Impact of Conversion from Conventional Pemafibrate to Novel Pemafibrate XR on Hypertriglyceridemia: An Observational Retrospective Study
Abstract
:1. Background
2. Methods
2.1. Patient Selection
2.2. Study Design
2.3. Biomarker Measurement
2.4. Other Clinical Data
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Pemafibrate Therapy
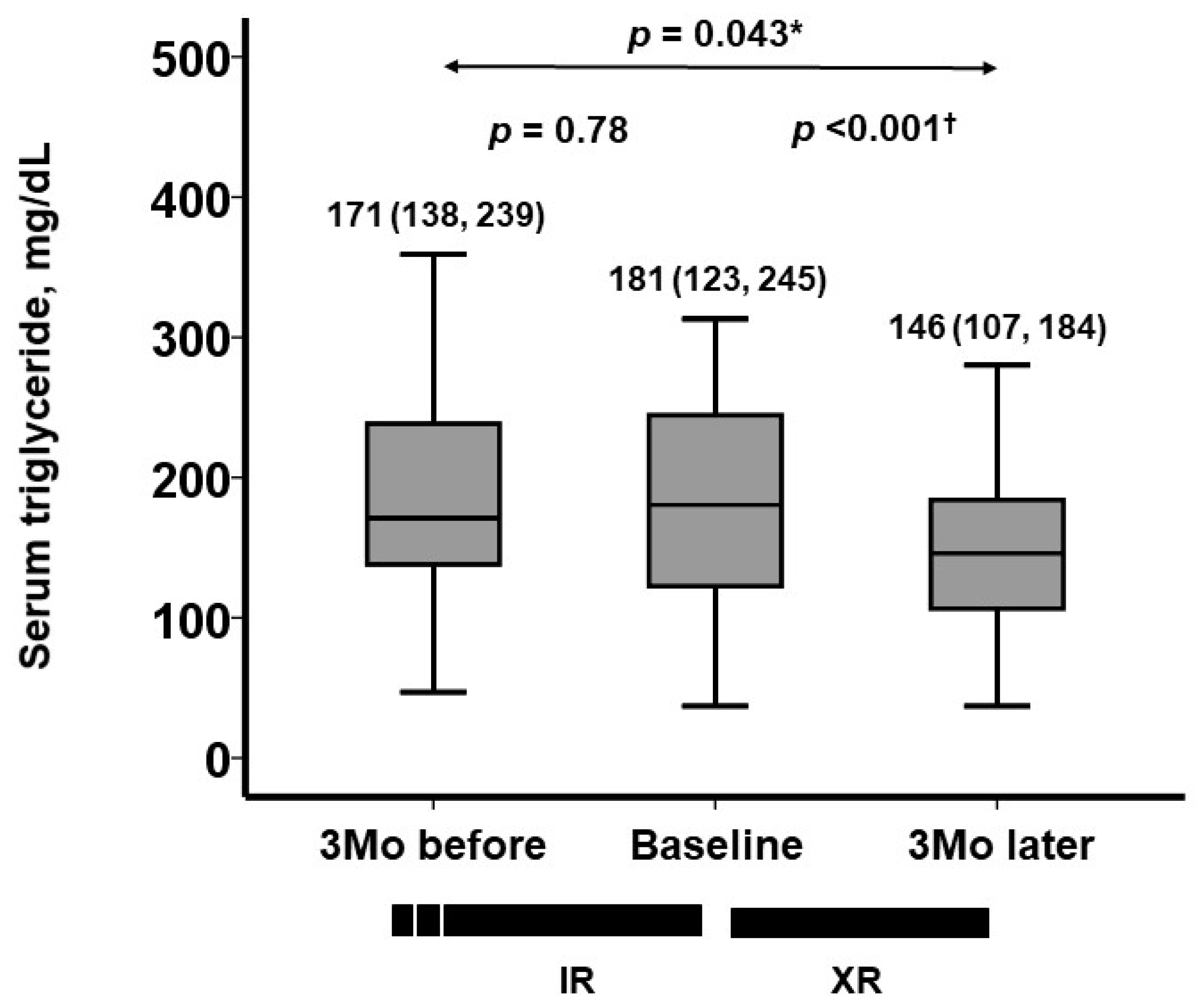
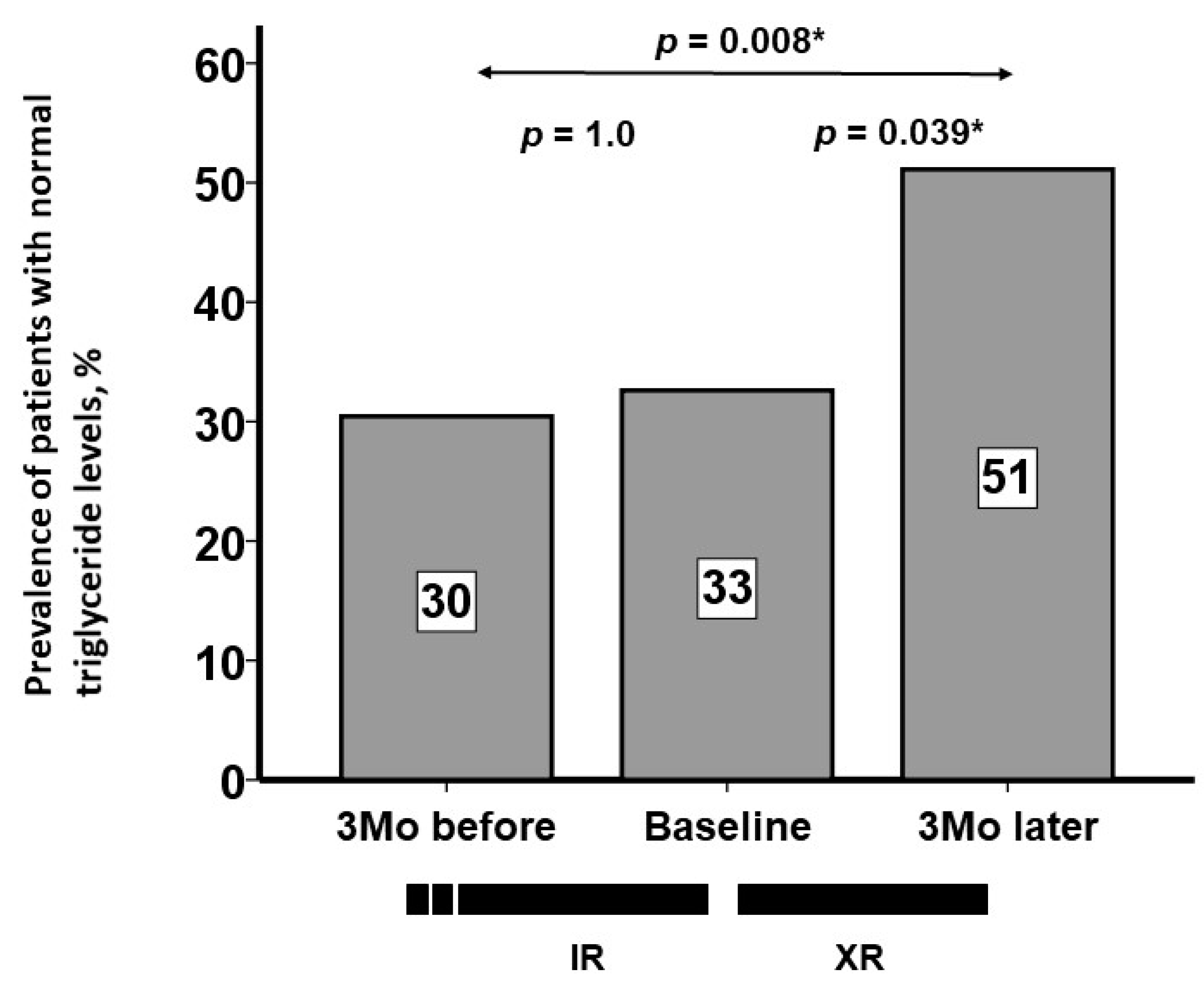
3.3. Trajectory of Serum Triglyceride Levels
3.4. Trajectory of Other Biomarkers
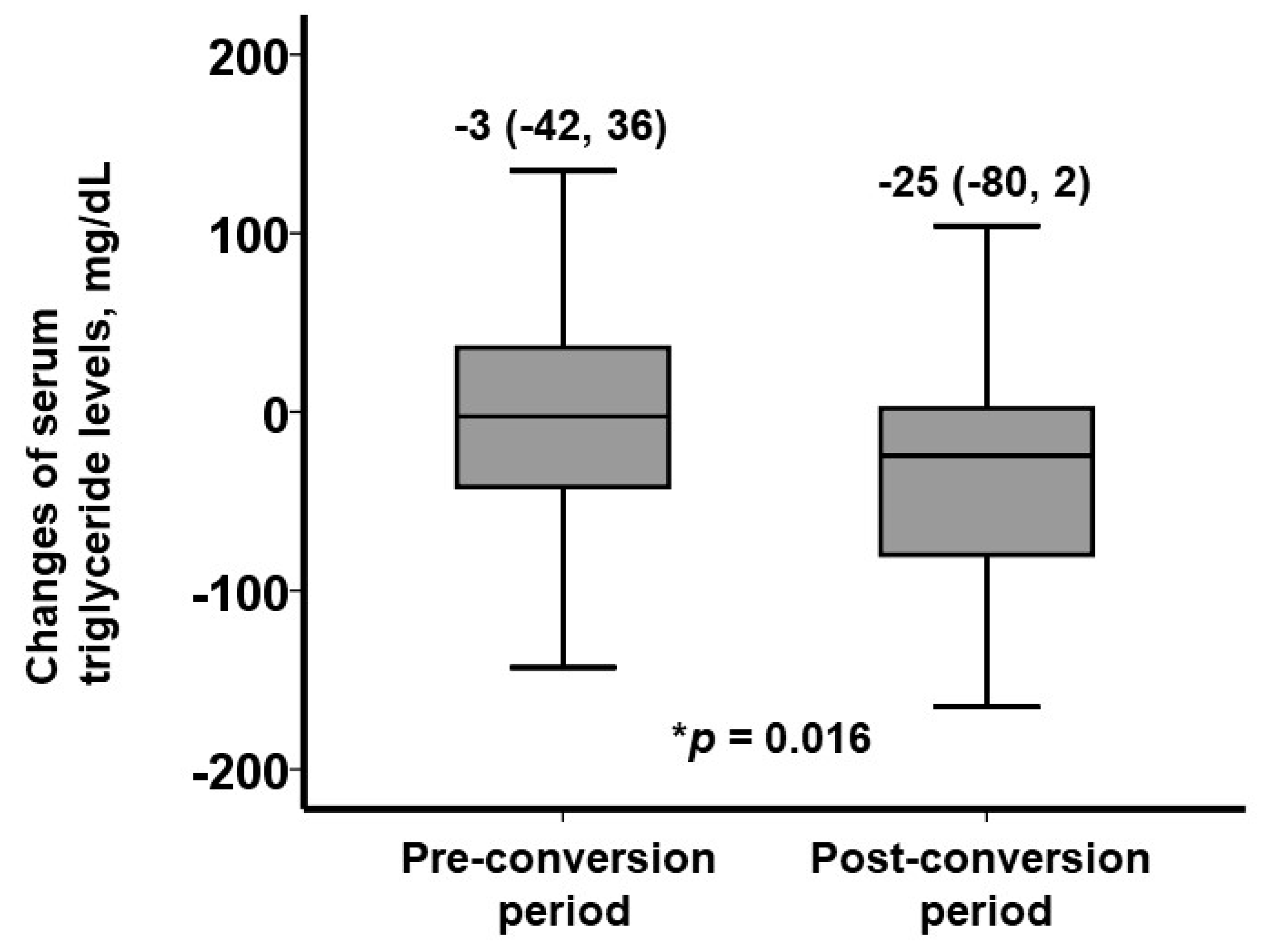
3.5. Factors Associated with a Decrease in Serum Triglyceride Levels Following Conversion
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cholesterol Treatment Trialists C; Fulcher, J.; O’Connell, R.; Voysey, M.; Emberson, J.; Blackwell, L.; Mihaylova, B.; Simes, J.; Collins, R.; Kirby, A.; et al. Efficacy and safety of LDL-lowering therapy among men and women: Meta-analysis of individual data from 174,000 participants in 27 randomised trials. Lancet 2015, 385, 1397–1405. [Google Scholar] [PubMed]
- Kazi, D.S.; Penko, J.M.; Bibbins-Domingo, K. Statins for Primary Prevention of Cardiovascular Disease: Review of Evidence and Recommendations for Clinical Practice. Med. Clin. N. Am. 2017, 101, 689–699. [Google Scholar] [CrossRef] [PubMed]
- Barter, P.J.; Rye, K.A. Cardioprotective properties of fibrates: Which fibrate, which patients, what mechanism? Circulation 2006, 113, 1553–1555. [Google Scholar] [CrossRef] [PubMed]
