Abstract
Background: Postimplantation syndrome (PIS) is a known inflammatory response following endovascular stent placement, yet comparative data between venous and arterial stenting remains limited. This study seeks to evaluate the incidence, characteristics, and clinical implications of PIS across these two distinct vascular territories. Methods: We retrospectively analyzed 191 patients who underwent either venous (n = 36) or arterial (n = 155) stent placement. Data collection encompassed demographic profiles, perioperative laboratory findings, and clinical outcomes. The primary endpoint was the incidence of PIS, defined as the presence of fever (≥38 °C), leukocytosis, and elevated C-reactive protein (CRP) within 30 days postprocedure. Secondary outcomes included length of hospital and ICU stay, incidence of endoleaks, reintervention rates, and 30-day mortality. Comparative statistical analyses were conducted to assess differences between the venous and arterial stent groups. Results: PIS was observed more frequently in arterial stent patients, as evidenced by significantly elevated postoperative white blood cell counts at 24 and 48 h (p = 0.046 and p = 0.014, respectively), along with borderline CRP increases (p = 0.052). Fever occurrence peaked at 72 and 96 h postprocedure, predominantly in the arterial cohort. Furthermore, patients with arterial stents had significantly longer hospital stays (5.59 ± 0.46 days vs. 3.42 ± 0.36 days; p = 0.0018) and a higher rate of 30-day endoleaks (7.1% vs. 0%; p = 0.005). Despite similar mortality and major adverse cardiac event (MACE) rates between groups, arterial stent patients exhibited a greater need for reintervention. While PIS was less common among venous stent recipients, its potential impact on postoperative recovery warrants careful monitoring. Conclusions: Arterial stenting is associated with a higher incidence of PIS and a more pronounced systemic inflammatory response, contributing to longer hospitalization and increased postoperative complications. Although venous stent patients experience PIS less frequently, its occurrence should not be overlooked, as it may influence overall recovery and clinical outcomes. Recognition and management of PIS in both venous and arterial stent patients are critical to improving patient care and optimizing procedural success.
1. Introduction
Post-implantation syndrome (PIS) is a significant clinical phenomenon that occurs following the placement of vascular stents, marked by a systemic inflammatory response. This syndrome, though widely recognized in arterial stenting, remains less understood in the context of venous stents. The lack of data on venous stents creates a critical gap in understanding how different vascular environments—arterial and venous—affect the development and severity of PIS. Moreover, while arterial stents have long been a cornerstone in managing aorto-occlusive diseases, the inflammatory responses that accompany their implantation, such as fever, elevated white blood cell (WBC) counts, and increased C-reactive protein (CRP) levels, remain a clinical challenge [1,2,3,4,5].
PIS was first characterized by Velazquez in 1990 as a condition involving postoperative fever, elevated WBC counts, and elevated CRP levels without an underlying infection. The syndrome is especially noted following endovascular aneurysm repair (EVAR), and its incidence ranges from 13% to 60% in different studies. Although PIS has traditionally been viewed as a self-limiting condition, emerging research suggests that it can be associated with serious complications, such as prolonged hospital stay, acute kidney injury, and adverse cardiovascular events. This underscores the need for better management strategies, especially in high-risk patients. In the setting of endovascular procedures, such as thoracic endovascular aortic repair (TEVAR) and EVAR, PIS often manifests within the first 72 h post-implantation. The inflammatory response following these procedures can vary significantly depending on factors such as the extent of vascular trauma, type of endograft, operative time, and the volume of contrast medium used. Research has highlighted that complex procedures, like TEVAR for Type B aortic dissection (TBAD), present a higher risk for PIS due to the extensive tissue manipulation and inflammatory cascade triggered by multiple endograft deployments. However, the clinical impact of PIS remains a topic of debate. Studies, including those by Xie et al., have shown mixed results regarding the long-term outcomes of patients with PIS. For instance, while some research suggests that PIS does not significantly affect long-term survival, others have identified PIS as an independent risk factor for major adverse events (MAEs) and all-cause mortality within the first year post-EVAR [1,2].
This study aims to systematically compare the incidence and severity of PIS between venous and arterial stent placements. By analyzing inflammatory profiles and clinical outcomes, we seek to identify the differences in post-implantation inflammatory responses across these vascular environments. Understanding these distinctions could lead to the development of targeted strategies to mitigate PIS, ultimately improving patient outcomes following both venous and arterial stent placements.
2. Materials and Methods
2.1. Study Design and Patient Selection
This study was designed as a retrospective analysis comparing the incidence and severity of post-implantation syndrome (PIS) following venous and arterial stenting procedures. A total of 191 patients who underwent endovascular stent placement between January 2018 and January 2024 were included. The median follow-up duration was 18 months, with a minimum follow-up of 8 months and a maximum follow-up of 32 months.
