Oral Contraceptives Interact with Adiposity-Associated Markers in Patients with Multiple Sclerosis
Abstract
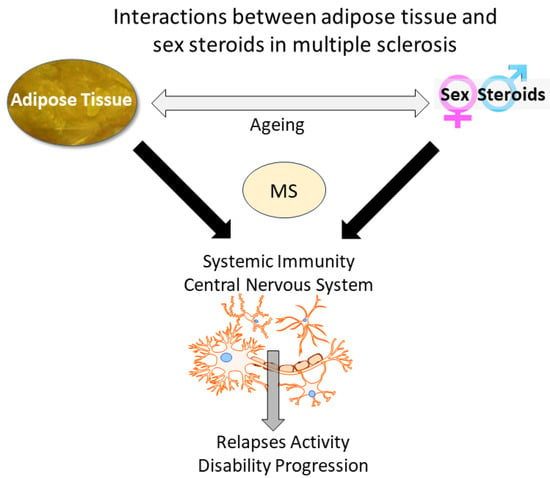
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Biochemical Analysis
2.3. Statistical Methods
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thompson, A.J.; Baranzini, S.E.; Geurts, J.; Hemmer, B.; Ciccarelli, O. Multiple Sclerosis. Lancet 2018, 391, 1622–1636. [Google Scholar] [CrossRef] [PubMed]
- Correale, J.; Marrodan, M. Multiple Sclerosis and Obesity: The Role of Adipokines. Front. Immunol. 2022, 13, 1038393. [Google Scholar] [CrossRef] [PubMed]
- Loonstra, F.C.; Falize, K.F.; de Ruiter, L.R.J.; Schoonheim, M.M.; Strijbis, E.M.M.; Killestein, J.; de Vries, H.E.; Uitdehaag, B.M.J.; Rijnsburger, M. Adipokines in Multiple Sclerosis Patients Are Related to Clinical and Radiological Measures. J. Neurol. 2023, 270, 2018–2030. [Google Scholar] [CrossRef]
- Chitnis, T.; Graves, J.; Weinstock-Guttman, B.; Belman, A.; Olsen, C.; Misra, M.; Aaen, G.; Benson, L.; Candee, M.; Gorman, M.; et al. Distinct Effects of Obesity and Puberty on Risk and Age at Onset of Pediatric MS. Ann. Clin. Transl. Neurol. 2016, 3, 897–907. [Google Scholar] [CrossRef] [PubMed]
- Harroud, A.; Morris, J.A.; Forgetta, V.; Mitchell, R.; Smith, G.D.; Sawcer, S.J.; Richards, J.B. Effect of Age at Puberty on Risk of Multiple Sclerosis: A Mendelian Randomization Study. Neurology 2019, 92, E1803–E1810. [Google Scholar] [CrossRef] [PubMed]
- Golden, L.C.; Voskuhl, R. The Importance of Studying Sex Differences in Disease: The Example of Multiple Sclerosis. J. Neurosci. Res. 2017, 95, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Sena, A.; Macedo, A.; Ferret-Sena, V.; Capela, C.; Pedrosa, R. Serum Lipoprotein Profile Is Associated With Protective Effects of Oral Contraceptive Use on Multiple Sclerosis Severity: A Cross-Sectional Study. Front. Neurol. 2019, 10, 60. [Google Scholar] [CrossRef]
- Fantuzzi, G. Adiponectin in Inflammatory and Immune-Mediated Diseases. Cytokine 2013, 64, 1–10. [Google Scholar] [CrossRef]
- Di Carlo, C.; Tommaselli, G.A.; De Rosa, N.; Fabozzi, A.; Santoro, R.; Bifulco, G.; Sparice, S.; Nappi, C. Plasma Leptin and Adiponectin Levels in Hormone Replacement Therapy and Contraception: Effects of Different Progestogens. Fertil. Steril. 2011, 96, 214–219. [Google Scholar] [CrossRef]
- Fallah, S.; Sanjary Pour, M.; Rabbani Chadegani, A.; Korani, M. Adiponectin, Leptin and Lipid Profiles Evaluation in Oral Contraceptive Pill Consumers. Arch. Gynecol. Obstet. 2012, 285, 1747–1752. [Google Scholar] [CrossRef]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of Multiple Sclerosis: 2017 Revisions of the McDonald Criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Roxburgh, R.H.S.R.; Seaman, S.R.; Masterman, T.; Hensiek, A.E.; Sawcer, S.J.; Vukusic, S.; Achiti, I.; Confavreux, C.; Coustans, M.; le Page, E.; et al. Multiple Sclerosis Severity Score: Using Disability and Disease Duration to Rate Disease Severity. Neurology 2005, 64, 1144–1151. [Google Scholar] [CrossRef]
