger, V.; Chamberlain, J.; Francis, S. Neutrophil Elastase Promotes Interleukin-1β Secretion from Human Coronary Endothelium. J. Biol. Chem. 2015, 290, 24067–24078. [Google Scholar] [CrossRef] [PubMed]
- Saffarzadeh, M.; Juenemann, C.; Queisser, M.A.; Lochnit, G.; Barreto, G.; Galuska, S.P.; Lohmeyer, J.; Preissner, K.T. Neutrophil Extracellular Traps Directly Induce Epithelial and Endothelial Cell Death: A Predominant Role of Histones. PLoS ONE 2012, 7, e32366. [Google Scholar] [CrossRef]
- Fischer, K.; Guensch, D.P.; Jung, B.; King, I.; Von Tengg-Kobligk, H.; Giannetti, N.; Eberle, B.; Friedrich, M.G. Insights into Myocardial Oxygenation and Cardiovascular Magnetic Resonance Tissue Biomarkers in Heart Failure with Preserved Ejection Fraction. Circ. Heart Fail. 2022, 15, E008903. [Google Scholar] [CrossRef]
- Oancea, A.-F.; Morariu, P.C.; Buburuz, A.M.; Miftode, I.-L.; Miftode, R.S.; Mitu, O.; Jigoranu, A.; Floria, D.-E.; Timpau, A.; Vata, A.; et al. Spectrum of Non-Obstructive Coronary Artery Disease and Its Relationship with Atrial Fibrillation. J. Clin. Med. 2024, 13, 4921. [Google Scholar] [CrossRef]
- Dumont, B.L.; Neagoe, P.E.; Charles, E.; Villeneuve, L.; Ninni, S.; Tardif, J.C.; Räkel, A.; White, M.; Sirois, M.G. Low-Density Neutrophils and Neutrophil Extracellular Traps (NETs) Are New Inflammatory Players in Heart Failure. Can. J. Cardiol. 2024, 40, 1524–1535. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, W.; Lu, C.; Ma, Z.; Jiang, S.; Gu, C.; Acuña-Castroviejo, D.; Yang, Y. Targeting NLRP3 (Nucleotide-Binding Domain, Leucine- Rich-Containing Family, Pyrin Domain-Containing-3) Inflammasome in Cardiovascular Disorders. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 2765–2779. [Google Scholar] [CrossRef]
- Chen, S.; Yu, Z.; Wen, W.; Chen, J.; Lu, K. NLRP3 Expression and Its Predictive Role in Heart Failure with Preserved Ejection Fraction among Non-Valvular Atrial Fibrillation Patients. Cardiology 2024, 1–7. [Google Scholar] [CrossRef]
- Cheng, X.; Zhao, H.; Wen, X.; Li, G.; Guo, S.; Zhang, D. NLRP3-Inflammasome Inhibition by MCC950 Attenuates Cardiac and Pulmonary Artery Remodelling in Heart Failure with Preserved Ejection Fraction. Life Sci. 2023, 333, 122185. [Google Scholar] [CrossRef]
- Deng, Y.; Xie, M.; Li, Q.; Xu, X.; Ou, W.; Zhang, Y.; Xiao, H.; Yu, H.; Zheng, Y.; Liang, Y.; et al. Targeting Mitochondria-Inflammation Circuit by β-Hydroxybutyrate Mitigates HFpEF. Circ. Res. 2021, 128, 232–245. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.R.; Lee, S.G.; Kim, S.H.; Kim, J.H.; Choi, E.; Cho, W.; Rim, J.H.; Hwang, I.; Lee, C.J.; Lee, M.; et al. SGLT2 Inhibition Modulates NLRP3 Inflammasome Activity via Ketones and Insulin in Diabetes with Cardiovascular Disease. Nat. Commun. 2020, 11, 2127. [Google Scholar] [CrossRef] [PubMed]