The study was conducted in accordance with the guidelines of the Declaration of Helsinki and was approved by the Research Ethics Board of Ankara University (approval date: 19 September 2024, protocol no:2024/577). Written informed consent was obtained from all patients. Demographic and clinical information were extracted from the electronic health record system.
Of these, 36 patients received venous stents and 155 patients underwent arterial stenting. The primary aim was to compare the inflammatory response and clinical outcomes associated with PIS in both groups.
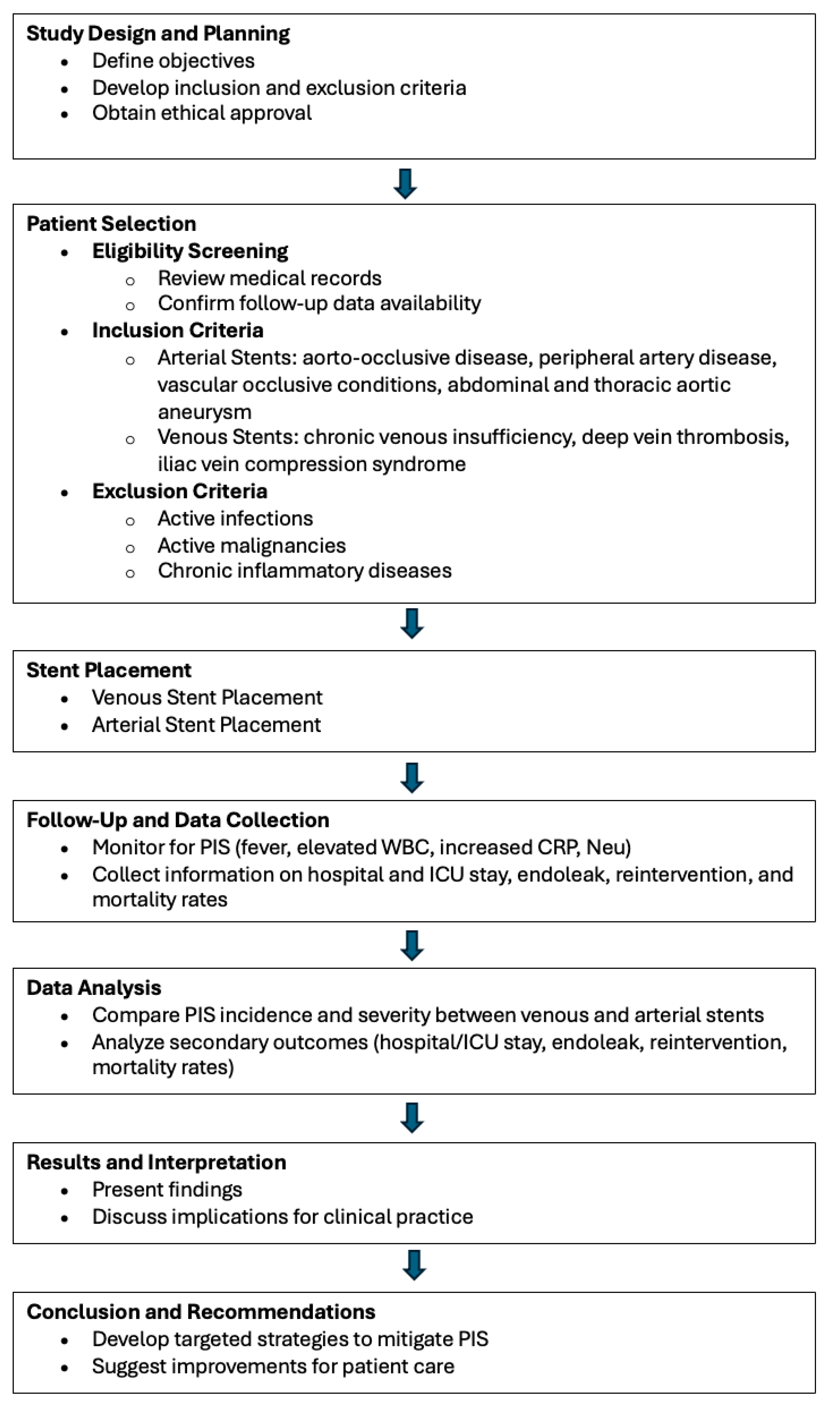
Patients were selected based on the availability of complete medical records and follow-up data. Inclusion criteria for arterial stent placement included patients undergoing stenting for aorto-occlusive disease, peripheral artery disease, or other vascular occlusive conditions. For venous stenting, patients with indications such as chronic venous insufficiency, deep vein thrombosis, or iliac vein compression syndrome were included. Exclusion criteria for both groups involved patients with active infections, malignancies, or chronic inflammatory diseases that could confound the assessment of PIS (Figure 1).