- Ferri, N.; Corsini, A.; Sirtori, C.; Ruscica, M. PPAR-α agonists are still on the rise: An update on clinical and experimental findings. Expert Opin. Investig. Drugs 2017, 26, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Fruchart, J.C. Selective peroxisome proliferator-activated receptor alpha modulators (SPPARMalpha): The next generation of peroxisome proliferator-activated receptor alpha-agonists. Cardiovasc. Diabetol. 2013, 12, 82. [Google Scholar] [CrossRef] [PubMed]
- Arai, H.; Yamashita, S.; Yokote, K.; Araki, E.; Suganami, H.; Ishibashi, S.; on behalf of the K-877 Study Group. Efficacy and Safety of Pemafibrate Versus Fenofibrate in Patients with High Triglyceride and Low HDL Cholesterol Levels: A Multicenter, Placebo-Controlled, Double-Blind, Randomized Trial. J. Atheroscler. Thromb. 2018, 25, 521–538. [Google Scholar] [CrossRef] [PubMed]
- Izumihara, R.; Nomoto, H.; Kito, K.; Yamauchi, Y.; Omori, K.; Shibayama, Y.; Yanagiya, S.; Miya, A.; Kameda, H.; Cho, K.Y.; et al. Switching from Conventional Fibrates to Pemafibrate Has Beneficial Effects on the Renal Function of Diabetic Subjects with Chronic Kidney Disease. Diabetes Metab. J. 2024, 48, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Kamimura, T.; Hounslow, N.; Suganami, H.; Tanigawa, R. Drug-drug interactions between pemafibrate and statins on pharmacokinetics in healthy male volunteers: Open-label, randomized, 6-sequence, 3-period crossover studies. Clin. Transl. Sci. 2024, 17, e13900. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, S.; Hirano, T.; Shimano, H.; Tsukamoto, K.; Yoshida, M.; Yoshida, H. Managing hypertriglyceridemia for cardiovascular disease prevention: Lessons from the PROMINENT trial. Eur. J. Clin. Investig. 2024, 54, e14227. [Google Scholar] [CrossRef] [PubMed]