- Kolijn, D.; Pabel, S.; Tian, Y.; Lódi, M.; Herwig, M.; Carrizzo, A.; Zhazykbayeva, S.; Kovács, Á.; Fülöp, G.; Falcão-Pires, I.; et al. Empagliflozin Improves Endothelial and Cardiomyocyte Function in Human Heart Failure with Preserved Ejection Fraction via Reduced Pro-Inflammatory-Oxidative Pathways and Protein Kinase Gα Oxidation. Cardiovasc. Res. 2021, 117, 495–507. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, N.; Fu, P.; An, Y.; Sun, F.; Wang, C.; Han, X.; Zhang, Y.; Yu, X.; Liu, Y. Dapagliflozin Attenuates Heart Failure with Preserved Ejection Fraction Remodeling and Dysfunction by Elevating β-Hydroxybutyrate-Activated Citrate Synthase. J. Cardiovasc. Pharmacol. 2023, 82, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Wu, J.; Ye, H.; Zhang, X.; Wang, L. Application Value of Systemic Inflammatory Indexes in the Clinical Evaluation of Patients with Heart Failure with Preserved Ejection Fraction (HFpEF). Medicina 2022, 58, 1473. [Google Scholar] [CrossRef]
- Verma, R.; Moroney, M.; Hibino, M.; Mazer, C.D.; Connelly, K.A.; Yan, A.T.; Quan, A.; Teoh, H.; Verma, S.; Puar, P. Baseline Neutrophil-to-Lymphocyte Ratio and Efficacy of SGLT2 Inhibition with Empagliflozin on Cardiac Remodelling. ESC Heart Fail. 2023, 10, 2127–2133. [Google Scholar] [CrossRef]


| Variable | All Patients (n = 479) | Combined Endpoint Met (n = 267) | Combined Endpoint Not Met (n = 212) | p-Value |
|---|---|---|---|---|
| Demographics | ||||
| Age, median (IQR) | 74.3 (69.2–78.3) | 73.9 (68.5–78.6) | 74.5 (69.3–78.3) | 0.734 |
| Male sex, n (%) | 133 (27.8%) | 88 (33.0%) | 45 (21.2%) | 0.004 |
| Clinical parameters | ||||
| BMI, median (IQR) | 29.1 (25.3–33.3) | 29.3 (25.7–33.8) | 28.8 (25.0–32.0) | 0.212 |
| NYHA functional class | <0.001 | |||
| 1 | 17 (3.7%) | 4 (1.6%) | 13 (6.3%) | |
| 2 | 159 (34.8%) | 59 (23.5%) | 100 (48.5%) | |
| 3 | 281 (61.5%) | 188 (74.9%) | 93 (45.2%) | |
| Heart rate, bpm, median (IQR) | 70 (62–80) | 72 (62–81) | 68 (61–79) | 0.155 |
| BP systolic, mmHg, median (IQR) | 140 (125–155) | 140 (122–156) | 140 (125–151) | 0.908 |
| BP diastolic, mmHg, median (IQR) | 80 (70–87) | 78 (69–86) | 80 (70–88) | 0.036 |
| NT-proBNP, pg/mL, median (IQR) | 1068 (415–2062) | 1498 (720–2577) | 643 (319–1232) | <0.001 |
| Creatinine, mg/dL, median (IQR) | 1.09 (0.88–1.37) | 1.17 (0.93–1.50) | 0.98 (0.84–1.20) | <0.001 |
| eGFR, mL/min/1.73 m2, median (IQR) | 55 (41–71) | 49 (37–66) | 62 (46–76) | <0.001 |
| Heart failure with preserved ejection fraction scores | ||||
| H2FPEF-Score | 0.132 | |||
| ≤3 | 99 (20.7%) | 44 (16.5%) | 55 (25.9%) | |
| 4–6 | 231 (48.2%) | 134 (50.2%) | 97 (45.8%) | |
| 7–9 | 149 (31.1%) | 89 (33.3%) | 60 (28.3%) | |
| HFA-PEFF-Score | <0.001 | |||
| ≤2 | 55 (11.5%) | 21 (7.9%) | 34 (16.0%) | |
| 3–4 | 124 (25.9%) | 57 (21.3%) | 67 (31.6%) | |
| 5–6 | 300 (62.6%) | 189 (70.8) | 111 (52.4%) | |
| Comorbidities and medical history | ||||
