Treating Anxiety-Based Cognitive Distortions Pertaining to Somatic Perception for Better Chronic Pain Outcomes: A Recommendation for Better Practice in the Present Day and the Cyber Age of Medicine
Abstract
:1. Introduction
2. Anxiety-Based Cognitive Distortions Pertaining to Somatic Perception (ABCD-SPs)
2.1. An Overview of the Role of ABCD-SPs in the Negative Sequelae of CNCP
2.2. ABCD-SP Validated Assessment Tools
2.2.1. The Fear-Avoidance Beliefs Questionnaire (FAB)
2.2.2. The Pain Catastrophizing Scale (PCS)
2.2.3. The Tampa Scale of Kinesiophobia (TSK)
3. Pathology of Anxiety-Based Cognitive Distortions Pertaining to Somatic Perception—Proposed Mechanisms
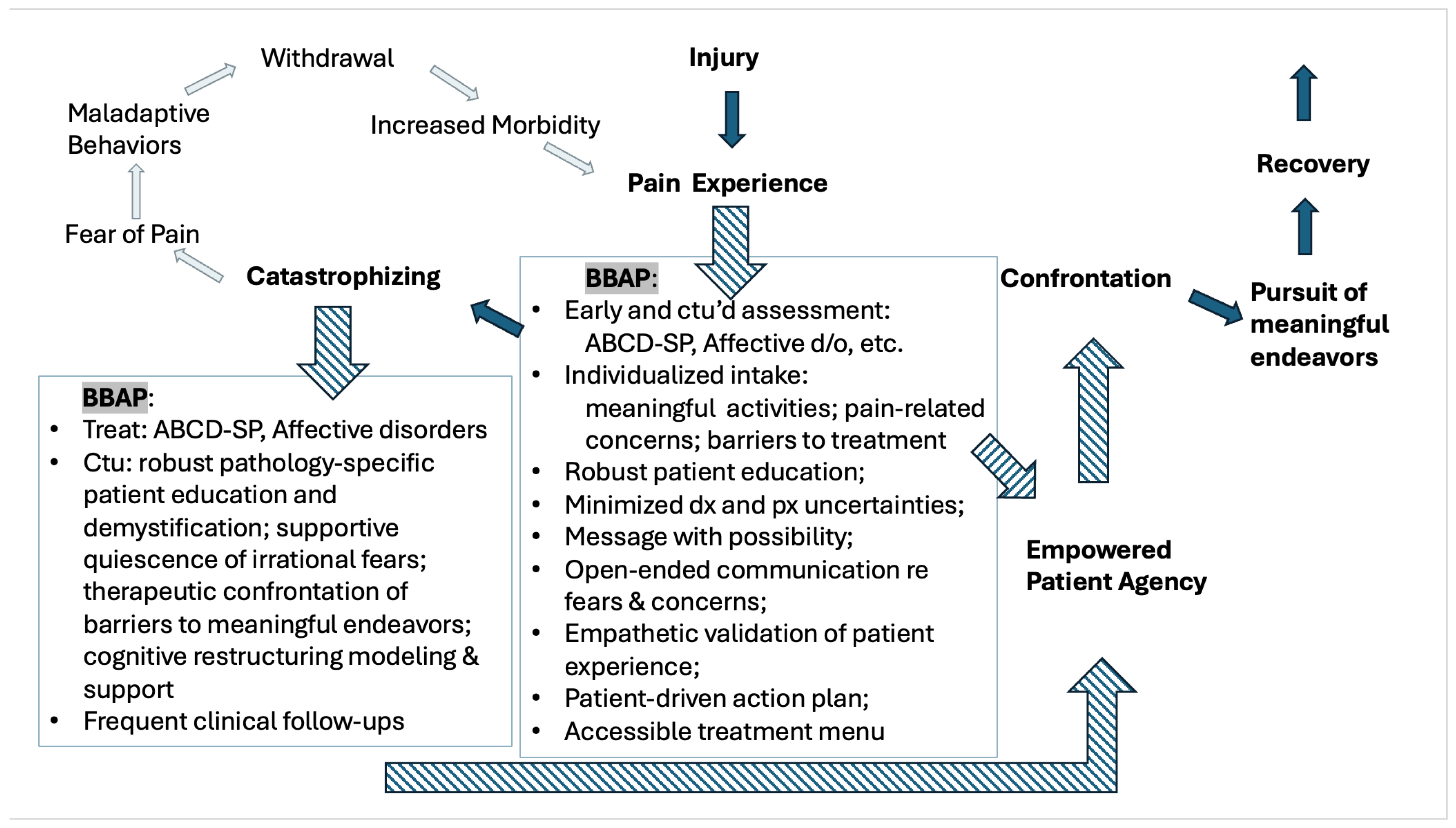
4. The Call for a Belief and Behavior Action Plan—Theoretical Considerations
5. Creating a Belief and Behavior Action Plan—Clinical Considerations
6. Creating a Belief and Behavior Action Plan—Recommendations and Practical Considerations
- Utilize standardized assessments and short-answer questionnaires upon initial evaluation, and periodically in follow-up, to assess and monitor the potential for ABCD-SPs to interfere with rehabilitation:
- Standardized assessments:
- Short-answer questionnaires to catalog patients’ perceptions regarding the following:
- Activities of meaning, which can help accomplish the following goals:
- Lay the groundwork to create an individualized care plan.
- Strategize support and diminish negative impact on these activities.
- Better motivate patient participation [24].
- Aid in decreasing treatment plans rooted in implicit bias for populations heralding from a race, culture, gender, sexuality, or age group that differs from the provider [123].
- Implement an intentional BBAP inquiry and communication strategy and style in the clinical visit:
- Invest heavily in the first visit by performing a deep exploration and inquiry into the patient’s pain experience and their current pain-related beliefs and resulting behaviors (much of which can be initiated via the short-answer format suggested above) [101].
- Use validating active listening, which has been shown to increase patient adherence to care planning [125].
- Lean into, and address head-on, patient’s accounts of suffering and fear in the clinical setting to achieve the following:
- Dispel the ability of these sentiments to hijack adaptive recovery processes when the patient ruminates alone [93].
- Decrease the suffering of invisibility that patients with CNCP often face. While it is difficult for clinicians to focus on a patients’ suffering because of the accompanying sense of clinical impotence, and frequent lack of objective solutions, it has been suggested that simply witnessing the patient’s subjective suffering experience may decrease the severity of the same suffering [112].