- Toy, E.L.; Beaulieu, N.U.; McHale, J.M.; Welland, T.R.; Plauschinat, C.A.; Swensen, A.; Duh, M.S. Treatment of COPD: Relationships between daily dosing frequency, adherence, resource use, and costs. Respir. Med. 2010, 105, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Sadosky, A.; Srivastava, K.; Arora, A.; Kataria, A.; Cappelleri, J.C.; Peterson, A.M. Impact of reducing dosing frequency on adherence to oral therapies: A literature review and meta-analysis. Patient Prefer. Adherence 2013, 7, 419–434. [Google Scholar] [CrossRef] [PubMed]
- Arai, H.; Yamashita, S.; Araki, E.; Yokote, K.; Tanigawa, R.; Saito, A.; Yamasaki, S.; Suganami, H.; Ishibashi, S. Efficacy and Safety of Pemafibrate Extended-Release Tablet: A Phase 3, Multicenter, Randomized, Double-Blind, Active-Controlled, Parallel-Group Comparison Trial. J. Atheroscler. Thromb. 2024, 64677. [Google Scholar] [CrossRef] [PubMed]
- Akasaki, Y. Once-daily Extended-Release Pemafibrate Enhances Adherence and Triglyceride Control Over Twice-Daily Dosing. J. Atheroscler. Thromb. 2024, ED266. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, S.; Yamashita, S.; Arai, H.; Araki, E.; Yokote, K.; Suganami, H.; Fruchart, J.