Atrial Fibrillation in Patients with Hypertrophic Cardiomyopathy and Cardiac Amyloidosis: From Clinical Management to Catheter Ablation Indication
Abstract
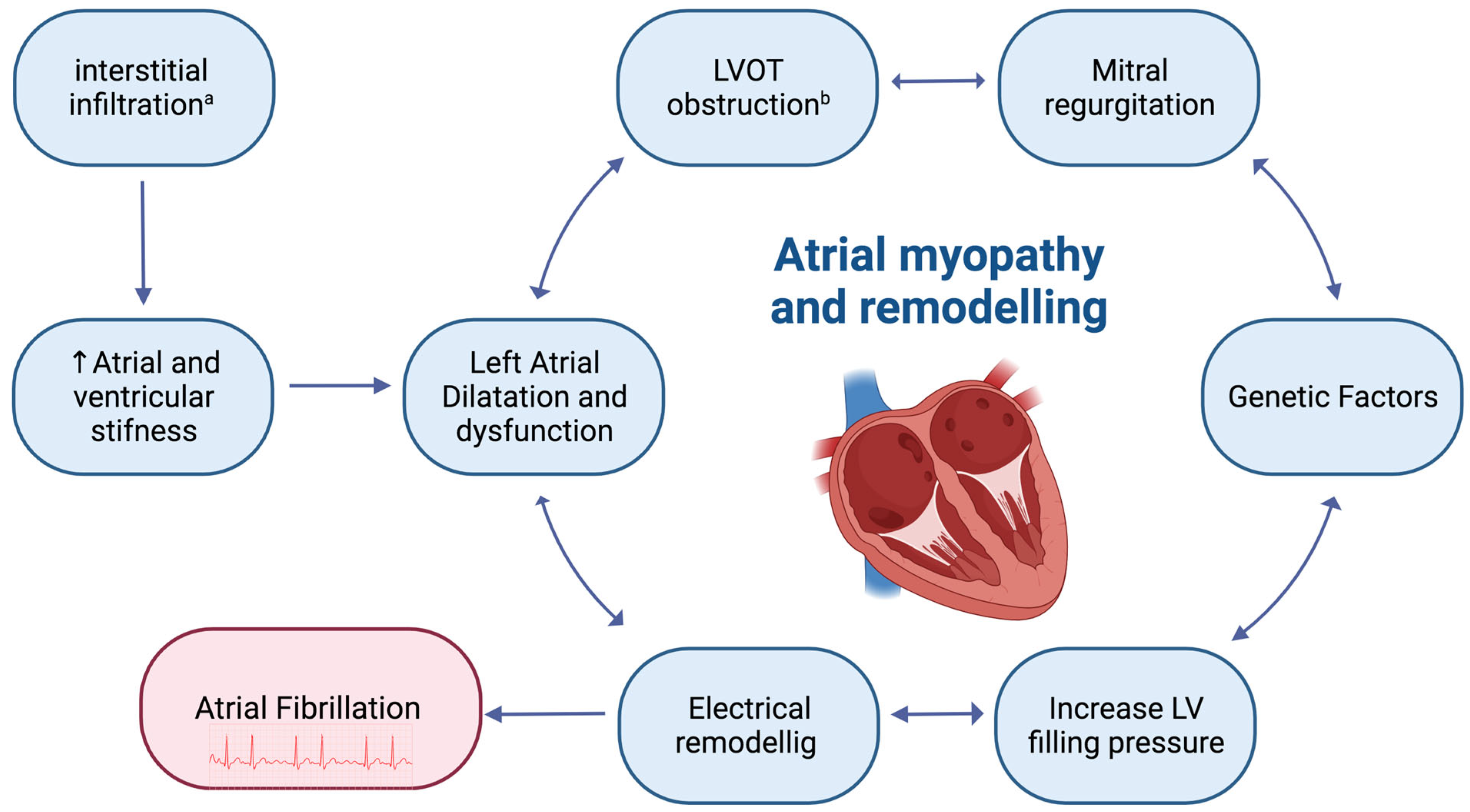
:1. Introduction: Hypertrophic Cardiomyopathy and Atrial Fibrillation
2. Pharmacological Management of AF in HCM
3. Non-Pharmacological Management of AF in HCM
4. Cardiac Amyloidosis and Atrial Fibrillation
5. Pharmacological Management of AF in CA
6. Non-Pharmacological Management of AF in CA
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elliott, P.M.; Anastasakis, A.; Borger, M.A.; Borggrefe, M.; Cecchi, F.; Charron, P.; Hagege, A.A.; Lafont, A.; Limongelli, G.; Mahrholdt, H.; et al. 2014 ESC Guidelines on Diagnosis and Management of Hypertrophic Cardiomyopathy: The Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur. Heart J. 2014, 35, 2733–2779. [Google Scholar] [CrossRef] [PubMed]
- Guttmann, O.P.; Rahman, M.S.; O’Mahony, C.; Anastasakis, A.; Elliott, P.M. Atrial Fibrillation and Thromboembolism in Patients with Hypertrophic Cardiomyopathy: Systematic Review. Heart 2014, 100, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Ommen, S.R.; Mital, S.; Burke, M.A.; Day, S.M.; Deswal, A.; Elliott, P.; Evanovich, L.L.; Hung, J.; Joglar, J.A.; Kantor, P.; et al. 2020 AHA/ACC Guideline for the Diagnosis and Treatment of Patients With Hypertrophic Cardiomyopathy: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2020, 142, e533–e557. [Google Scholar] [CrossRef]
- Tani, T.; Tanabe, K.; Ono, M.; Yamaguchi, K.; Okada, M.; Sumida, T.; Konda, T.; Fujii, Y.; Kawai, J.; Yagi, T.; et al. Left Atrial Volume and the Risk of Paroxysmal Atrial Fibrillation in Patients with Hypertrophic Cardiomyopathy. J. Am. Soc. Echocardiogr. 2004, 17, 644–648. [Google Scholar] [CrossRef] [PubMed]
- Garg, L.; Gupta, M.; Sabzwari, S.R.A.; Agrawal, S.; Agarwal, M.; Nazir, T.; Gordon, J.; Bozorgnia, B.; Martinez, M.W. Atrial Fibrillation in Hypertrophic Cardiomyopathy: Prevalence, Clinical Impact, and Management. Heart Fail. Rev. 2019, 24, 189–197. [Google Scholar] [CrossRef]
- Siontis, K.C.; Geske, J.B.; Ong, K.; Nishimura, R.A.; Ommen, S.R.; Gersh, B.J. Atrial Fibrillation in Hypertrophic Cardiomyopathy: Prevalence, Clinical Correlations, and Mortality in a Large High-Risk Population. J. Am. Heart Assoc. 2014, 3, e001002. [Google Scholar] [CrossRef]
- Guttmann, O.P.; Pavlou, M.; O’Mahony, C.; Monserrat, L.; Anastasakis, A.; Rapezzi, C.; Biagini, E.; Gimeno, J.R.; Limongelli, G.; Garcia-Pavia, P.; et al. Predictors of Atrial Fibrillation in Hypertrophic Cardiomyopathy. Heart Br. Card. Soc. 2017, 103, 672–678. [Google Scholar] [CrossRef]