- Be cognizant of both the implicit and explicit messaging inherent in communications imparted by the clinician to the patient about diagnosis and prognosis. Positive self-perceptions and health-related optimism correlate with improved pain suffering, pain-related disability [92,95,97,126,127,128], and even increased longevity [129]. When possible and appropriate, choose vocabulary and descriptors that de-escalate the patient’s perceived threat of nociceptive input, and which highlight functional and meaningful possibility.
- Message with mindfulness of potential emotional trauma-affected hyperarousal and increased sensitivity to pain [130].
- Increase healthcare literacy and promote pathology demystification:
- Ask patients to paraphrase their understanding of their injury, pain, and pathology. Note terminology used and connect medical terminology to patient’s perceptions and descriptions to promote demystification [57,92]. Correct misconceptions while maintaining patient-generated frame of reference and terminology, when appropriate.
- Consider inviting a call and paraphrased repeat opportunity between the clinician and the patient to improve comprehension of pathology and related care plan.
- Assuming the standard use of language interpreters to bridge translation barriers, also employ visual aids and physical models to engage multiple patient learning style preferences to explain not only pathology but also the mechanisms of pain symptomatology in an effort to demystify and decrease anxiety related to somatic perceptions.
- Orient to when fear of catastrophe is warranted.
- Debrief previous urgent, or emergent, clinical visits to seek pain treatment. Discuss causational factors and a care plan for future episodes in the form of improved medication organization, strategized BBAP interventions, a change in medication regimen for more effective analgesia, a change in formulary or treatment type for improved access, etc.
- Orient to “red flag” signs and symptoms that medically warrant emergent attention and educate to differentiate from chronic, stable stimuli.
- BBAP components should include the following:
- Cultivate an empowering, patient-driven action plan (to complement the encompassing medical and interdisciplinary treatment plan) containing the following elements:
- Facilitation of a menu of active, self-care options to address various pain levels and flares. Include features accessible in and out of the home, and which represent treatment modalities from a variety of psychosocial domains: behavioral, physical, social, medical, spiritual, occupational, etc.
- Minimized barriers and avoidance of the “gate keeper” perception of clinical treatment options where possible—within the confines of evidence-based care—which inherently promote a role of helplessness, perceptions of scarcity, and an external locus of control. Instead, promote care planning options that are autonomously administered and are rooted in patient agency, including the following:
- Orient to a home exercise program that de-amplifies pain suffering via an assortment of activities rooted in multiple psychosocial domains [62].
- Plan a care regimen creatively, and individually, around potential socioeconomic barriers to treatment access (transportation, mobility, coverage, cost, etc.) by choosing generic medications, refilling for longer durations, providing telemedicine, etc. [131].
7. Discussion
7.1. Anticipated Objections to BBAP Implementation—Financial Disincentivization
7.2. Anticipated Objections to BBAP Implementation—Scope of Practice Creep
7.3. BBAP Relevance and Potential: Medicine in the Cyber Age
8. Limitations
9. Conclusions
Funding
Conflicts of Interest
References
- Rikard, S.M. Chronic Pain Among Adults—United States, 2019–2021. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.J.; Hillner, B.E. The Cost of Pain. JAMA Netw. Open 2019, 2, e191532. [Google Scholar] [CrossRef] [PubMed]
- Von Korff, M.; Scher, A.I.; Helmick, C.; Carter-Pokras, O.; Dodick, D.W.; Goulet, J.; Hamill-Ruth, R.; LeResche, L.; Porter, L.; Tait, R.; et al. United States National Pain Strategy for Population Research: Concepts, Definitions, and Pilot Data. J. Pain. 2016, 17, 1068–1080. [Google Scholar] [CrossRef] [PubMed]
- Bifulco, L.; Anderson, D.R.; Blankson, M.L.; Channamsetty, V.; Blaz, J.W.; Nguyen-Louie, T.T.; Scholle, S.H. Evaluation of a Chronic Pain Screening Program Implemented in Primary Care. JAMA Netw. Open 2021, 4, e2118495. [Google Scholar] [CrossRef] [PubMed]
- Dahlhamer, J.; Lucas, J.; Zelaya, C.; Nahin, R.; Mackey, S.; DeBar, L.; Kerns, R.; Von Korff, M.; Porter, L.; Helmick, C. Prevalence of Chronic Pain and High-Impact Chronic Pain Among Adults—United States, 2016. MMWR Morb. Mortal. Wkly. Rep. 2018, 67, 1001–1006. [Google Scholar] [CrossRef]
- MPR. Opioid Refugees: Patients Adrift in Search of Pain Relief. Available online: https://www.empr.com/home/mpr-first-report/painweek-2013/opioid-refugees-patients-adrift-in-search-of-pain-relief/ (accessed on 6 May 2020).
- Silva, M.J.; Kelly, Z. The Escalation of the Opioid Epidemic Due to COVID-19 and Resulting Lessons About Treatment Alternatives. Am. J. Manag. Care 2020, 26, e202–e204. [Google Scholar] [CrossRef]
- Silva, M.J.; Coffee, Z.; Goza, J.; Rumril, K. Microinduction to Buprenorphine from Methadone for Chronic Pain: Outpatient Protocol with Case Examples. J. Pain Palliat. Care Pharmacother. 2022, 36, 40–48. [Google Scholar] [CrossRef]
- Dowell, D.; Haegerich, T.M.; Chou, R. CDC Guideline for Prescribing Opioids for Chronic Pain—United States, 2016. MMWR Recomm. Rep. 2016, 65, 1624–1645. Available online: https://www.cdc.gov/mmwr/volumes/65/rr/rr6501e1.htm (accessed on 20 September 2024). [CrossRef]
- Davenport, T.; Kalakota, R. The potential for artificial intelligence in healthcare. Future Healthc. J. 2019, 6, 94–98. [Google Scholar] [CrossRef]
- Sahni, N.; Stein, G.; Zemmel, R.; Cutler, D.M. The Potential Impact of Artificial Intelligence on Healthcare Spending 2023; National Bureau of Economic Research: Cambridge, MA, USA, 2023; Available online: https://papers.ssrn.com/abstract=4334926 (accessed on 10 September 2024).
- Alcon, C.; Bergman, E.; Humphrey, J.; Patel, R.M.; Wang-Price, S. The Relationship between Pain Catastrophizing and Cognitive Function in Chronic Musculoskeletal Pain: A Scoping Review. Pain Res. Manag. 2023, 2023, 5851450. [Google Scholar] [CrossRef]
- Brouwer, B.; Waardenburg, S.; Jacobs, C.; Overdijk, M.; Leue, C.; Köke, A.; Kuijk, S.V.; Van Kleef, M.; Van Zundert, J.; De Meij, N. Biopsychosocial baseline values of 15,000 patients suffering from chronic pain: Dutch DataPain study. Reg. Anesth. Pain Med. 2020, 45, 774–782. [Google Scholar] [CrossRef] [PubMed]
- APA Dictionary of Psychology. Available online: https://dictionary.apa.org/ (accessed on 19 July 2024).