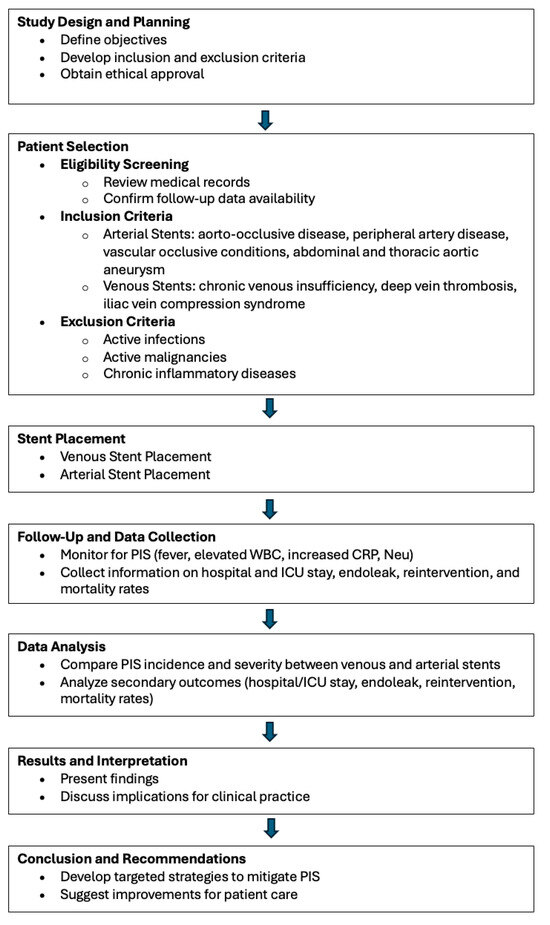
Figure 1.
Study design and process.
The flowchart illustrates the study’s overall process from design to conclusion. It begins with the planning phase, where study objectives and criteria are defined, followed by patient selection based on inclusion and exclusion criteria. The flow proceeds through the stent placement phase, separating venous and arterial stents, and then into the follow-up and data collection stage. The data analysis compares outcomes between different stent types, leading to the interpretation of results. Finally, the chart concludes with recommendations for clinical practice based on the study’s findings.
2.2. Data Collection and Variables
Data were collected retrospectively from institutional electronic health records (EHRs). The following demographic and clinical variables were recorded: age, sex, comorbidities (e.g., diabetes mellitus, hypertension, hyperlipidemia), smoking status, and procedural details, including stent type, size, and location. For each patient, data regarding the development of PIS were collected, with PIS being defined by the presence of fever (>38 °C), elevated white blood cell (WBC) count (>12,000 cells/μL), or elevated C-reactive protein (CRP) levels (>10 mg/L) within 72 h post-procedure.
2.3. Post-Implantation Syndrome Assessment
The primary outcome measure was the incidence of PIS in both venous and arterial stent groups. The diagnosis of PIS was made based on clinical and laboratory findings. Fever was defined as an axillary temperature >38 °C measured on two consecutive occasions within 48–72 h post-procedure. Laboratory parameters, such as WBC count and CRP levels, were recorded pre-procedurally and at 24, 48, 72, 96, and 120 h post-stent placement. Any additional signs of systemic inflammation, including malaise, tachycardia, or hypotension, were also evaluated.
2.4. Stent Types and Material Properties
In the realm of vascular interventions, various stent brands are employed, each offering unique material properties that influence performance and clinical outcomes. PTFE and polyester are common materials used in stent grafts due to their durability and biocompatibility. In our study, we utilized several stent brands, including Endurant and Minerva, both manufactured by Medtronic in Ireland, which leverage PTFE to mitigate inflammatory responses. Additionally, Bentley stents, produced by Bentley InnoMed GmbH in Germany, utilize polyester for flexibility and strength in treating peripheral vascular diseases.
The Ankura stent (Shenzhen, China), developed by Lifetech, is specifically designed for thoracic aortic interventions, while the Myra stent (India), manufactured by Meril Life Sciences, is recognized for its hybrid architecture in various peripheral applications. The Castor stent, produced by Endovastec, is notable for its design aimed at treating thoracic aortic conditions, particularly aortic dissection involving arch lesions. It is the world’s first branched stent graft capable of simultaneously repairing the aorta and supra-arch branch arteries through minimally invasive techniques.
Lastly, the Atropos stent (Dublin, Ireland), also manufactured by Medtronic, is designed for various vascular conditions, enhancing flexibility and adaptability during deployment. The Abre stent (Dublin, Ireland), also from Medtronic, is a non-covered nitinol stent utilized in our study specifically for venous interventions. Unlike PTFE and polyester stents, the Abre stent’s design minimizes complications, such as migration and postimplantation syndrome (PIS). This differentiation in material usage underscores the importance of selecting appropriate stent types based on the specific clinical scenarios presented in vascular procedures [6,7,8].