- Reneau, J.; Goldblatt, M.; Gould, J.; Kindel, T.; Kastenmeier, A.; Higgins, R.; Rosemary Rengel, L.; Schoyer, K.; James, R.; Obi, B.; et al. Effect of Adiposity on Tissue-Specific Adiponectin Secretion. PLoS ONE 2018, 13, e0198889. [Google Scholar] [CrossRef] [PubMed]
- Devorak, J.; Mokry, L.E.; Morris, J.A.; Forgetta, V.; Davey Smith, G.; Sawcer, S.; Richards, J.B. Large Differences in Adiponectin Levels Have No Clear Effect on Multiple Sclerosis Risk: A Mendelian Randomization Study. Mult. Scler. 2017, 23, 1461–1468. [Google Scholar] [CrossRef] [PubMed]
- Harroud, A.; Manousaki, D.; Butler-Laporte, G.; Mitchell, R.E.; Davey Smith, G.; Richards, J.B.; Baranzini, S.E. The Relative Contributions of Obesity, Vitamin D, Leptin, and Adiponectin to Multiple Sclerosis Risk: A Mendelian Randomization Mediation Analysis. Mult. Scler. J. 2021, 27, 1994–2000. [Google Scholar] [CrossRef]
- Kvistad, S.S.; Myhr, K.M.; Holmøy, T.; Benth, J.Š.; Wergeland, S.; Beiske, A.G.; Bjerve, K.S.; Hovdal, H.; Midgard, R.; Sagen, J.V.; et al. Serum Levels of Leptin and Adiponectin Are Not Associated with Disease Activity or Treatment Response in Multiple Sclerosis. J. Neuroimmunol. 2018, 323, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Baharnoori, M.; Wilson, R.; Saxena, S.; Gonzalez, C.T.; Sotiropoulos, M.G.; Keyhanian, K.; Healy, B.C.; Chitnis, T. Altered Adipokine Levels Are Associated with Dimethyl Fumarate Treatment in Multiple Sclerosis Patients. Mult. Scler. Relat. Disord. 2021, 56, 103311. [Google Scholar] [CrossRef]
- Çoban, A.; Düzel, B.; Tüzün, E.; Tamam, Y. Investigation of the Prognostic Value of Adipokines in Multiple Sclerosis. Mult. Scler. Relat. Disord. 2017, 15, 11–14. [Google Scholar] [CrossRef]
- Keyhanian, K.; Saxena, S.; Gombolay, G.; Healy, B.C.; Misra, M.; Chitnis, T. Adipokines Are Associated with Pediatric Multiple Sclerosis Risk and Course. Mult. Scler. Relat. Disord. 2019, 36, 101384. [Google Scholar] [CrossRef]
- Signoriello, E.; Lus, G.; Polito, R.; Casertano, S.; Scudiero, O.; Coletta, M.; Monaco, M.L.; Rossi, F.; Nigro, E.; Daniele, A. Adiponectin Profile at Baseline Is Correlated to Progression and Severity of Multiple Sclerosis. Eur. J. Neurol. 2019, 26, 348–355. [Google Scholar] [CrossRef]
- Nyirenda, M.H.; Fadda, G.; Healy, L.M.; Mexhitaj, I.; Poliquin-Lasnier, L.; Hanwell, H.; Saveriano, A.W.; Rozenberg, A.; Li, R.; Moore, C.S.; et al. Pro-Inflammatory Adiponectin in Pediatric-Onset Multiple Sclerosis. Mult. Scler. 2021, 27, 1948–1959. [Google Scholar] [CrossRef] [PubMed]