- PCSManual_English.pdf. Available online: http://sullivan-painresearch.mcgill.ca/pdf/pcs/PCSManual_English.pdf (accessed on 8 May 2020).
- Waddell, G.; Newton, M.; Henderson, I.; Somerville, D.; Main, C.J. A Fear-Avoidance Beliefs Questionnaire (FABQ) and the role of fear-avoidance beliefs in chronic low back pain and disability. Pain 1993, 52, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Neblett, R.; Hartzell, M.M.; Mayer, T.G.; Bradford, E.M.; Gatchel, R.J. Establishing clinically meaningful severity levels for the Tampa Scale for Kinesiophobia (TSK-13). Eur. J. Pain 2016, 20, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, M.J.L.; Martel, M.O.; Tripp, D.; Savard, A.; Crombez, G. The relation between catastrophizing and the communication of pain experience. Pain 2006, 122, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Linton, S.J.; Shaw, W.S. Impact of Psychological Factors in the Experience of Pain. Phys. Ther. 2011, 91, 700–711. [Google Scholar] [CrossRef]
- Waddell, G.; Somerville, D.; Henderson, I.; Newton, M. Objective clinical evaluation of physical impairment in chronic low back pain. Spine 1992, 17, 617–628. [Google Scholar] [CrossRef]
- Philips, H.; Jahanshahi, M. The components of pain behaviour report. Behav. Res. Ther. 1986, 24, 117–125. [Google Scholar] [CrossRef]
- Silva, M.J.; Coffee, Z.; Ho Alex Yu, C.; Martel, M.O. Anxiety and Fear Avoidance Beliefs and Behavior May Be Significant Risk Factors for Chronic Opioid Analgesic Therapy Reliance for Patients with Chronic Pain—Results from a Preliminary Study. Pain Med. 2021, 9, 2106–2116. [Google Scholar] [CrossRef]
- Silva, M.J.; Coffee, Z.; Yu, C.H.A.; Hu, J. Changes in Psychological Outcomes after Cessation of Full Mu Agonist Long-Term Opioid Therapy for Chronic Pain. J. Clin. Med. 2023, 12, 1354. [Google Scholar] [CrossRef]
- Silva, M.J.; Coffee, Z.; Yu, C.H. Prolonged Cessation of Chronic Opioid Analgesic Therapy: A Multidisciplinary Intervention. Am. J. Manag. Care 2022, 28, 60–65. [Google Scholar] [CrossRef]
- Borkum, J. Maladaptive Cognitions and Chronic Pain: Epidemiology, Neurobiology, and Treatment. J. Ration.-Emotive Cogn.-Behav. Ther. 2010, 28, 4–24. [Google Scholar] [CrossRef]
- Silva, M.J.; Coffee, Z.; Yu, C.H. The Correlation of Psychological Questionnaire Response Changes after Cessation of Chronic Opioid Analgesic Therapy in Patients with Chronic Pain. 2020; Manuscript Submitted for Publication. [Google Scholar]
- National Institute on Drug Abuse. Prescription Opioids DrugFacts. Available online: https://www.drugabuse.gov/publications/drugfacts/prescription-opioids (accessed on 19 October 2020).
- Wedam, E.F.; Bigelow, G.E.; Johnson, R.E.; Nuzzo, P.A.; Haigney, M.C.P. QT-interval effects of methadone, levomethadyl, and buprenorphine in a randomized trial. Arch. Intern. Med. 2007, 167, 2469–2475. [Google Scholar] [CrossRef] [PubMed]
- Antony, T.; Alzaharani, S.Y.; El-Ghaiesh, S.H. Opioid-induced hypogonadism: Pathophysiology, clinical and therapeutics review. Clin. Exp. Pharmacol. Physiol. 2020, 47, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Eisenstein, T.K.; Rogers, T.J. Drugs of Abuse. In Neuroimmune Pharmacology; Ikezu, T., Gendelman, H.E., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 661–678. [Google Scholar] [CrossRef]
- Lei, L.; Gong, X.; Wen, C.; Zeng, S.; Lei, Q. Research progress on the effects of opioids on the immune system. Trends Anaesth. Crit. Care 2024, 57, 101372. [Google Scholar] [CrossRef]
- NORCO® Hydrocodone Bitartrate and Acetaminophen Tablet. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/040099s023lbl.pdf (accessed on 20 September 2024).
- Chu, L.F.; Angst, M.S.; Clark, D. Opioid-induced hyperalgesia in humans: Molecular mechanisms and clinical considerations. Clin. J. Pain 2008, 24, 479–496. [Google Scholar] [CrossRef]
- Lee, M.; Silverman, S.M.; Hansen, H.; Patel, V.B.; Manchikanti, L. A comprehensive review of opioid-induced hyperalgesia. Pain. Physician 2011, 14, 145–161. [Google Scholar] [CrossRef]
- Rudd, R.A. Increases in Drug and Opioid-Involved Overdose Deaths—United States, 2010–2015. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 1445–1452. [Google Scholar] [CrossRef]
- Xu, J. Mortality in the United States, 2018. NCHS Data Briefs 2020, 8, 355. [Google Scholar]
- National Institute on Drug Abuse. Overdose Death Rates. Available online: https://www.drugabuse.gov/drug-topics/trends-statistics/overdose-death-rates (accessed on 9 June 2021).