2.5. Statistical Analysis
All continuous variables were assessed for normality with the Shapiro–Wilk test and by visual inspection of histograms. Variables following a normal distribution were expressed as means and standard deviations, while non-parametric variables were reported as medians with interquartile ranges. Differences between groups were tested using the Kruskall–Wallis test and the Mann–Whitney U test, as appropriate. Categorical variables were expressed as percentages and analyzed using the chi-squared test. A p-value of less than 0.05 was considered statistically significant. All tests were two-tailed, and statistical analyses were performed using SPSS software (Version 20.0, Chicago, IL, USA).
3. Results
A total of 191 patients were included in this analysis, with 36 patients receiving venous stents and 155 patients receiving arterial stents. Among those with arterial stents, 63 had PTFE grafts and 92 had polyester grafts. The mean age was comparable across the groups, with patients in the venous stent group having an average age of 51.25 ± 13.35 years, while those in the arterial stent group had an average age of 66.5 ± 11.7 years. When comparing PTFE and polyester grafts, there was no significant difference in age between the two groups (65.51 ± 10.81 vs. 67.25 ± 12.32 years, p = 0.503).
The proportion of male patients was significantly higher in the arterial stent group compared to the venous stent group (89.67% vs. 61.11%, p < 0.001). Among those with arterial stents, the proportion of males was slightly higher in the PTFE group (92.06%) compared to the polyester group (88.04%), although this difference was not statistically significant (p = 0.251).
The distribution of initial diagnoses varied significantly between venous and arterial stent patients (p < 0.001). All patients in the venous stent group were treated for deep vein thrombosis (DVT), whereas the arterial stent group had a mix of patients with peripheral arterial disease (PAD) (72.25%), abdominal aortic aneurysm (AAA) (23.22%), and thoracic aortic aneurysm (TAA) (4.51%). Among those with arterial stents, the PTFE group had a higher proportion of patients with PAD (84.13%) compared to the polyester group (64.13%). The prevalence of AAA was higher in the polyester group (29.35% vs. 14.29%, p = 0.005).
The prevalence of risk factors and comorbidities, including previous coronary artery disease (CAD), chronic obstructive pulmonary disease (COPD), hypertension (HTN), diabetes mellitus (DM), and malignancy, was similar across all groups. There were no statistically significant differences between PTFE and polyester grafts in terms of these comorbidities, including the prevalence of hypertension (26.98% vs. 39.13%, p = 0.330).
Preoperative white blood cell (WBC) counts were not significantly different between venous and arterial stent groups (p = 0.069) or between PTFE and polyester grafts (p = 0.715). Other preoperative parameters, including neutrophil counts, C-reactive protein (CRP) levels, platelet counts, and hematocrit (Hct) levels, also showed no significant differences between PTFE and polyester grafts. Postoperatively, both groups experienced similar trends in laboratory values over time, with no significant differences between PTFE and polyester stents in terms of WBC, neutrophil, CRP, platelet, or hematocrit levels at any time point.
Procedure times were longer in the venous stent group compared to the arterial stent group (35.1 ± 6.3 min vs. 30.1 ± 1.94 min, p = 0.018). However, there was no significant difference between PTFE and polyester grafts in arterial stents (33.24 ± 3.63 min vs. 27.99 ± 2.82 min, p = 0.251). The contrast medium volume used during the procedure was also similar between the two graft types (103.40 ± 6.91 mL for PTFE vs. 95.04 ± 5.81 mL for polyester, p = 0.310).
Hospital stay was significantly longer for patients with PTFE grafts compared to polyester grafts (6.15 ± 0.8 days vs. 4.51 ± 0.32 days, p < 0.001). Additionally, ICU stay duration was longer in the PTFE group (2.22 ± 0.7 days vs. 1.24 ± 0.15 days, p = 0.001).
There was no significant difference in the incidence of type I or type II endoleaks between PTFE and polyester grafts (4.76% vs. 3.26%, p = 0.374). Similarly, the 30-day reintervention rate was higher in the PTFE group (19.04% vs. 8.69%), but this difference did not reach statistical significance (p = 0.283). The 30-day mortality rate was higher in the polyester group (3.26% vs. 1.58%, p = 0.033), suggesting a potentially increased risk of early mortality with polyester grafts (Table 1).

Table 1.
Demographic and Clinical Outcome Insights: A Comparative Analysis of Venous vs. Arterial Stents and PTFE vs. Polyester Stents.
This table compares demographic information and outcomes for patients receiving venous and arterial stents, as well as PTFE and polyester stents. The data include patient age, gender, comorbidities, and follow-up durations. Additionally, the table presents post-implantation syndrome (PIS) incidence, hospital and ICU stay durations, endoleak rates, reintervention rates, and mortality rates for each stent type. Values are presented as means (± standard deviation) for continuous variables and frequencies (percentages) for categorical variables.