- Olivotto, I.; Cecchi, F.; Casey, S.A.; Dolara, A.; Traverse, J.H.; Maron, B.J. Impact of Atrial Fibrillation on the Clinical Course of Hypertrophic Cardiomyopathy. Circulation 2001, 104, 2517–2524. [Google Scholar] [CrossRef]
- Carrick, R.T.; Maron, M.S.; Adler, A.; Wessler, B.; Hoss, S.; Chan, R.H.; Sridharan, A.; Huang, D.; Cooper, C.; Drummond, J.; et al. Development and Validation of a Clinical Predictive Model for Identifying Hypertrophic Cardiomyopathy Patients at Risk for Atrial Fibrillation: The HCM-AF Score. Circ. Arrhythm. Electrophysiol. 2021, 14, e009796. [Google Scholar] [CrossRef]
- Lee, H.-J.; Kim, H.-K.; Jung, J.-H.; Han, K.-D.; Lee, H.; Park, J.-B.; Kim, H.M.; Kim, Y.-J.; Ommen, S.R. Novel Oral Anticoagulants for Primary Stroke Prevention in Hypertrophic Cardiomyopathy Patients With Atrial Fibrillation. Stroke 2019, 50, 2582–2586. [Google Scholar] [CrossRef]
- Jung, H.; Yang, P.S.; Jang, E.; Yu, H.T.; Kim, T.H.; Uhm, J.S.; Kim, J.Y.; Pak, H.N.; Lee, M.H.; Joung, B.; et al. Effectiveness and Safety of Non-Vitamin K Antagonist Oral Anticoagulants in Patients with Atrial Fibrillation with Hypertrophic Cardiomyopathy: A Nationwide Cohort Study. Chest 2019, 155, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.; Yang, P.S.; Sung, J.H.; Jang, E.; Yu, H.T.; Kim, T.H.; Uhm, J.S.; Kim, J.Y.; Pak, H.N.; Lee, M.H.; et al. Hypertrophic Cardiomyopathy in Patients with Atrial Fibrillation: Prevalence and Associated Stroke Risks in a Nationwide Cohort Study. Thromb. Haemost. 2019, 119, 285–293. [Google Scholar] [CrossRef]
- Jung, H.; Sung, J.H.; Yang, P.S.; Jang, E.; Yu, H.T.; Kim, T.H.; Pak, H.N.; Lee, M.H.; Joung, B.; Lip, G.Y.H. Stroke Risk Stratification for Atrial Fibrillation Patients with Hypertrophic Cardiomyopathy. J. Am. Coll. Cardiol. 2018, 72, 2409–2411. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.A.S.; Maron, M.S.; Estes, N.A.M.; Price, L.L.; Rowin, E.J.; Maron, B.J.; Link, M.S. Safety, Side Effects and Relative Efficacy of Medications for Rhythm Control of Atrial Fibrillation in Hypertrophic Cardiomyopathy. Am. J. Cardiol. 2019, 123, 1859–1862. [Google Scholar] [CrossRef]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.-A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the Diagnosis and Management of Atrial Fibrillation Developed in Collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the Diagnosis and Management of Atrial Fibrillation of the European Society of Cardiology (ESC) Developed with the Special Contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef] [PubMed]