-C.; Kodama, T.; K-877-04 Study Group. Effects of K-877, a novel selective PPARalpha modulator (SPPARMalpha), in dyslipidaemic patients: A randomized, double blind, active- and placebo-controlled, phase 2 trial. Atherosclerosis 2016, 249, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Choudhry, N.K.; Kronish, I.M.; Vongpatanasin, W.; Ferdinand, K.C.; Pavlik, V.N.; Egan, B.M.; Schoenthaler, A.; Miller, N.H.; Hyman, D.J.; on behalf of the American Heart Association Council on Hypertension; et al. Medication Adherence and Blood Pressure Control: A Scientific Statement From the American Heart Association. Hypertension 2022, 79, e1–e14. [Google Scholar] [CrossRef] [PubMed]
- Claxton, A.J.; Cramer, J.; Pierce, C. A systematic review of the associations between dose regimens and medication compliance. Clin. Ther. 2001, 23, 1296–1310. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Zhao, Y.; Yang, X.; Li, Y.; Han, M.; Qie, R.; Huang, S.; Wu, X.; Zhang, Y.; Wu, Y.; et al. Adherence to antihypertensive medication and cardiovascular disease events in hypertensive patients: A dose–response meta-analysis of 2 769 700 participants in cohort study. QJM Int. J. Med. 2021, 115, 279–286. [Google Scholar] [CrossRef] [PubMed]
| n = 46 | |
|---|---|
| Age, years | 62 (52, 70) |
| Men | 29 (63%) |
| Body mass index | 25.9 (22.2, 30.1) |
| Heart failure | 27 (59%) |
| Hypertension | 4 (9%) |
| Dyslipidemia | 46 (100%) |
| Atrial fibrillation | 3 (7%) |
| Diabetes mellitus | 30 (65%) |
| Chronic kidney disease | 13 (28%) |
| Hemodialysis | 0 (0%) |
| Statin use | 15 (33%) |
| Ezetimib use | 5 (11%) |
| Anti-hypertension medication | 3 (7%) |
| Anti-diabetic medication | 28 (61%) |