- George, S.Z.; Fritz, J.M.; Bialosky, J.E.; Donald, D.A. The effect of a fear-avoidance-based physical therapy intervention for patients with acute low back pain: Results of a randomized clinical trial. Spine (Phila Pa 1976) 2003, 28, 2551–2560. [Google Scholar] [CrossRef]
- Miller, R.P.; Kori, S.H.; Todd, D.D. The Tampa Scale: A Measure of Kinisophobia. Clin. J. Pain 1991, 7, 51. [Google Scholar] [CrossRef]
- Hudes, K. The Tampa Scale of Kinesiophobia and neck pain, disability and range of motion: A narrative review of the literature. J. Can. Chiropr. Assoc. 2011, 55, 222–232. [Google Scholar] [PubMed]
- Adams, H.; Ellis, T.; Stanish, W.D.; Sullivan, M.J.L. Psychosocial factors related to return to work following rehabilitation of whiplash injuries. J. Occup. Rehabil. 2007, 17, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, M.J.L.; Bishop, S.R.; Pivik, J. The Pain Catastrophizing Scale: Development and Validation. Psychol. Assess. 1995, 7, 524–532. [Google Scholar] [CrossRef]
- Vlaeyen, J.W.; Kole-Snijders, A.M.; Rotteveel, A.M.; Ruesink, R.; Heuts, P.H. The role of fear of movement/(re)injury in pain disability. J. Occup. Rehabil. 1995, 5, 235–252. [Google Scholar] [CrossRef]
- Crombez, G.; Vlaeyen, J.W.; Heuts, P.H.; Lysens, R. Pain-related fear is more disabling than pain itself: Evidence on the role of pain-related fear in chronic back pain disability. Pain 1999, 80, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Wertli, M.M.; Eugster, R.; Held, U.; Steurer, J.; Kofmehl, R.; Weiser, S. Catastrophizing—A prognostic factor for outcome in patients with low back pain: A systematic review. Spine J. 2014, 14, 2639–2657. [Google Scholar] [CrossRef]
- Helmerhorst, G.T.T.; Vranceanu, A.-M.; Vrahas, M.; Smith, M.; Ring, D. Risk Factors for Continued Opioid Use One to Two Months after Surgery for Musculoskeletal Trauma. JBJS 2014, 96, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Arteta, J.; Cobos, B.; Hu, Y.; Jordan, K.; Howard, K. Evaluation of How Depression and Anxiety Mediate the Relationship between Pain Catastrophizing and Prescription Opioid Misuse in a Chronic Pain Population. Pain Med. 2016, 17, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Martel, M.O.; Jamison, R.N.; Wasan, A.D.; Edwards, R.R. The Association Between Catastrophizing and Craving in Patients with Chronic Pain Prescribed Opioid Therapy: A Preliminary Analysis. Pain Med. 2014, 15, 1757–1764. [Google Scholar] [CrossRef]
- Martel, M.O.; Wasan, A.D.; Jamison, R.N.; Edwards, R.R. Catastrophic thinking and increased risk for prescription opioid misuse in patients with chronic pain. Drug Alcohol. Depend. 2013, 132, 335–341. [Google Scholar] [CrossRef]
- Seminowicz, D.A.; Davis, K.D. Cortical responses to pain in healthy individuals depends on pain catastrophizing. Pain 2006, 120, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Seminowicz, D.A.; Shpaner, M.; Keaser, M.L.; Krauthamer, G.M.; Mantegna, J.; Dumas, J.A.; Newhouse, P.A.; Filippi, C.G.; Keefe, F.J.; Naylor, M.R. Cognitive-behavioral therapy increases prefrontal cortex gray matter in patients with chronic pain. J. Pain 2013, 14, 1573–1584. [Google Scholar] [CrossRef] [PubMed]
- Burton, A.K.; Waddell, G.; Tillotson, K.M.; Summerton, N. Information and advice to patients with back pain can have a positive effect. A randomized controlled trial of a novel educational booklet in primary care. Spine 1999, 24, 2484–2491. [Google Scholar] [CrossRef]
- Vlaeyen, J.W.S.; Morley, S. Cognitive-behavioral treatments for chronic pain: What works for whom? Clin. J. Pain 2005, 21, 1–8. [Google Scholar] [CrossRef]
- Jellema, P.; van der Horst, H.E.; Vlaeyen, J.W.S.; Stalman, W.A.B.; Bouter, L.M.; van der Windt, D.A.W.M. Predictors of Outcome in Patients with (Sub)Acute Low Back Pain Differ across Treatment Groups. Spine 2006, 31, 1699–1705. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, M.J.L.; Adams, H.; Rhodenizer, T.; Stanish, W.D. A psychosocial risk factor--targeted intervention for the prevention of chronic pain and disability following whiplash injury. Phys. Ther. 2006, 86, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Vlaeyen, J.W.; Linton, S.J. Fear-avoidance and its consequences in chronic musculoskeletal pain: A state of the art. Pain 2000, 85, 317–332. [Google Scholar] [CrossRef]
- Leeuw, M.; Goossens, M.E.J.B.; Linton, S.J.; Crombez, G.; Boersma, K.; Vlaeyen, J.W.S. The fear-avoidance model of musculoskeletal pain: Current state of scientific evidence. J. Behav. Med. 2007, 30, 77–94. [Google Scholar] [CrossRef]
- Fritz, J.M.; George, S.Z. Identifying psychosocial variables in patients with acute work-related low back pain: The importance of fear-avoidance beliefs. Phys. Ther. 2002, 82, 973–983. [Google Scholar] [CrossRef]
- George, S.Z.; Fritz, J.M.; Erhard, R.E. A comparison of fear-avoidance beliefs in patients with lumbar spine pain and cervical spine pain. Spine 2001, 26, 2139–2145. [Google Scholar] [CrossRef]
- George, S.Z.; Fritz, J.M.; McNeil, D.W. Fear-avoidance beliefs as measured by the fear-avoidance beliefs questionnaire: Change in fear-avoidance beliefs questionnaire is predictive of change in self-report of disability and pain intensity for patients with acute low back pain. Clin. J. Pain 2006, 22, 197–203. [Google Scholar] [CrossRef]
- Cleland, J.A.; Fritz, J.M.; Brennan, G.P. Predictive validity of initial fear avoidance beliefs in patients with low back pain receiving physical therapy: Is the FABQ a useful screening tool for identifying patients at risk for a poor recovery? Eur. Spine J. 2008, 17, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Ryum, T.; Stiles, T.C. Changes in pain catastrophizing, fear-avoidance beliefs, and pain self-efficacy mediate changes in pain intensity on disability in the treatment of chronic low back pain. Pain Rep. 2023, 8, e1092. [Google Scholar] [CrossRef] [PubMed]