- Dragasis, S.; Vlachos, K.; Kariki, O.; Koskina, S.; Zygouri, A.; Patsiotis, I.G.; Anastasakis, A.; Athanasopoulos, G.; Ritsatos, K.; Letsas, K.; et al. Atrial Fibrillation in Hypertrophic Cardiomyopathy–A Contemporary Mini-Review. Hell. J. Cardiol. HJC Hell. Kardiol. Ep. 2022, 67, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Saksena, S.; Slee, A.; Waldo, A.L.; Freemantle, N.; Reynolds, M.; Rosenberg, Y.; Rathod, S.; Grant, S.; Thomas, E.; Wyse, D.G. Cardiovascular Outcomes in the AFFIRM Trial: An Assessment of Individual Antiarrhythmic Drug Therapies Compared to Rate Control Using Propensity Score Matched Analyses. J. Am. Coll. Cardiol. 2011, 58, 1975–1985. [Google Scholar] [CrossRef]
- Santangeli, P.; Di Biase, L.; Themistoclakis, S.; Raviele, A.; Schweikert, R.A.; Lakkireddy, D.; Mohanty, P.; Bai, R.; Mohanty, S.; Pump, A.; et al. Catheter Ablation of Atrial Fibrillation in Hypertrophic Cardiomyopathy: Long-Term Outcomes and Mechanisms of Arrhythmia Recurrence. Circ. Arrhythm. Electrophysiol. 2013, 6, 1089–1094. [Google Scholar] [CrossRef]
- Derejko, P.; Polańska, M.; Chojnowska, L.; Michałowska, I.; Wójcik, A.; Piotrowicz, E.; Lech, A.; Kłopotowski, M.; Baranowski, R.; Przybylski, A.; et al. Catheter Ablation of Atrial Fibrillation in Patients with Hypertrophic Cardiomyopathy: Atrial Fibrillation Type Determines the Success Rate. Kardiol. Pol. 2013, 71, 17–24. [Google Scholar]
- Bassiouny, M.; Lindsay, B.D.; Lever, H.; Saliba, W.; Klein, A.; Banna, M.; Abraham, J.; Shao, M.; Rickard, J.; Kanj, M.; et al. Outcomes of Nonpharmacologic Treatment of Atrial Fibrillation in Patients with Hypertrophic Cardiomyopathy. Heart Rhythm. 2015, 12, 1438–1447. [Google Scholar] [CrossRef]
- Castagno, D.; Di Donna, P.; Olivotto, I.; Frontera, A.; Calò, L.; Scaglione, M.; Arretini, A.; Anselmino, M.; Giustetto, C.; De Ferrari, G.M.; et al. Transcatheter Ablation for Atrial Fibrillation in Patients with Hypertrophic Cardiomyopathy: Long-Term Results and Clinical Outcomes. J. Cardiovasc. Electrophysiol. 2021, 32, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Dinshaw, L.; Münkler, P.; Schäffer, B.; Klatt, N.; Jungen, C.; Dickow, J.; Tamenang, A.; Schleberger, R.; Pecha, S.; Pinnschmidt, H.; et al. Ablation of Atrial Fibrillation in Patients With Hypertrophic Cardiomyopathy: Treatment Strategy, Characteristics of Consecutive Atrial Tachycardia and Long-Term Outcome. J. Am. Heart Assoc. 2021, 10, e017451. [Google Scholar] [CrossRef]