- Bove, R.; Musallam, A.; Xia, Z.; Baruch, N.; Messina, S.; Healy, B.C.; Chitnis, T. Longitudinal BMI Trajectories in Multiple Sclerosis: Sex Differences in Association with Disease Severity. Mult. Scler. Relat. Disord. 2016, 8, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Bove, R.; Rankin, K.; Chua, A.S.; Saraceno, T.; Sattarnezhad, N.; Greeke, E.; Stuart, F.; LaRussa, A.; Glanz, B.I.; Chitnis, T. Oral Contraceptives and MS Disease Activity in a Contemporary Real-World Cohort. Mult. Scler. 2018, 24, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Otero-Romero, S.; Carbonell-Mirabent, P.; Midaglia, L.; Zuluaga, M.; Galán, I.; Cobo-Calvo, A.; Rio, J.; Arrambide, G.; Vidal-Jordana, A.; Castillo, J.; et al. Oral Contraceptives Do Not Modify the Risk of a Second Attack and Disability Accrual in a Prospective Cohort of Women with a Clinically Isolated Syndrome and Early Multiple Sclerosis. Mult. Scler. 2022, 28, 950–957. [Google Scholar] [CrossRef] [PubMed]
- Foong, Y.C.; Aitken, D.; Winzenberg, T.; Otahal, P.; Scott, D.; Jones, G. The Association between Physical Activity and Reduced Body Fat Lessens with Age—Results from a Cross-Sectional Study in Community-Dwelling Older Adults. Exp. Gerontol. 2014, 55, 107–112. [Google Scholar] [CrossRef]
- Graves, J.S.; Krysko, K.M.; Hua, L.H.; Absinta, M.; Franklin, R.J.M.; Segal, B.M. Ageing and Multiple Sclerosis. Lancet Neurol. 2023, 22, 66–77. [Google Scholar] [CrossRef]
- Tur, C.; Carbonell-Mirabent, P.; Cobo-Calvo, Á.; Otero-Romero, S.; Arrambide, G.; Midaglia, L.; Castilló, J.; Vidal-Jordana, Á.; Rodríguez-Acevedo, B.; Zabalza, A.; et al. Association of Early Progression Independent of Relapse Activity with Long-Term Disability after a First Demyelinating Event in Multiple Sclerosis. JAMA Neurol. 2023, 80, 151–160. [Google Scholar] [CrossRef]
- Hellwig, K.; Chen, L.H.; Stancyzk, F.Z.; Langer-Gould, A.M. Oral Contraceptives and Multiple Sclerosis/Clinically Isolated Syndrome Susceptibility. PLoS ONE 2016, 11, e0149094. [Google Scholar] [CrossRef]
| Characteristics | COC− (n = 30) | COC+ (n = 32) | p-Value | ||
|---|---|---|---|---|---|
| M (SD) | Median (IQR) | M (SD) | Median (IQR) | ||
| Age (years) | 34.70 (8.47) | 35.00 (12) | 31.84 (6.53) | 31.00 (10) | 0.109 |
| Disease onset (years) | 30.10 (8.43) | 30.00 (13) | 27.16 (6.21) | 27.00 (9) | 0.150 |
| Relapses (n, %) a | 27 (90%) | ----- | 29 (90.6%) | ----- | 0.667 |
| MSSS | 4.00 (2.46) | 3.62 (3.86) | 2.04 (1.72) | 1.61(2.14) | 0.001 |
| EDSS | 2.00 (1.20) | 2.00 (2.4) | 1.03 (0.80) | 1.00 (1.0) | 0.001 |
| BMI | 23.33 (11.72) | 23.00 (7) | 24.81 (5.03) | 24.50 (9) | 0.241 |
| Waist circumference (cm) | 78.70 (11.72) | 79.00 (21) | 76.72 (11.39) | 77.00 (18) | 0.599 |
| Waist/hip | 0.78 (0.07) | 0.80 (0.10) | 0.77 (0.08) | 0.76 (0.11) | 0.317 |
| Leptin (ng/mL) | 19.27 (14.05) | 15.00 (22.10) | 21.17 (14.54) | 20.35 (25.2) | 0.375 |
| Leptin/BMI | 0.78 (0.47) | 0.68 (0.90) | 0.81 (0.42) | 0.74 (0.69) | 0.499 |
| APN (µg/mL) | 10.26 (3.81) | 10.10 (5.65) | 7.93 (3.26) | 7.50 (2.70) | 0.016 |