| Atrial fibrillation, n (%) | 289 (60.3%) | 170 (63.7%) | 119 (56.1%) | 0.094 |
| Significant coronary artery disease, n (%) | 153 (32.0%) | 100 (37.5%) | 53 (25.1%) | 0.004 |
| Myocardial infarction, n (%) | 36 (7.6%) | 24 (9.1%) | 12 (5.8%) | 0.177 |
| Diabetes mellitus, n (%) | 164 (34.3%) | 114 (42.9%) | 50 (23.6%) | <0.001 |
| Arterial hypertension, n (%) | 441 (92.3%) | 252 (94.4%) | 189 (89.6%) | 0.051 |
| Heart failure medication | ||||
| Beta receptor antagonists, n (%) | 345 (72.2%) | 200 (74.9%) | 145 (68.7%) | 0.134 |
| ACEi/AT1i, n (%) | 319 (66.7%) | 176 (65.9%) | 143 (67.8%) | 0.669 |
| Mineralocorticoid receptor antagonists, n (%) | 206 (43.2%) | 128 (47.9%) | 78 (37.1%) | 0.018 |
| Sodium-glucose-cotransporter 2 inhibitors, n (%) | 18 (3.8%) | 3 (1.1%) | 15 (7.1%) | <0.001 |
| Echocardiographic markers | ||||
| IVS, mm, median (IQR) | 12 (11–14) | 12 (11–14) | 12 (11–14) | 0.888 |
| RWT, median (IQR) | 0.48 (0.4–0.55) | 0.49 (0.41–0.56) | 0.47 (0.38–0.55) | 0.055 |
| LV ejection fraction, %, median (IQR) | 60 (55–66) | 60 (55–66) | 60 (54–66) | 0.990 |
| LA volume index, mL/m2, median (IQR) | 40 (30–53) | 40 (32–55) | 38 (29–48) | 0.002 |
| LV stroke volume index, mL/m2, median (IQR) | 24 (19–30) | 25 (19–31) | 23 (18–29) | 0.035 |
| LV mass index, g/m2, median (IQR) | 94 (78–115) | 94 (79–115) | 96 (75–114) | 0.426 |
| E/A, median (IQR) | 1.19 (0.84–2.04) | 1.42 (0.92–2.28) | 1.07 (0.79–1.55) | 0.001 |
| E/e’, median (IQR) | 12.1 (9.1–15.1) | 13.6 (9.8–15.6) | 10.9 (8.6–14.0) | 0.086 |
| sysPAP, mmHg, median (IQR) | 53 (43–67) | 62 (48–74) | 46 (37–57) | <0.001 |
| LV global longitudinal strain, -%, mean (SD) | 16.6 (3.8) | 16.1 (3.6) | 17.2 (3.9) | 0.019 |
| Markers of inflammation | ||||
| Leukocytes, G/L, median (IQR) | 7.3 (5.9–8.7) | 7.3 (5.7–8.9) | 7.2 (5.9–8.4) | 0.335 |
| Neutrophils, G/L, median (IQR) | 4.7 (3.9–6.0) | 4.9 (4.0–6.1) | 4.5 (3.7–5.8) | 0.038 |
| Monocytes, G/L, median (IQR) | 0.6 (0.5–0.7) | 0.6 (0.5–0.7) | 0.6 (0.5–0.7) | 0.037 |
| Lymphocytes, G/L, median (IQR) | 1.5 (1.1–1.9) | 1.4 (1.0–1.8) | 1.6 (1.2–2.0) | <0.001 |
| Thrombocytes, G/L, median (IQR) | 226 (187–270) | 223 (185–269) | 228 (195–272) | 0.172 |
| C-reactive protein, mg/dL, median (IQR) | 0.37 (0.16–0.8) | 0.49 (0.21–1.12) | 0.28 (0.11–0.57) | <0.001 |
| NLR, median (IQR) | 3.2 (2.3–4.8) | 3.6 (2.5–5.1) | 2.8 (2.2–3.9) | <0.001 |
| MLR, median (IQR) | 0.40 (0.31–0.57) | 0.43 (0.33–0.64) | 0.38 (0.29–0.46) | <0.001 |
| PIV, median (IQR) | 433 (272–696) | 469 (307–840) | 385 (249–605) | <0.001 |
| Variable | HR | 95%-CI | p-Value | HR | 95%-CI | p-Value | HR | 95%-CI | p-Value | HR | 95%-CI | p-Value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate NLR | Multivariate MLR | Multivariate PIV | |||||||||
| NLR median | 1.76 | 1.38–2.24 | <0.001 | 1.81 | 1.22–2.69 | 0.003 | ||||||
| MLR median | 1.46 | 1.14–1.86 | 0.003 | 1.57 | 1.06–2.34 | 0.026 | ||||||