- Di Donna, P.; Olivotto, I.; Delcrè, S.D.L.; Caponi, D.; Scaglione, M.; Nault, I.; Montefusco, A.; Girolami, F.; Cecchi, F.; Haissaguerre, M.; et al. Efficacy of Catheter Ablation for Atrial Fibrillation in Hypertrophic Cardiomyopathy: Impact of Age, Atrial Remodelling, and Disease Progression. Europace 2010, 12, 347–355. [Google Scholar] [CrossRef]
- Lapenna, E.; Pozzoli, A.; De Bonis, M.; La Canna, G.; Nisi, T.; Nascimbene, S.; Vicentini, L.; Di Sanzo, S.; Del Forno, B.; Schiavi, D.; et al. Mid-Term Outcomes of Concomitant Surgical Ablation of Atrial Fibrillation in Patients Undergoing Cardiac Surgery for Hypertrophic Cardiomyopathy. Eur. J. Cardio Thoracic Surg. 2017, 51, 1112–1118. [Google Scholar] [CrossRef]
- Meng, Y.; Zhang, Y.; Liu, P.; Zhu, C.; Lu, T.; Hu, E.; Yang, Q.; Nie, C.; Wang, S. Clinical Efficacy and Safety of Cox-Maze IV Procedure for Atrial Fibrillation in Patients With Hypertrophic Obstructive Cardiomyopathy. Front. Cardiovasc. Med. 2021, 8, 720950. [Google Scholar] [CrossRef]
- Hodges, K.; Tang, A.; Rivas, C.G.; Umana-Pizano, J.; Chemtob, R.; Desai, M.Y.; Gillinov, A.M.; Smedira, N.; Wierup, P. Surgical Ablation of Atrial Fibrillation in Hypertrophic Obstructive Cardiomyopathy: Outcomes of a Tailored Surgical Approach. J. Card. Surg. 2020, 35, 2957–2964. [Google Scholar] [CrossRef]
- Quintana, E.; Cox, J.L. Surgical Management of Atrial Fibrillation at the Time of Septal Myectomy. Ann. Cardiothorac. Surg. 2017, 6, 386–393. [Google Scholar] [CrossRef]
- Garcia-Pavia, P.; Rapezzi, C.; Adler, Y.; Arad, M.; Basso, C.; Brucato, A.; Burazor, I.; Caforio, A.L.P.; Damy, T.; Eriksson, U.; et al. Diagnosis and Treatment of Cardiac Amyloidosis: A Position Statement of the ESC Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2021, 42, 1554–1568. [Google Scholar] [CrossRef]
- Muchtar, E.; Dispenzieri, A.; Magen, H.; Grogan, M.; Mauermann, M.; McPhail, E.D.; Kurtin, P.J.; Leung, N.; Buadi, F.K.; Dingli, D.; et al. Systemic Amyloidosis from A (AA) to T (ATTR): A Review. J. Intern. Med. 2021, 289, 268–292. [Google Scholar] [CrossRef]
- Zhao, D.-S.; Shen, Y.; Zhang, Q.; Lin, G.; Lu, Y.-H.; Chen, B.-T.; Shi, L.-S.; Huang, J.-F.; Lu, H.-H. Outcomes of Catheter Ablation of Atrial Fibrillation in Patients with Hypertrophic Cardiomyopathy: A Systematic Review and Meta-Analysis. Europace 2016, 18, 508–520. [Google Scholar] [CrossRef]
- Laptseva, N.; Rossi, V.A.; Sudano, I.; Schwotzer, R.; Ruschitzka, F.; Flammer, A.J.; Duru, F. Arrhythmic Manifestations of Cardiac Amyloidosis: Challenges in Risk Stratification and Clinical Management. J. Clin. Med. 2023, 12, 2581. [Google Scholar] [CrossRef] [PubMed]