- Boersma, K.; Linton, S.; Overmeer, T.; Jansson, M.; Vlaeyen, J.; de Jong, J. Lowering fear-avoidance and enhancing function through exposure in vivo. A multiple baseline study across six patients with back pain. Pain 2004, 108, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Besen, E.; Gaines, B.; Linton, S.J.; Shaw, W.S. The role of pain catastrophizing as a mediator in the work disability process following acute low back pain. J. Appl. Biobehav. Res. 2017, 22, e12085. [Google Scholar] [CrossRef]
- De Jong, J.R.; Vlaeyen, J.W.S.; Onghena, P.; Goossens, M.E.J.B.; Geilen, M.; Mulder, H. Fear of movement/(re)injury in chronic low back pain: Education or exposure in vivo as mediator to fear reduction? Clin. J. Pain 2005, 21, 9–17, discussion 69–72. [Google Scholar] [CrossRef]
- Ryum, T.; Hartmann, H.; Borchgrevink, P.; De Ridder, K.; Stiles, T.C. The effect of in-session exposure in Fear-Avoidance treatment of chronic low back pain: A randomized controlled trial. Eur. J. Pain 2021, 25, 171–188. [Google Scholar] [CrossRef]
- Vlaeyen, J.W.S.; de Jong, J.; Geilen, M.; Heuts, P.H.T.G.; van Breukelen, G. The treatment of fear of movement/(re)injury in chronic low back pain: Further evidence on the effectiveness of exposure in vivo. Clin. J. Pain 2002, 18, 251–261. [Google Scholar] [CrossRef]
- Simons, L.E. Fear of pain in children and adolescents with neuropathic pain and complex regional pain syndrome. Pain 2016, 157, S90. [Google Scholar] [CrossRef]
- Yuan, Y.; Schreiber, K.; Flowers, K.M.; Edwards, R.; Azizoddin, D.; Ashcraft, L.; Newhill, C.E.; Hruschak, V. The relationship between emotion regulation and pain catastrophizing in patients with chronic pain. Pain Med. 2024, 25, 468–477. [Google Scholar] [CrossRef]
- Rosenberg, J.C.; Schultz, D.M.; Duarte, L.E.; Rosen, S.M.; Raza, A. Increased pain catastrophizing associated with lower pain relief during spinal cord stimulation: Results from a large post-market study. Neuromodulation 2015, 18, 277–284, discussion 284. [Google Scholar] [CrossRef] [PubMed]
- Pinto, P.R.; McIntyre, T.; Ferrero, R.; Almeida, A.; Araújo-Soares, V. Predictors of acute postsurgical pain and anxiety following primary total hip and knee arthroplasty. J. Pain 2013, 14, 502–515. [Google Scholar] [CrossRef] [PubMed]
- Høvik, L.H.; Winther, S.B.; Foss, O.A.; Gjeilo, K.H. Preoperative pain catastrophizing and postoperative pain after total knee arthroplasty: A prospective cohort study with one year follow-up. BMC Musculoskelet. Disord. 2016, 17, 214. [Google Scholar] [CrossRef]
- Khan, R.S.; Ahmed, K.; Blakeway, E.; Skapinakis, P.; Nihoyannopoulos, L.; Macleod, K.; Sevdalis, N.; Ashrafian, H.; Platt, M.; Darzi, A.; et al. Catastrophizing: A predictive factor for postoperative pain. Am. J. Surg. 2011, 201, 122–131. [Google Scholar] [CrossRef]
- Teunis, T.; Bot, A.G.J.; Thornton, E.R.; Ring, D. Catastrophic Thinking Is Associated with Finger Stiffness after Distal Radius Fracture Surgery. J. Orthop. Trauma. 2015, 29, e414–e420. [Google Scholar] [CrossRef] [PubMed]
- Gibson, E.; Sabo, M.T. Can pain catastrophizing be changed in surgical patients? A scoping review. Can. J. Surg. 2018, 61, 311–318. [Google Scholar] [CrossRef]
- Chimenti, R.L.; Pacha, M.S.; Glass, N.A.; Frazier, M.; Bowles, A.O.; Valantine, A.D.; Archer, K.R.; Wilken, J.M. Elevated Kinesiophobia Is Associated with Reduced Recovery from Lower Extremity Musculoskeletal Injuries in Military and Civilian Cohorts. Phys. Ther. 2021, 102, pzab262. [Google Scholar] [CrossRef]
- Örücü Atar, M.; Demir, Y.; Tekin, E.; Kılınç Kamacı, G.; Korkmaz, N.; Aydemir, K. Kinesiophobia and associated factors in patients with traumatic lower extremity amputation. Turk. J. Phys. Med. Rehabil. 2022, 68, 493–500. [Google Scholar] [CrossRef]
- Roelofs, J.; Goubert, L.; Peters, M.; Vlaeyen, J.; Crombez, G. The Tampa Scale for Kinesiophobia: Further examination of psychometric properties in patients with chronic low back pain and fibromyalgia. Eur. J. Pain 2004, 8, 495–502. [Google Scholar] [CrossRef]
- Varallo, G.; Scarpina, F.; Giusti, E.M.; Cattivelli, R.; Guerrini Usubini, A.; Capodaglio, P.; Castelnuovo, G. Does Kinesiophobia Mediate the Relationship between Pain Intensity and Disability in Individuals with Chronic Low-Back Pain and Obesity? Brain Sci. 2021, 11, 684. [Google Scholar] [CrossRef]
- Van Bogaert, W.; Coppieters, I.; Kregel, J.; Nijs, J.; De Pauw, R.; Meeus, M.; Cagnie, B.; Danneels, L.; Malfliet, A. Influence of Baseline Kinesiophobia Levels on Treatment Outcome in People with Chronic Spinal Pain. Phys. Ther. 2021, 101, pzab076. [Google Scholar] [CrossRef] [PubMed]
- Saracoglu, I.; Arik, M.I.; Afsar, E.; Gokpinar, H.H. The effectiveness of pain neuroscience education combined with manual therapy and home exercise for chronic low back pain: A single-blind randomized controlled trial. Physiother. Theory Pract. 2022, 38, 868–878. [Google Scholar] [CrossRef] [PubMed]
- Malfliet, A.; Kregel, J.; Meeus, M.; Roussel, N.; Danneels, L.; Cagnie, B.; Dolphens, M.; Nijs, J. Blended-Learning Pain Neuroscience Education for People with Chronic Spinal Pain: Randomized Controlled Multicenter Trial. Phys. Ther. 2018, 98, 357–368. [Google Scholar] [CrossRef]
- Bodes Pardo, G.; Lluch Girbés, E.; Roussel, N.A.; Gallego Izquierdo, T.; Jiménez Penick, V.; Pecos Martín, D. Pain Neurophysiology Education and Therapeutic Exercise for Patients with Chronic Low Back Pain: A Single-Blind Randomized Controlled Trial. Arch. Phys. Med. Rehabil. 2018, 99, 338–347. [Google Scholar] [CrossRef]
- Kim, H.; Lee, S. Effects of pain neuroscience education on kinesiophobia in patients with chronic pain: A systematic review and meta-analysis. Phys. Ther. Rehabil. Sci. 2020, 9, 309–317. [Google Scholar] [CrossRef]
- Andias, R.; Neto, M.; Silva, A.G. The effects of pain neuroscience education and exercise on pain, muscle endurance, catastrophizing and anxiety in adolescents with chronic idiopathic neck pain: A school-based pilot, randomized and controlled study. Physiother. Theory Pract. 2018, 34, 682–691. [Google Scholar] [CrossRef]