In conclusion, patients with arterial stents experienced higher rates of PIS, as indicated by elevated WBC, neutrophil, and CRP levels, as well as longer hospital stays and higher postoperative complications such as endoleak. These findings suggest that arterial stent placement may be associated with a greater inflammatory response compared to venous stent placement.
In patients who developed PIS, our treatment approach primarily focused on managing the inflammatory response and associated symptoms. Antipyretics, such as paracetamol, were administered to control fever, while intravenous fluids were used to maintain adequate hydration and stabilize hemodynamic parameters. In cases of significant leukocytosis or elevated C-reactive protein (CRP) levels, a short course of broad-spectrum antibiotics was initiated to rule out any potential infectious component. Regular monitoring of vital signs, laboratory parameters, and clinical symptoms was carried out to assess the progression of PIS. Additionally, patients with prolonged or severe symptoms were evaluated for potential complications, such as endoleak or thrombus formation, which may have required further intervention or imaging studies.
4. Discussion
Postimplantation syndrome (PIS) is a systemic inflammatory response that occurs following the implantation of stents or endografts, typically characterized by fever, leukocytosis, and elevated inflammatory markers, such as C-reactive protein (CRP) in the absence of infection. In our study, we examined the incidence of PIS in patients undergoing venous and arterial stent placements, and our findings demonstrate significant differences in the inflammatory responses between these two groups. These results are consistent with the existing literature on PIS following endovascular aneurysm repair (EVAR), which highlights variations in PIS incidence based on procedural type, stent material, and patient characteristics [9].
Our analysis revealed that arterial stent patients were more prone to developing PIS, with higher postoperative WBC and neutrophil counts, along with more pronounced CRP elevation, when compared to patients with venous stents. This finding corroborates previous studies that suggest polyester grafts, often used in arterial stents, are associated with higher rates of PIS due to their proinflammatory properties. In contrast, venous stents, which typically utilize less inflammatory materials such as PTFE, exhibited a lower incidence of PIS, highlighting the influence of graft composition on postoperative outcomes [10].
4.1. Inflammatory Response and PIS Pathophysiology
The pathophysiology of PIS remains poorly understood, but the prevailing hypothesis is that the inflammatory response is triggered by the interaction between the stent material and the vascular endothelium, leading to the release of proinflammatory cytokines, such as interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α). In our study, arterial stent patients experienced more significant and sustained inflammatory responses, as evidenced by elevated CRP and neutrophil counts over several days postoperatively. This aligns with studies that have demonstrated a strong association between polyester grafts and heightened inflammatory reactions, as polyester is known to provoke a more robust foreign body response compared to PTFE grafts [11,12,13,14].
Moreover, our findings suggest that the inflammatory response following arterial stent placement is not only more severe but also more prolonged. Fever, a key marker of PIS, was more frequently observed in arterial stent patients, particularly at 72 and 96 h postoperatively. This prolonged febrile response is indicative of sustained systemic inflammation, which may be due to the larger surface area of the stent and the nature of the graft material, as well as the underlying vascular pathology being treated (e.g., arterial occlusive disease). These factors likely contribute to the increased incidence of PIS in arterial stent patients, as previously suggested by studies on EVAR [9].
4.2. Clinical Implications and Patient Outcomes
The clinical impact of PIS on patient outcomes has been the subject of debate. While some studies suggest that PIS is a transient and benign condition, our study demonstrates that PIS in arterial stent patients is associated with longer hospital stays and higher rates of postoperative complications, such as endoleaks and reintervention. Although PIS was not directly correlated with increased mortality or major adverse cardiac events (MACEs) in our cohort, its association with prolonged hospitalization and greater resource utilization underscores the clinical importance of early recognition and management of PIS [14,15].
Previous studies have suggested that the type of stent graft used may influence the long-term outcomes of patients with PIS. For instance, polyester grafts, which were more commonly used in our arterial stent group, have been associated with higher rates of inflammation and a greater risk of complications, such as endoleaks and stent-related infections. This is consistent with our findings, where patients with arterial stents experienced more frequent endoleaks and required more reinterventions compared to those with venous stents. The proinflammatory nature of polyester grafts may also explain the higher incidence of fever and leukocytosis in these patients [16,17].
4.3. Role of Graft Material and Procedure Type
The influence of graft material on the development of PIS is well-documented in the literature, with polyester being linked to a more pronounced inflammatory response than PTFE. Our study supports this, as arterial stent patients, many of whom received polyester grafts, had significantly higher levels of inflammatory markers and experienced more complications than venous stent patients, who were more likely to receive PTFE grafts [18].