- Gilstrap, L.G.; Dominici, F.; Wang, Y.; El-Sady, M.S.; Singh, A.; Di Carli, M.F.; Falk, R.H.; Dorbala, S. Epidemiology of Cardiac Amyloidosis-Associated Heart Failure Hospitalizations Among Fee-for-Service Medicare Beneficiaries in the United States. Circ. Heart Fail. 2019, 12, e005407. [Google Scholar] [CrossRef]
- Longhi, S.; Quarta, C.C.; Milandri, A.; Lorenzini, M.; Gagliardi, C.; Manuzzi, L.; Bacchi-Reggiani, M.L.; Leone, O.; Ferlini, A.; Russo, A.; et al. Atrial Fibrillation in Amyloidotic Cardiomyopathy: Prevalence, Incidence, Risk Factors and Prognostic Role. Amyloid 2015, 22, 147–155. [Google Scholar] [CrossRef]
- Papathanasiou, M.; Jakstaite, A.-M.; Oubari, S.; Siebermair, J.; Wakili, R.; Hoffmann, J.; Carpinteiro, A.; Hagenacker, T.; Thimm, A.; Rischpler, C.; et al. Clinical Features and Predictors of Atrial Fibrillation in Patients with Light-Chain or Transthyretin Cardiac Amyloidosis. ESC Heart Fail. 2022, 9, 1740–1748. [Google Scholar] [CrossRef]
- Gertz, M.A.; Dispenzieri, A.; Sher, T. Pathophysiology and Treatment of Cardiac Amyloidosis. Nat. Rev. Cardiol. 2015, 12, 91–102. [Google Scholar] [CrossRef]
- Vergaro, G.; Aimo, A.; Rapezzi, C.; Castiglione, V.; Fabiani, I.; Pucci, A.; Buda, G.; Passino, C.; Lupón, J.; Bayes-Genis, A.; et al. Atrial Amyloidosis: Mechanisms and Clinical Manifestations. Eur. J. Heart Fail. 2022, 24, 2019–2028. [Google Scholar] [CrossRef]
- Nochioka, K.; Quarta, C.C.; Claggett, B.; Roca, G.Q.; Rapezzi, C.; Falk, R.H.; Solomon, S.D. Left Atrial Structure and Function in Cardiac Amyloidosis. Eur. Heart J. Cardiovasc. Imag. 2017, 18, 1128–1137. [Google Scholar] [CrossRef]
- Bandera, F.; Martone, R.; Chacko, L.; Ganesananthan, S.; Gilbertson, J.A.; Ponticos, M.; Lane, T.; Martinez-Naharro, A.; Whelan, C.; Quarta, C.; et al. Clinical Importance of Left Atrial Infiltration in Cardiac Transthyretin Amyloidosis. JACC Cardiovasc. Imaging 2022, 15, 17–29. [Google Scholar] [CrossRef]
- Lyne, J.C.; Petryka, J.; Pennell, D.J. Atrial Enhancement by Cardiovascular Magnetic Resonance in Cardiac Amyloidosis. Eur. Heart J. 2008, 29, 212. [Google Scholar] [CrossRef]
- Leeson, C.P.; Myerson, S.G.; Walls, G.B.; Neubauer, S.; Ormerod, O.J. Atrial Pathology in Cardiac Amyloidosis: Evidence from ECG and Cardiovascular Magnetic Resonance. Eur. Heart J. 2006, 27, 1670. [Google Scholar] [CrossRef]