- Lin, L.-H.; Lin, T.-Y.; Chang, K.-V.; Wu, W.-T.; Özçakar, L. Pain neuroscience education for reducing pain and kinesiophobia in patients with chronic neck pain: A systematic review and meta-analysis of randomized controlled trials. Eur. J. Pain 2024, 28, 231–243. [Google Scholar] [CrossRef]
- Wood, L.; Bejarano, G.; Csiernik, B.; Miyamoto, G.C.; Mansell, G.; Hayden, J.A.; Lewis, M.; Cashin, A.G. Pain catastrophising and kinesiophobia mediate pain and physical function improvements with Pilates exercise in chronic low back pain: A mediation analysis of a randomised controlled trial. J. Physiother. 2023, 69, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Savych, B.; Neumark, D.; Lea, R. Do Opioids Help Injured Workers Recover and Get Back to Work? The Impact of Opioid Prescriptions on Duration of Temporary Disability. Ind. Relat. J. Econ. Soc. 2019, 58, 549–590. [Google Scholar] [CrossRef]
- Grattan, A.; Sullivan, M.D.; Saunders, K.W.; Campbell, C.I.; Von Korff, M.R. Depression and Prescription Opioid Misuse among Chronic Opioid Therapy Recipients with No History of Substance Abuse. Ann. Fam. Med. 2012, 10, 304–311. [Google Scholar] [CrossRef]
- Scherrer, J.F.; Ahmedani, B.; Autio, K.; Debar, L.; Lustman, P.J.; Miller-Matero, L.R.; Salas, J.; Secrest, S.; Sullivan, M.D.; Wilson, L.; et al. The Prescription Opioids and Depression Pathways Cohort Study. J. Psychiatr. Brain Sci. 2020, 5, 9. [Google Scholar] [CrossRef]
- Scherrer, J.F.; Salas, J.; Schneider, F.D.; Bucholz, K.K.; Sullivan, M.D.; Copeland, L.A.; Ahmedani, B.K.; Burroughs, T.; Lustman, P.J. Characteristics of new depression diagnoses in patients with and without prior chronic opioid use. J. Affect. Disord. 2017, 210, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Vlaeyen, J.W.S.; Crombez, G.; Linton, S.J. The fear-avoidance model of pain. Pain 2016, 157, 1588. [Google Scholar] [CrossRef]
- Vogel, J.A.; Rising, K.L.; Jones, J.; Bowden, M.L.; Ginde, A.A.; Havranek, E.P. Reasons Patients Choose the Emergency Department over Primary Care: A Qualitative Metasynthesis. J. Gen. Intern. Med. 2019, 34, 2610–2619. [Google Scholar] [CrossRef]
- Rogers, A.H.; Farris, S.G. A meta-analysis of the associations of elements of the fear-avoidance model of chronic pain with negative affect, depression, anxiety, pain-related disability and pain intensity. Eur. J. Pain 2022, 26, 1611–1635. [Google Scholar] [CrossRef] [PubMed]
- Zale, E.L.; Ditre, J.W. Pain-Related Fear, Disability, and the Fear-Avoidance Model of Chronic Pain. Curr. Opin. Psychol. 2015, 5, 24–30. [Google Scholar] [CrossRef]
- Felitti, V.J.; Anda, R.F.; Nordenberg, D.; Williamson, D.F.; Spitz, A.M.; Edwards, V.; Koss, M.P.; Marks, J.S. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am. J. Prev. Med. 1998, 14, 245–258. [Google Scholar] [CrossRef]
- Tidmarsh, L.V.; Harrison, R.; Ravindran, D.; Matthews, S.L.; Finlay, K.A. The Influence of Adverse Childhood Experiences in Pain Management: Mechanisms, Processes, and Trauma-Informed Care. Front Pain Res 2022, 3, 923866. [Google Scholar] [CrossRef]
- Fordyce, W.E.; Shelton, J.L.; Dundore, D.E. The modification of avoidance learning pain behaviors. J. Behav. Med. 1982, 5, 405–414. [Google Scholar] [CrossRef]
- Schmidt, A.J. Cognitive factors in the performance level of chronic low back pain patients. J. Psychosom. Res. 1985, 29, 183–189. [Google Scholar] [CrossRef]
- Rachman, S.; Lopatka, C. Accurate and inaccurate predictions of pain. Behav. Res. Ther. 1988, 26, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Rubak, S.; Sandbæk, A.; Lauritzen, T.; Christensen, B. Motivational interviewing: A systematic review and meta-analysis. Br. J. Gen. Pract. 2005, 55, 305–312. [Google Scholar]
- Webster, F.; Connoy, L.; Longo, R.; Ahuja, D.; Amtmann, D.; Anderson, A.; Ashton-James, C.E.; Boyd, H.; Chambers, C.T.; Cook, K.F.; et al. Patient Responses to the Term Pain Catastrophizing: Thematic Analysis of Cross-sectional International Data. J. Pain 2023, 24, 356–367. [Google Scholar] [CrossRef]
- U.P. Foundation. ‘Catastrophizing’: A Form of Pain Shaming. Available online: https://uspainfoundation.org/blog/catastrophizing-a-form-of-pain-shaming/ (accessed on 24 July 2024).
- Atkins, N.; Mukhida, K. The relationship between patients’ income and education and their access to pharmacological chronic pain management: A scoping review. Can. J. Pain 2022, 6, 142–170. [Google Scholar] [CrossRef]
- Maharaj, A.S.; Bhatt, N.V.; Gentile, J.P. Bringing It in the Room: Addressing the Impact of Racism on the Therapeutic Alliance. Innov. Clin. Neurosci. 2021, 18, 39–43. [Google Scholar]
- Strand, N.H.; Mariano, E.R.; Goree, J.H.; Narouze, S.; Doshi, T.L.; Freeman, J.A.; Pearson, A.C.S. Racism in Pain Medicine: We Can and Should Do More. Mayo Clin. Proc. 2021, 96, 1394–1400. [Google Scholar] [CrossRef] [PubMed]
- Amtmann, D.; Bamer, A.M.; Liljenquist, K.S.; Cowan, P.; Salem, R.; Turk, D.C.; Jensen, M.P. The Concerns About Pain (CAP) Scale: A Patient-Reported Outcome Measure of Pain Catastrophizing. J. Pain 2020, 21, 1198–1211. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, M.J.L.; Tripp, D.A. Pain Catastrophizing: Controversies, Misconceptions and Future Directions. J. Pain 2024, 25, 575–587. [Google Scholar] [CrossRef]
- Raja, S.N.; Carr, D.B.; Cohen, M.; Finnerup, N.B.; Flor, H.; Gibson, S.; Keefe, F.; Mogil, J.S.; Ringkamp, M.; Sluka, K.A.; et al. The Revised IASP definition of pain: Concepts, challenges, and compromises. Pain 2020, 161, 1976–1982. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services. Pain Management Best Practices Inter-Agency Task Force Report: Updates, Gaps, Inconsistencies, and Recommendations. Retrieved from U.S. Department of Health and Human Services Website. May 2019. Available online: https://www.hhs.gov/sites/default/files/pain-mgmt-best-practices-draft-final-report-05062019.pdf (accessed on 4 June 2024).