| Anti-platelet medication | 18 (39%) |
| 3Mo before Conversion | Baseline | p-Value vs. 3Mo before | 3Mo after Conversion | p-Value vs. Baseline | |
|---|---|---|---|---|---|
| Total cholesterol, mg/dL | 187 (154, 212) | 191 (164, 219) | 0.41 | 185 (161, 211) | 0.39 |
| LDL cholesterol, mg/dL | 100 (71, 118) | 97 (74, 117) | 0.54 | 99 (82, 114) | 0.75 |
| HDL cholesterol, mg/dL | 44 (34, 54) | 39 (32, 51) | 0.052 | 47 (40, 55) | <0.001 * |
| Triglyceride/HDL cholesterol ratio | 4.2 (2.6, 5.5) | 4.1 (2.9, 6.0) | 0.15 | 2.9 (2.0, 4.8) | 0.001 * |
| Triglyceride-rich lipoprotein, mg/dL | 29.5 (26.0, 51.0) | 33.5 (25.0, 51.0) | 0.25 | 30.5 (21.0, 40.0) | 0.034 * |
| Estimated small dense LDL cholesterol, mg/dL | 38.2 (30.9, 45.8) | 36.8 (31.6, 47.9) | 0.27 | 35.8 (31.3, 42.9) | 0.023 * |
| 3Mo before Conversion | Baseline | p-Value vs. 3Mo before | 3Mo after Conversion | p-Value vs. Baseline | |
|---|---|---|---|---|---|
| Hemoglobin, g/dL | 14.1 (13.3, 15.6) | 14.0 (12.7, 15.2) | 0.068 | 13.9 (12.5, 14.9) | 0.65 |
| Aspartate aminotransferase, IU/L | 23 (13, 34) | 23 (17, 36) | 0.19 | 22 (18, 33) | 0.19 |
| Alanine aminotransferase, IU/L | 21 (13, 37) | 21 (11, 37) | 0.93 | 19 (13, 33) | 0.93 |
| Gamma-glutamyl transpeptidase, IU/L | 29 (17, 40) | 29 (16, 36) | 0.13 | 24 (16, 36) | 0.13 |
| eGFR, mL/min/1.73 m2 | 69.5 (57.3, 82.8) | 66.9 (47.5, 78.9) | 0.56 | 71.3 (57.4, 78.6) | 0.56 |
| Sodium, mEq/L | 140 (138, 141) | 140 (139, 142) | 0.087 | 140 (139, 142) | 0.067 |
| Potassium, mEq/L | 4.3 (4.0, 4.5) | 4.2 (4.1, 4.4) | 0.59 | 4.3 (4.0, 4.5) | 0.59 |