These findings highlight the importance of considering graft material when planning endovascular interventions, as the choice of graft may have a significant impact on the postoperative inflammatory response and the risk of PIS [12,13,16].
Additionally, the duration and complexity of the procedure may also contribute to the development of PIS. Our data showed that arterial stent procedures, which tend to be more complex and longer in duration than venous stent procedures, were associated with a higher incidence of PIS. This is consistent with previous research suggesting that longer procedural times and the use of larger volumes of contrast media may increase the risk of PIS [4,19].
4.4. Role of Vascular Pathology in Post-Implantation Syndrome: Arteries vs. Veins
In addition to the material properties of the stents used, the underlying pathology of the vessels plays a significant role in the inflammatory response seen after endovascular interventions. Atherosclerosis, which is the primary disease process affecting arteries requiring stenting, is characterized by chronic inflammation. The inflammatory theory of atherosclerosis posits that the arterial wall becomes an active source of inflammatory mediators long before the intervention, contributing to a heightened inflammatory milieu even before stent placement [20]. This pre-existing inflammation likely exacerbates the post-implantation inflammatory response, thus contributing to a more pronounced presentation of PIS in arterial stenting. Conversely, thrombotic veins, though subject to clot formation, do not exhibit the same degree of chronic inflammation as the atherosclerotic arteries. While thrombus formation can trigger an acute inflammatory response, it is typically not a long-standing inflammatory process like atherosclerosis. As a result, venous stenting may lead to a less intense inflammatory reaction, and subsequently, a different PIS profile compared to arterial interventions. This fundamental difference between the inflammatory states of arteries and veins highlights the importance of considering vessel pathology when managing PIS, in addition to focusing on the stent material itself [21].
4.5. Role of Stent Migration in Post-Implantation Syndrome
Stent migration, a known complication, can exacerbate postimplantation syndrome (PIS) by triggering an inflammatory response due to vessel injury and mechanical irritation. The displacement of the stent can lead to further vascular damage, which may trigger or intensify the inflammatory cascade associated with PIS. In our study, no instances of stent migration were observed, largely due to the selection of stents longer than 10 cm. Shorter stents are more prone to migration due to insufficient anchorage within the vessel. By utilizing longer stents, we ensured better fixation, thereby reducing the risk of migration and its potential contribution to PIS. This strategic approach minimized both migration-related complications and the severity of PIS in our patient population [22,23].
4.6. Management of PIS and Future Research Directions
Given the association between PIS and adverse postoperative outcomes, effective management strategies are critical. In our study, patients with arterial stents required longer hospital stays, likely due to the need for close monitoring of inflammatory markers and management of complications such as endoleaks. Current management of PIS is largely supportive, focusing on hydration, antipyretics, and, in some cases, the use of corticosteroids or non-steroidal anti-inflammatory drugs (NSAIDs). However, the use of systemic anti-inflammatory medications remains controversial due to their potential side effects, particularly in high-risk patients [24,25,26].
Looking forward, targeted therapies that modulate the inflammatory response without broadly suppressing the immune system, such as interleukin inhibitors, may offer a promising approach to managing PIS. Further research is needed to identify specific biomarkers that can predict the development of PIS and guide treatment decisions. Additionally, the development of standardized diagnostic criteria for PIS would help clinicians better identify and manage this condition, reducing the risk of misdiagnosis and improving patient outcomes [27].
5. Limitations
Several limitations should be considered when interpreting our results. Variability in patient characteristics, including underlying comorbidities and concurrent medications, may influence the inflammatory response and complicate direct comparisons between venous and arterial stenting. A major limitation of this study is its retrospective design, which hinders the ability to assess the severity of inflammation in real-time. Prospective studies are recommended to provide more precise evaluations of the inflammatory response. The retrospective nature of some information collection may introduce biases. Future prospective studies with standardized protocols and controlled variables will help address these limitations and strengthen the evidence base.
6. Conclusions
In conclusion, our study highlights the significant differences in the incidence and severity of PIS between patients receiving venous and arterial stents. The findings suggest that arterial stent placement, particularly with polyester grafts, is associated with a more pronounced and prolonged inflammatory response, leading to increased postoperative complications and longer hospital stays. While PIS did not directly correlate with increased mortality or MACE in our study, its impact on patient recovery and resource utilization is substantial.
This study demonstrated that arterial stent placement is associated with a higher incidence of postimplantation syndrome (PIS), characterized by elevated inflammatory markers, prolonged hospital stays, and an increased rate of postoperative complications, such as endoleaks and reinterventions. Polyester grafts, commonly used in arterial stenting, appear to provoke a stronger inflammatory response compared to PTFE grafts used in venous stents. However, although the incidence of PIS was lower in patients receiving venous stents, the presence of PIS should not be disregarded in these cases, as it still has the potential to affect recovery and clinical outcomes. Recognizing the risk of PIS in both venous and arterial stent placements is crucial for improving patient management. Early identification and appropriate management of PIS can help mitigate its impact, regardless of the type of stent used. This emphasizes the need for careful postoperative monitoring in all stent patients to optimize recovery and minimize complications.