- Fumagalli, C.; Zampieri, M.; Argiro, A.; Musumeci, B.; Tini, G.; Di Bella, G.; Cipriani, A.; Porcari, A.; Canepa, M.; Merlo, M.; et al. Incidence and Factors Associated with de Novo Atrial Fibrillation in Patients with Wild-Type Transthyretin Cardiac Amyloidosis. Eur. Heart J. 2022, 43, ehac544-1787. [Google Scholar] [CrossRef]
- Sanchis, K.; Cariou, E.; Colombat, M.; Ribes, D.; Huart, A.; Cintas, P.; Fournier, P.; Rollin, A.; Carrié, D.; Galinier, M.; et al. Atrial Fibrillation and Subtype of Atrial Fibrillation in Cardiac Amyloidosis: Clinical and Echocardiographic Features, Impact on Mortality. Amyloid 2019, 26, 128–138. [Google Scholar] [CrossRef]
- Donnellan, E.; Wazni, O.M.; Hanna, M.; Elshazly, M.B.; Puri, R.; Saliba, W.; Kanj, M.; Vakamudi, S.; Patel, D.R.; Baranowski, B.; et al. Atrial Fibrillation in Transthyretin Cardiac Amyloidosis: Predictors, Prevalence, and Efficacy of Rhythm Control Strategies. JACC Clin. Electrophysiol. 2020, 6, 1118–1127. [Google Scholar] [CrossRef]
- Plehn, J.F.; Southworth, J.; Cornwell, G.G. Brief Report: Atrial Systolic Failure in Primary Amyloidosis. N. Engl. J. Med. 1992, 327, 1570–1573. [Google Scholar] [CrossRef] [PubMed]
- Arbelo, E.; Protonotarios, A.; Gimeno, J.R.; Arbustini, E.; Barriales-Villa, R.; Basso, C.; Bezzina, C.R.; Biagini, E.; Blom, N.A.; de Boer, R.A.; et al. 2023 ESC Guidelines for the Management of Cardiomyopathies. Eur. Heart J. 2023, 44, ehad194. [Google Scholar] [CrossRef] [PubMed]
- Donnellan, E.; Elshazly, M.B.; Vakamudi, S.; Wazni, O.M.; Cohen, J.A.; Kanj, M.; Hanna, M.; Baranowski, B.; Saliba, W.; Jaber, W. No Association Between CHADS-VASc Score and Left Atrial Appendage Thrombus in Patients With Transthyretin Amyloidosis. JACC Clin. Electrophysiol. 2019, 5, 1473–1474. [Google Scholar] [CrossRef]
- Kittleson, M.M.; Maurer, M.S.; Ambardekar, A.V.; Bullock-Palmer, R.P.; Chang, P.P.; Eisen, H.J.; Nair, A.P.; Nativi-Nicolau, J.; Ruberg, F.L. Cardiac Amyloidosis: Evolving Diagnosis and Management: A Scientific Statement From the American Heart Association. Circulation 2020, 142, e7–e22. [Google Scholar] [CrossRef]
- Di Lisi, D.; Di Caccamo, L.; Damerino, G.; Portelli, M.C.; Comparato, F.; Di Stefano, V.; Brighina, F.; Corrado, E.; Galassi, A.R.; Novo, G. Effectiveness and Safety of Oral Anticoagulants in Cardiac Amyloidosis: Lights and Shadows. Curr. Probl. Cardiol. 2023, 48, 101188. [Google Scholar] [CrossRef]
- Briasoulis, A.; Kourek, C.; Papamichail, A.; Loritis, K.; Bampatsias, D.; Repasos, E.; Xanthopoulos, A.; Tsougos, E.; Paraskevaidis, I. Arrhythmias in Patients with Cardiac Amyloidosis: A Comprehensive Review on Clinical Management and Devices. J. Cardiovasc. Dev. Dis. 2023, 10, 337. [Google Scholar] [CrossRef]