| Univariable Analysis | Multivariable Analysis | |||
|---|---|---|---|---|
| Odds Ratio (95% CI) | p-Value | Odds Ratio (95% CI) | p-Value | |
| Age > 70 years | 72.0 (0.82–62.9) | 0.074 | ||
| Men | 1.98 (0.57–6.79) | 0.28 | ||
| Once-daily prescription | 6.09 (1.17–31.6) | 0.031 * | 6.59 (1.03–42.2) | 0.047 * |
| Number of daily tablets | 0.87 (0.74–1.03) | 0.14 | ||
| Serum triglyceride, mg/dL | 1.02 (1.00–1.03) | 0.011 * | 1.02 (1.00–1.03) | 0.014 * |
| Heart failure | 1.63 (0.18–19.3) | 0.61 | ||
| Hypertension | 0.67 (0.20–2.31) | 0.535 | ||
| Atrial fibrillation | 1.19 (0.10–14.1) | 0.89 | ||
| Diabetes mellitus | 0.68 (0.19–2.46) | 0.56 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hida, Y.; Imamura, T.; Kinugawa, K. Impact of Conversion from Conventional Pemafibrate to Novel Pemafibrate XR on Hypertriglyceridemia: An Observational Retrospective Study. J. Clin. Med. 2024, 13, 5879. https://doi.org/10.3390/jcm13195879
Hida Y, Imamura T, Kinugawa K. Impact of Conversion from Conventional Pemafibrate to Novel Pemafibrate XR on Hypertriglyceridemia: An Observational Retrospective Study. Journal of Clinical Medicine. 2024; 13(19):5879. https://doi.org/10.3390/jcm13195879
Chicago/Turabian StyleHida, Yuki, Teruhiko Imamura, and Koichiro Kinugawa. 2024. "Impact of Conversion from Conventional Pemafibrate to Novel Pemafibrate XR on Hypertriglyceridemia: An Observational Retrospective Study" Journal of Clinical Medicine 13, no. 19: 5879. https://doi.org/10.3390/jcm13195879