Future research should focus on refining diagnostic criteria for PIS and exploring targeted therapies to mitigate the inflammatory response and improve patient outcomes.
Author Contributions
Conceptualization, N.D. and E.O.; Methodology, N.D., E.O. and A.I.H.; Software, N.D.; Formal analysis, N.D. and A.K.; Investigation, N.D., A.K., N.P., A.A. and E.K.Y.; Resources, N.D., N.P., A.A. and E.K.Y.; Data curation, N.D., A.K. and N.P.; Writing—original draft, N.D.; Writing—review & editing, E.O. and Z.E.; Supervision, E.O. and Z.E. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of Ankara University.(protocol code 2024/577 and date of approval 19.09.2024).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author due to privacy and ethical reasons.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zlatanovic, P.; Busch, A. Post-Implantation Syndrome After Complex Endovascular Abdominal Aneurysm Repair: Not So Important After All? Eur. J. Vasc. Endovasc. Surg. 2023, 66, 813. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Lin, X.; Wu, Q.; Xie, Y.; Zhang, Z.; Qiu, Z.; Chen, L. Risk prediction and prognostic analysis of post-implantation syndrome after thoracic endovascular aortic repair. Sci. Rep. 2024, 14, 17376. [Google Scholar] [CrossRef] [PubMed]
- Soares Ferreira, R.; Oliveira-Pinto, J.; Ultee, K.; Voute, M.T.; Oliveira, N.; Hoeks, S.; Verhagen, H.J.M.; Gonçalves, F.B. Long term outcomes of post-implantation syndrome after endovascular aneurysm repair. Eur. J. Vasc. Endovasc. Surg. 2021, 62, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, O.; Di Girolamo, A.; Belli, C.; Gattuso, R.; Baratta, F.; Gossetti, B.; Alunno, A.; Irace, L. Incidence of post-implantation syndrome with different endovascular aortic aneurysm repair modalities and devices and related etiopathogenetic implications. Ann. Vasc. Surg. 2020, 63, 155–161. [Google Scholar] [CrossRef]
- Sousa, J.; Vilares, A.T. Postimplantation syndrome is not associated with myocardial injury after noncardiac surgery after endovascular aneurysm repair. Ann. Vasc. Surg. 2020, 68, 275–282. [Google Scholar] [CrossRef]
- Black, S.; Sapoval, M.; Dexter, D.J.; Gibson, K.; Kolluri, R.; Razavi, M.; Freitas, D.J.; Wang, H.; Brucato, S.; Murphy, E.; et al. Three-Year Outcomes of the Abre Venous Self-Expanding Stent System in Patients with Symptomatic Iliofemoral Venous Outflow Obstruction. JVasc Interv. Radiol. 2024, 35, 664–675. [Google Scholar] [CrossRef]
- Borhani, S.; Hassanajili, S.; Tafti, S.H.A.; Rabbani, S. Cardiovascular stents: Overview, evolution, and next generation. Prog. Biomater. 2018, 7, 175–205. [Google Scholar] [CrossRef]
- Pan, C.; Han, Y.; Lu, J. Structural Design of Vascular Stents: A review. Micromachines 2021, 12, 770. [Google Scholar] [CrossRef]
- Oddi, F.M.; Vacca, F.; Ciattaglia, R.; Fresilli, M.; Fazzini, S.; Ippoliti, A. Polyester stent graft devices and higher risk of post-implantation syndrome after EVAR: Single-center analysis of 367 patients. Ann. Vasc. Surg. 2021, 75, 455–460. [Google Scholar] [CrossRef]
- Romanelli, A.; Langone, A.; Vicinanza, V.; Gammaldi, R. Postimplantation Syndrome after Traumatic Internal Carotid Artery Pseudoaneurysm Repair with Stent. J. Neuroanaesth. Crit. Care 2024, 1, 1–74. [Google Scholar] [CrossRef]
- Brocca, A.; Virzì, G.M.; de Cal, M.; Giavarina, D.; Carta, M.; Ronco, C. Elevated levels of procalcitonin and interleukin-6 are linked with postoperative complications in cardiac surgery. Scand. J. Surg. 2017, 106, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Voute, M.T.; Bastos Gonçalves, F.M.; van de Luijtgaarden, K.M.; Nulent, C.G.; Hoeks, S.E.; Stolker, R.J.; Verhagen, H.J.M. Stent graft composition plays a material role in the postimplantation syndrome. J. Vasc. Surg. 2012, 56, 1503–1509. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Choi, J.H.; Kim, E.J. Volume of mural thrombus plays a role in the elevation of inflammatory markers after endovascular aortic repair. J. Cardiothorac. Surg. 2018, 13, 27. [Google Scholar] [CrossRef] [PubMed]