- Bean, D.J.; Dryland, A.; Rashid, U.; Tuck, N.L. The Determinants and Effects of Chronic Pain Stigma: A Mixed Methods Study and the Development of a Model. J. Pain 2022, 23, 1749–1764. [Google Scholar] [CrossRef]
- Koesling, D.; Bozzaro, C. Chronic pain patients’ need for recognition and their current struggle. Med. Health Care Philos. 2021, 24, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Licciardone, J.C.; Tran, Y.; Ngo, K.; Toledo, D.; Peddireddy, N.; Aryal, S. Physician Empathy and Chronic Pain Outcomes. JAMA Netw. Open 2024, 7, e246026. [Google Scholar] [CrossRef]
- Zaman, T.; Striebel, J. Opioid Refugees: A Diverse Population Continues to Emerge; California Society of Addiction Medicine: San Francisco, CA, USA, 2015; p. 16. [Google Scholar]
- The Georgia Straight. Opioid Refugees: How the Fentanyl Crisis Led to a Backlash against Doctors That’s Leaving People in Pain. Available online: https://www.straight.com/news/1043911/opioid-refugees-how-fentanyl-crisis-led-backlash-against-doctors-thats-leaving-people (accessed on 6 May 2020).
- [Report]|The Pain Refugees, by Brian Goldstone. Available online: https://harpers.org/archive/2018/04/the-pain-refugees/ (accessed on 6 May 2020).
- Burke, S.E.; Dovidio, J.F.; Przedworski, J.M.; Hardeman, R.R.; Perry, S.P.; Phelan, S.M.; Nelson, D.B.; Burgess, D.J.; Yeazel, M.W.; van Ryn, M. Do Contact and Empathy Mitigate Bias against Gay and Lesbian People among Heterosexual Medical Students? A Report from Medical Student CHANGES. Acad. Med. 2015, 90, 645–651. [Google Scholar] [CrossRef]
- Abd-Elsayed, A.; Heyer, A.M.; Schatman, M.E. Disparities in the Treatment of the LGBTQ Population in Chronic Pain Management. J. Pain Res. 2021, 14, 3623–3625. [Google Scholar] [CrossRef]
- Bailey, Z.D.; Krieger, N.; Agénor, M.; Graves, J.; Linos, N.; Bassett, M.T. Structural racism and health inequities in the USA: Evidence and interventions. Lancet 2017, 389, 1453–1463. [Google Scholar] [CrossRef] [PubMed]
- Hall, W.J.; Chapman, M.V.; Lee, K.M.; Merino, Y.M.; Thomas, T.W.; Payne, B.K.; Eng, E.; Day, S.H.; Coyne-Beasley, T. Implicit Racial/Ethnic Bias among Health Care Professionals and Its Influence on Health Care Outcomes: A Systematic Review. Am. J. Public. Health 2015, 105, e60–e76. [Google Scholar] [CrossRef] [PubMed]
- Atkins, R. Instruments Measuring Perceived Racism/Racial Discrimination: Review and Critique of Factor Analytic Techniques. Int. J. Health Serv. Plan Adm. Eval. 2014, 44, 711. [Google Scholar] [CrossRef]
- Wang, M.L.; Jacobs, O. From Awareness to Action: Pathways to Equity in Pain Management. Health Equity 2023, 7, 416–418. [Google Scholar] [CrossRef]
- Edgoose, J.; Quiogue, M.; Sidhar, K. How to Identify, Understand, and Unlearn Implicit Bias in Patient Care. FPM 2019, 26, 29–33. [Google Scholar]
- Perugino, F.; De Angelis, V.; Pompili, M.; Martelletti, P. Stigma and Chronic Pain. Pain Ther. 2022, 11, 1085–1094. [Google Scholar] [CrossRef]
- Linton, S.J.; Boersma, K.; Vangronsveld, K.; Fruzzetti, A. Painfully reassuring? The effects of validation on emotions and adherence in a pain test. Eur. J. Pain 2012, 16, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Hanssen, M.M.; Peters, M.L.; Vlaeyen, J.W.S.; Meevissen, Y.M.C.; Vancleef, L.M.G. Optimism lowers pain: Evidence of the causal status and underlying mechanisms. Pain 2013, 154, 53–58. [Google Scholar] [CrossRef] [PubMed]
- P. Forum. COVID-19 Pandemic Impact on Patients, Families & Individuals in Recovery from a SUD. Available online: https://www.addictionpolicy.org/post/covid-19-pandemic-impact-on-patients-families-individuals-in-recovery-fromsubstance-use-disorder (accessed on 15 June 2021).
- Goubert, L.; Crombez, G.; Van Damme, S. The role of neuroticism, pain catastrophizing and pain-related fear in vigilance to pain: A structural equations approach. Pain 2004, 107, 234. [Google Scholar] [CrossRef]
- Levy, B.R.; Slade, M.D.; Kunkel, S.R.; Kasl, S.V. Longevity increased by positive self-perceptions of aging. J. Pers. Soc. Psychol. 2002, 83, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Yamin, J.B.; Meints, S.M.; Edwards, R.R. Beyond pain catastrophizing: Rationale and recommendations for targeting trauma in the assessment and treatment of chronic pain. Expert. Rev. Neurother. 2024, 24, 231–234. [Google Scholar] [CrossRef] [PubMed]
- Maly, A.; Vallerand, A.H. Neighborhood, Socioeconomic, and Racial Influence on Chronic Pain. Pain. Manag. Nurs. 2018, 19, 14–22. [Google Scholar] [CrossRef]
- Cognitive Restructuring: Steps, Technique, and Examples. Available online: https://www.medicalnewstoday.com/articles/cognitive-restructuring (accessed on 19 March 2024).
- Newman, M.G.; Erickson, T.; Przeworski, A.; Dzus, E. Self-help and minimal-contact therapies for anxiety disorders: Is human contact necessary for therapeutic efficacy? J. Clin. Psychol. 2003, 59, 251–274. [Google Scholar] [CrossRef]
- Mayo-Wilson, E.; Montgomery, P. Media-delivered cognitive behavioural therapy and behavioural therapy (self-help) for anxiety disorders in adults. Cochrane Database Syst. Rev. 2013, 9, CD005330. [Google Scholar] [CrossRef]
- Somatoform Disorders|AAFP. Available online: https://www.aafp.org/pubs/afp/issues/2007/1101/p1333.html (accessed on 7 August 2024).