- Ruberg, F.L.; Berk, J.L. Transthyretin (TTR) Cardiac Amyloidosis. Circulation 2012, 126, 1286–1300. [Google Scholar] [CrossRef]
- Yamada, S.; Yoshihisa, A.; Hijioka, N.; Kamioka, M.; Kaneshiro, T.; Yokokawa, T.; Misaka, T.; Ishida, T.; Takeishi, Y. Autonomic Dysfunction in Cardiac Amyloidosis Assessed by Heart Rate Variability and Heart Rate Turbulence. Ann. Noninvasive Electrocardiol. 2020, 25, e12749. [Google Scholar] [CrossRef] [PubMed]
- Gertz, M.A.; Skinner, M.; Connors, L.H.; Falk, R.H.; Cohen, A.S.; Kyle, R.A. Selective Binding of Nifedipine to Amyloid Fibrils. Am. J. Cardiol. 1985, 55, 1646. [Google Scholar] [CrossRef]
- Cassidy, J.T. Cardiac Amyloidosis. Two Cases with Digitalis Sensitivity. Ann. Intern. Med. 1961, 55, 989–994. [Google Scholar] [CrossRef] [PubMed]
- Assaf, A.; Mekhael, M.; Noujaim, C.; Chouman, N.; Younes, H.; Kreidieh, O.; Marrouche, N.; Donnellan, E. Conduction System Disease in Cardiac Amyloidosis. In Trends in Cardiovascular Medicine; Elsevier: Amsterdam, The Netherlands, 2023. [Google Scholar] [CrossRef]
- Barbhaiya, C.R.; Kumar, S.; Baldinger, S.H.; Michaud, G.F.; Stevenson, W.G.; Falk, R.; John, R.M. Electrophysiologic Assessment of Conduction Abnormalities and Atrial Arrhythmias Associated with Amyloid Cardiomyopathy. Heart Rhythm 2016, 13, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Tan, N.Y.; Mohsin, Y.; Hodge, D.O.; Lacy, M.Q.; Packer, D.L.; Dispenzieri, A.; Grogan, M.; Asirvatham, S.J.; Madhavan, M.; McLEOD, C.J. Catheter Ablation for Atrial Arrhythmias in Patients With Cardiac Amyloidosis. J. Cardiovasc. Electrophysiol. 2016, 27, 1167–1173. [Google Scholar] [CrossRef]
- Donnellan, E.; Wazni, O.; Kanj, M.; Elshazly, M.B.; Hussein, A.; Baranowski, B.; Hanna, M.; Patel, D.; Trulock, K.; Martyn, M.; et al. Atrial Fibrillation Ablation in Patients with Transthyretin Cardiac Amyloidosis. Eurospace 2020, 22, 259–264. [Google Scholar] [CrossRef]
- Alhassan, H.A.; Kainat, A.; Donohue, J.; Baumgartner, S.J.; Akunor, H.; Saba, S.; Jain, S.; Soman, P. Safety of Catheter Ablation Therapy for Atrial Fibrillation in Cardiac Amyloidosis. J. Am. Heart Assoc. 2023, 12, e029339. [Google Scholar] [CrossRef]
- Ullah, W.; Ruge, M.; Hajduczok, A.G.; Kochar, K.; Frisch, D.R.; Pavri, B.B.; Alvarez, R.; Rajapreyar, I.N.; Brailovsky, Y. Adverse Outcomes of Atrial Fibrillation Ablation in Heart Failure Patients with and without Cardiac Amyloidosis: A Nationwide Readmissions Database Analysis (2015–2019). Eur. Heart J. Open 2023, 3, oead026. [Google Scholar] [CrossRef]