- Moulakakis, K.G.; Alepaki, M.; Sfyroeras, G.S.; Antonopoulos, C.N.; Giannakopoulos, T.G.; Kakisis, J.; Karakitsos, P.; Liapis, C.D. The impact of endograft type on inflammatory response after endovascular treatment of abdominal aortic aneurysm. J. Vasc. Surg. 2013, 57, 668–677. [Google Scholar] [CrossRef]
- Kwon, H.; Ko, G.Y.; Kim, M.J.; Han, Y.; Noh, M.; Kwon, T.W.; Cho, Y.P. Effects of postimplantation systemic inflammatory response on long-term clinical out- comes after endovascular aneurysm repair of an abdominal aortic aneurysm. Medicine 2016, 95, 4532. [Google Scholar] [CrossRef]
- Swartbol, P.; Norgren, L.; Parsson, H.; Truedsson, L. Endovascular abdominal aortic aneurysm repair induces significant alter- ations in surface adhesion molecule expression on donor white blood cells exposed to patient plasma. Eur. J. Vasc. Endovasc. Surg. 1997, 14, 48–59. [Google Scholar] [CrossRef]
- Syk, J.; Brunkwall, J.; Ivancev, K.; Linblad, B.; Montgomery, A.; Wellander, E.; Wisniewski, J.; Risberg, B. Postoperative fever, bowel ischaemia and cytokine response to abdominal aortic aneurysm repairda comparison between endovascular and open surgery. Eur. J. Vasc. Endovascular Surg. 1999, 15, 398–405. [Google Scholar] [CrossRef]
- Lo, R.C.; Buck, D.B.; Herrmann, J.; Hamdan, A.D.; Wyers, M.; Patel, V.I.; Fillinger, M.; Schermerhorn, M.L. Risk factors and consequences of persistent type II endoleaks. J. Vasc. Surg. 2016, 63, 895–901. [Google Scholar] [CrossRef]
- Jeong, M.J.; Kwon, H.; Ko, G.Y.; Gwon, D.; Kim, M.J.; Han, Y.; Kwon, T.W.; Cho, Y.P. New predictors of aneurysm sac behavior after endovascular aortic aneurysm repair. Eur. Radiol. 2019, 29, 6591–6599. [Google Scholar] [CrossRef]
- Libby, P. Inflammation in atherosclerosis. Nature 2002, 420, 868–874. [Google Scholar] [CrossRef]
- Ross, R. Atherosclerosis—An inflammatory disease. N. Engl. J. Med. 1999, 340, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Sayed, M.H.; Salem, M.; Desai, K.R.; Q’Sullivan, G.J.; Black, S.A. A review of the incidence, outcome, and management of venous stent migration. J. Vasc. Surg. Venous Lymhat Disord. 2022, 10, 482–490. [Google Scholar] [CrossRef]
- Natha, B.; Black, S.A. Migration and Other Venous Stenting Complications: How to Identify and Avoid Them. Endovasc. Today. 2022, 21, 46–47. [Google Scholar]
- Arnaoutoglou, E.; Kouvelos, G.; Papa, N.; Gartzonika, K.; Milionis, H.; Koulouras, V.; Matsagkas, M. Prospective evaluation of postimplantation syndrome evolution on patient outcomes after endovascular aneurysm repair for abdominal aortic aneurysm. J. Vasc. Surg. 2016, 63, 1248–1255. [Google Scholar] [CrossRef]
- Bischoff, M.S.; Hafner, S.; Able, T.; Peters, A.S.; Hyhlik-Durr, A.; Bockler, D. Incidence and treatment of postimplantation syndrome after endovascular repair of infrarenal aortic aneurysms. Gefasschirurgie 2013, 18, 381–387. [Google Scholar] [CrossRef]
- Doria, M.; Manoranjithan, S.; Scoville, C.; Vogel, T.R.; Cheung, S.; Calvagna, C.; Lepidi, S.; Bath, J. Systematic review of risk factors and outcomes of post-implantation syndrome following endovascular repair. J. Vasc. Surg. 2023, 79, 1240–1250. [Google Scholar] [CrossRef]
- Arnaoutoglou, E.; Kouvelos, G.; Milionis, H.; Mavridis, A.; Kolaitis, N.; Papa, N.; Papadopoulos, G.; Matsagkas, M. Post-implantation syndrome following endovascular abdominal aortic aneurysm repair: Preliminary data. Interact. Cardiovasc. Thorac. Surg. 2011, 12, 609–614. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).