- Catastrophizing Misinterpretations Predict Somatoform-Related Symptoms and New Onsets of Somatoform Disorders—ScienceDirect. Available online: https://www.sciencedirect.com/science/article/abs/pii/S0022399915300271 (accessed on 7 August 2024).
- Seto, H.; Nakao, M. Relationships between catastrophic thought, bodily sensations and physical symptoms. Biopsychosoc. Med. 2017, 11, 28. [Google Scholar] [CrossRef]
- Cate Polacek, M.; Christopher, R.; Michelle Mann, B.S.; Margarita Udall, M.P.H.; Terri Craig, P.; Michael Deminski, M.S.; Nila, A.; Sathe, M.A. Healthcare Professionals’ Perceptions of Challenges to Chronic Pain Management. Am. J. Manag. Care 2020, 26, e135–e139. Available online: https://www.ajmc.com/view/healthcare-professionals-perceptions-of-challenges-to-chronic-pain-management (accessed on 5 August 2024).
- Woolford, S.J.; Resnicow, K.; Davis, M.M.; Nichols, L.P.; Wasserman, R.C.; Harris, D.; Gebremariam, A.; Shone, L.; Fiks, A.G.; Chang, T. Cost-effectiveness of a motivational interviewing obesity intervention versus usual care in pediatric primary care offices. Obesity 2022, 30, 2265–2274. [Google Scholar] [CrossRef] [PubMed]
- Olmstead, T.A.; Yonkers, K.A.; Forray, A.; Zimbrean, P.; Gilstad-Hayden, K.; Martino, S. Cost and cost-effectiveness of three strategies for implementing motivational interviewing for substance misuse on medical inpatient units. Drug Alcohol. Depend. 2020, 214, 108156. [Google Scholar] [CrossRef] [PubMed]
- Schütze, R.; Rees, C.; Smith, A.; Slater, H.; Campbell, J.M.; O’Sullivan, P. How Can We Best Reduce Pain Catastrophizing in Adults with Chronic Noncancer Pain? A Systematic Review and Meta-Analysis. J. Pain 2018, 19, 233–256. [Google Scholar] [CrossRef]
- Darnall, B.D. Psychological Treatment for Chronic Pain: Improving Access and Integration. Psychol. Sci. Public. Interest. 2021, 22, 45–51. [Google Scholar] [CrossRef]
- Bizzo, B.C.; Almeida, R.R.; Michalski, M.H.; Alkasab, T.K. Artificial Intelligence and Clinical Decision Support for Radiologists and Referring Providers. J. Am. Coll. Radiol. 2019, 16, 1351–1356. [Google Scholar] [CrossRef] [PubMed]
- Hershberger, P.J.; Pei, Y.; Bricker, D.A.; Crawford, T.N.; Shivakumar, A.; Vasoya, M.; Medaramitta, R.; Rechtin, M.; Bositty, A.; Wilson, J.F. Advancing Motivational Interviewing Training with Artificial Intelligence: ReadMI. Adv. Med. Educ. Pract. 2021, 12, 613–618. [Google Scholar] [CrossRef]
- Nurmi, J.; Knittle, K.; Ginchev, T.; Khattak, F.; Helf, C.; Zwickl, P.; Castellano-Tejedor, C.; Lusilla-Palacios, P.; Costa-Requena, J.; Ravaja, N.; et al. Engaging Users in the Behavior Change Process with Digitalized Motivational Interviewing and Gamification: Development and Feasibility Testing of the Precious App. JMIR Mhealth Uhealth 2020, 8, e12884. [Google Scholar] [CrossRef]
- Saiyed, A.; Layton, J.; Borsari, B.; Cheng, J.; Kanzaveli, T.; Tsvetovat, M.; Satterfield, J. Technology-Assisted Motivational Interviewing: Developing a Scalable Framework for Promoting Engagement with Tobacco Cessation Using NLP and Machine Learning. Procedia Comput. Sci. 2022, 206, 121–131. [Google Scholar] [CrossRef]
- Sawyer, C.; McKeon, G.; Hassan, L.; Onyweaka, H.; Martinez Agulleiro, L.; Guinart, D.; Torous, J.; Firth, J. Digital health behaviour change interventions in severe mental illness: A systematic review. Psychol. Med. 2023, 53, 6965–7005. [Google Scholar] [CrossRef]
| Fear-Avoidance Beliefs Questionnaire—Work and Physical Activity (FAB-W and -PA) [16,22,38] | Two subscales (FAB-W: 0–42; FAB-PA 0–24) in which higher scores indicate more severe pain and disability due to fear-avoidance beliefs about work and physical activity, respectively. Various score thresholds have been documented as associated with clinical relevancy and specific negative chronicity of CNCP. Higher scores have been associated with poor physical and manual therapy results and low return-to-work rates after an injury. |
| Tampa Scale of Kinesiophobia (TKS) [39,40] | A measure of fear of movement and reinjury. Scores range from 17 to 68, with higher scores being of higher severity. Higher TKS scores have been correlated with higher disability and pain scores. |
| Pain Catastrophizing Scale (PCS) [24,41,42] | Assesses levels of catastrophizing. In initial validation, a score of 30 or more correlated with high unemployment, self-declared “total” disability, and clinical depression. However, various lower score thresholds have been documented as associated with clinical relevancy for specific negative chronicity of CNCP. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, M.J. Treating Anxiety-Based Cognitive Distortions Pertaining to Somatic Perception for Better Chronic Pain Outcomes: A Recommendation for Better Practice in the Present Day and the Cyber Age of Medicine. J. Clin. Med. 2024, 13, 5923. https://doi.org/10.3390/jcm13195923
Silva MJ. Treating Anxiety-Based Cognitive Distortions Pertaining to Somatic Perception for Better Chronic Pain Outcomes: A Recommendation for Better Practice in the Present Day and the Cyber Age of Medicine. Journal of Clinical Medicine. 2024; 13(19):5923. https://doi.org/10.3390/jcm13195923
Chicago/Turabian StyleSilva, Marcelina Jasmine. 2024. "Treating Anxiety-Based Cognitive Distortions Pertaining to Somatic Perception for Better Chronic Pain Outcomes: A Recommendation for Better Practice in the Present Day and the Cyber Age of Medicine" Journal of Clinical Medicine 13, no. 19: 5923. https://doi.org/10.3390/jcm13195923