| PIV median | 1.67 | 1.30–2.13 | <0.001 | 1.64 | 1.10–2.46 | 0.015 | ||||||
| Age | 1.01 | 1.00–1.03 | 0.093 | |||||||||
| Male sex | 1.50 | 1.12–1.88 | 0.004 | 1.98 | 1.31–3.00 | 0.001 | 1.91 | 1.26–2.89 | 0.002 | 2.01 | 1.32–3.05 | 0.001 |
| BMI | 1.02 | 1.00–1.04 | 0.137 | |||||||||
| NYHA | 2.36 | 1.87–2.98 | <0.001 | 1.83 | 1.31–2.57 | <0.001 | 1.86 | 1.34–2.60 | <0.001 | 1.80 | 1.28–2.54 | <0.001 |
| NT-proBNP | 1.00 | 1.00–1.00 | <0.001 | 1.00 | 1.00–1.00 | 0.233 | 1.00 | 1.00–1.00 | 0.410 | 1.00 | 1.00–1.00 | 0.240 |
| eGFR | 0.98 | 0.98–0.99 | <0.001 | 0.99 | 0.98–1.00 | 0.005 | 0.99 | 0.98–1.00 | 0.004 | 0.99 | 0.98–1.0 | 0.005 |
| CRP | 1.08 | 1.03–1.14 | 0.001 | 1.01 | 0.91–1.12 | 0.852 | 1.02 | 0.92–1.13 | 0.731 | 1.01 | 0.90–1.12 | 0.930 |
| AF | 1.32 | 1.03–1.70 | 0.029 | 0.63 | 0.42–0.96 | 0.029 | 0.68 | 0.45–1.02 | 0.064 | 0.68 | 0.46–1.02 | 0.063 |
| DM | 1.92 | 1.51–2.46 | <0.001 | 1.82 | 1.24–2.69 | 0.002 | 1.92 | 1.30–2.83 | 0.001 | 1.92 | 1.30–2.83 | 0.001 |
| sysPAP | 1.03 | 1.02–1.04 | <0.001 | 1.03 | 1.02–1.04 | <0.001 | 1.03 | 1.02–1.04 | <0.001 | 1.03 | 1.02–1.04 | <0.001 |
| LV GLS | 1.05 | 1.01–1.10 | 0.022 | 1.00 | 0.95–1.05 | 0.976 | 1.01 | 0.96–1.06 | 0.803 | 1.00 | 0.95–1.05 | 0.970 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poledniczek, M.; Kronberger, C.; List, L.; Gregshammer, B.; Willixhofer, R.; Ermolaev, N.; Duca, F.; Binder, C.; Rettl, R.; Badr Eslam, R.; et al. Leukocyte Indices as Markers of Inflammation and Predictors of Outcome in Heart Failure with Preserved Ejection Fraction. J. Clin. Med. 2024, 13, 5875. https://doi.org/10.3390/jcm13195875
Poledniczek M, Kronberger C, List L, Gregshammer B, Willixhofer R, Ermolaev N, Duca F, Binder C, Rettl R, Badr Eslam R, et al. Leukocyte Indices as Markers of Inflammation and Predictors of Outcome in Heart Failure with Preserved Ejection Fraction. Journal of Clinical Medicine. 2024; 13(19):5875. https://doi.org/10.3390/jcm13195875
Chicago/Turabian StylePoledniczek, Michael, Christina Kronberger, Luca List, Bernhard Gregshammer, Robin Willixhofer, Nikita Ermolaev, Franz Duca, Christina Binder, René Rettl, Roza Badr Eslam, and et al. 2024. "Leukocyte Indices as Markers of Inflammation and Predictors of Outcome in Heart Failure with Preserved Ejection Fraction" Journal of Clinical Medicine 13, no. 19: 5875. https://doi.org/10.3390/jcm13195875
APA StylePoledniczek, M., Kronberger, C., List, L., Gregshammer, B., Willixhofer, R., Ermolaev, N., Duca, F., Binder, C., Rettl, R., Badr Eslam, R., Camuz Ligios, L., Nitsche, C., Hengstenberg, C., Kastner, J., Bergler-Klein, J., & Kammerlander, A. A. (2024). Leukocyte Indices as Markers of Inflammation and Predictors of Outcome in Heart Failure with Preserved Ejection Fraction. Journal of Clinical Medicine, 13(19), 5875. https://doi.org/10.3390/jcm13195875