| Author | Study Design | No. of Patients | Persistent AF % | Ablation Procedure | Occurrence of AT, % | Freedom of AF/AT, % | No. of Procedures | FU Duration, y |
|---|---|---|---|---|---|---|---|---|
| Di Donna et al. (2010) [23] | Retrospective multicenter | 61 | 43 | PVI, roof, mitral line, CTI (in 15 patients) | 15 | 67 | 1.5 | 2.4 ± 1.3 |
| Santangeli et al. (2013) [18] | Prospective multicenter | 43 | 72 | PVI, box lesion, SVC isolation, CFAE, non-PV trigger | 37 | 94 | 1.6 ± 0.7 | 1.3 (0.7–1.6) |
| Derejko et al. (2013) [19] | Prospective observational | 30 | 53 | PVI, CTI, mitral line, roof, CFAE | n.a | 53 | 1.4 | 1.9 ± 1.2 |
| Bassiouny et al. (2015) [20] | Retrospective single-center | CA 79 (54), SA 68 (46) | 42 | PVI, mitral line, roof, CTI, Cox-Maze | 8 | 46 | 1.2 | 2.9 (1.2–5) |
| Dinshaw et al. (2020) [22] | Retrospective single-center | 65 | 79 | PVI, CFAE, AT as appropriate | 38 | 60 | 1.9 ± 1.2 | 4.0 ± 2.7 |
| Castagno et al. (2021) [21] | Prospective multicenter | 116 | 63 | PVI, LA LINES, CFAE | 70 | 61 | 1.6 | 6.1 ± 3.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mistrulli, R.; Ferrera, A.; Muthukkattil, M.L.; Battistoni, A.; Gallo, G.; Barbato, E.; Spera, F.R.; Magrì, D. Atrial Fibrillation in Patients with Hypertrophic Cardiomyopathy and Cardiac Amyloidosis: From Clinical Management to Catheter Ablation Indication. J. Clin. Med. 2024, 13, 501. https://doi.org/10.3390/jcm13020501
Mistrulli R, Ferrera A, Muthukkattil ML, Battistoni A, Gallo G, Barbato E, Spera FR, Magrì D. Atrial Fibrillation in Patients with Hypertrophic Cardiomyopathy and Cardiac Amyloidosis: From Clinical Management to Catheter Ablation Indication. Journal of Clinical Medicine. 2024; 13(2):501. https://doi.org/10.3390/jcm13020501
Chicago/Turabian StyleMistrulli, Raffaella, Armando Ferrera, Melwyn Luis Muthukkattil, Allegra Battistoni, Giovanna Gallo, Emanuele Barbato, Francesco Raffaele Spera, and Damiano Magrì. 2024. "Atrial Fibrillation in Patients with Hypertrophic Cardiomyopathy and Cardiac Amyloidosis: From Clinical Management to Catheter Ablation Indication" Journal of Clinical Medicine 13, no. 2: 501. https://doi.org/10.3390/jcm13020501
APA StyleMistrulli, R., Ferrera, A., Muthukkattil, M. L., Battistoni, A., Gallo, G., Barbato, E., Spera, F. R., & Magrì, D. (2024). Atrial Fibrillation in Patients with Hypertrophic Cardiomyopathy and Cardiac Amyloidosis: From Clinical Management to Catheter Ablation Indication. Journal of Clinical Medicine, 13(2), 501. https://doi.org/10.3390/jcm13020501