| APN/BMI | 0.45 (0.20) | 0.45 (0.26) | 0.33 (0.14) | 0.34 (0.08) | 0.013 |
| Smokers (n, %) a | 10 (33.3%) | ----- | 12 (37.5%) | ----- | 0.732 |
| Variables | β | t | p | F (df) | p | R2ajust |
|---|---|---|---|---|---|---|
| Model 1 (MSSS as dependent variable) | ||||||
| Step 1 | 3.448 (4.2) | 0.016 | 0.175 | |||
| Leptin/BMI | 0.274 | 1.682 | 0.100 | |||
| APN/BMI | 0.355 | 2.419 | 0.020 | |||
| Waist circumference | 0.188 | 0.841 | 0.405 | |||
| Waist/hip | 0.183 | 0.950 | 0.347 | |||
| Step 2 | 5.234 (5.41) | ≤0.001 | 0.315 | |||
| Leptin/BMI | 0.240 | 1.610 | 0.115 | |||
| APN/BMI | 0.154 | 1.032 | 0.308 | |||
| Waist circumference | 0.136 | 0.665 | 0.510 | |||
| Waist/hip | 0.077 | 0.428 | 0.671 | |||
| COC | −0.431 | −3.093 | 0.004 | |||
| Step 3 | 4.792 (8.38) | ≤0.001 | 0.397 | |||
| Leptin/BMI | 0.194 | 1.321 | 0.194 | |||
| APN/BMI | 0.051 | 0.338 | 0.737 | |||
| Waist circumference | 0.018 | 0.090 | 0.928 | |||
| Waist/hip | 0.070 | 0.411 | 0.683 | |||
| COC | −0.391 | −2.951 | 0.005 | |||
| Age | 0.335 | 2.365 | 0.023 | |||
| Disease Duration | −0.318 | −2.400 | 0.021 | |||
| Number of relapses | −0.043 | −0.330 | 0.743 | |||
| Model 2 (number of relapses as dependent variable) * | ||||||
| Step 1 | 2.426 (4.42) | 0.063 | 0.110 | |||
| Leptin/BMI | 0.159 | 0.938 | 0.353 | |||
| APN/BMI | −0.353 | −2.310 | 0.026 | |||
| Waist circumference | 0.060 | 0.257 | 0.798 | |||
| Waist/hip | −0.179 | −0.894 | 0.377 | |||
| Step 2 | 1.913 (5.41) | 0.113 | 0.090 | |||
| Leptin/BMI | 0.155 | 0.905 | 0.371 | |||
| APN/BMI | −0.373 | −2.173 | 0.036 | |||
| Waist circumference | 0.055 | 0.231 | 0.818 | |||
| Waist/hip | −0.190 | −0.920 | 0.363 | |||
| COC | −0.044 | −0.272 | 0.787 | |||
| Step 3 | 1.799 (7.39) | 0.115 | 0.108 | |||
| Leptin/BMI | 0.074 | 0.414 | 0.681 | |||
| APN/BMI | −0.372 | −2.143 | 0.038 | |||
| Waist circumference | 0.134 | 0.550 | 0.585 | |||
| Waist/hip | −0.158 | −0.767 | 0.448 | |||
| COC | −0.067 | −0.414 | 0.681 | |||
| Age | −0.169 | −0.992 | 0.327 | |||
| Disease Duration | −0.143 | −0.894 | 0.377 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferret-Sena, V.; Ramos, C.; Cascais, M.J.; Capela, C.; Sena, A. Oral Contraceptives Interact with Adiposity-Associated Markers in Patients with Multiple Sclerosis. J. Clin. Med. 2024, 13, 464. https://doi.org/10.3390/jcm13020464
Ferret-Sena V, Ramos C, Cascais MJ, Capela C, Sena A. Oral Contraceptives Interact with Adiposity-Associated Markers in Patients with Multiple Sclerosis. Journal of Clinical Medicine. 2024; 13(2):464. https://doi.org/10.3390/jcm13020464
Chicago/Turabian StyleFerret-Sena, Véronique, Catarina Ramos, Maria João Cascais, Carlos Capela, and Armando Sena. 2024. "Oral Contraceptives Interact with Adiposity-Associated Markers in Patients with Multiple Sclerosis" Journal of Clinical Medicine 13, no. 2: 464. https://doi.org/10.3390/jcm13020464