Insight on Exercise-Induced Heart Remodeling in Different Track and Field Disciplines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Transthoracic Echocardiogram
2.2. Statistical Analysis
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fagard, R. Athlete’s Heart. Heart 2003, 89, 1455–1461. [Google Scholar] [CrossRef] [PubMed]
- D’Ascenzi, F.; Pelliccia, A.; Natali, B.M.; Zacà, V.; Cameli, M.; Alvino, F.; Malandrino, A.; Palmitesta, P.; Zorzi, A.; Corrado, D.; et al. Morphological and Functional Adaptation of Left and Right Atria Induced by Training in Highly Trained Female Athletes. Circ. Cardiovasc. Imaging 2014, 7, 222–229. [Google Scholar] [CrossRef]
- D’Ascenzi, F.; Anselmi, F.; Focardi, M.; Mondillo, S. Atrial Enlargement in the Athlete’s Heart: Assessment of Atrial Function May Help Distinguish Adaptive from Pathologic Remodeling. J. Am. Soc. Echocardiogr. 2018, 31, 148–157. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, A.; Riegler, L.; Golia, E.; Cocchia, R.; Scarafile, R.; Salerno, G.; Pezzullo, E.; Nunziata, L.; Citro, R.; Cuomo, S.; et al. Range of Right Heart Measurements in Top-Level Athletes: The Training Impact. Int. J. Cardiol. 2013, 164, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Jurcut, R.; Giusca, S.; La Gerche, A.; Vasile, S.; Ginghina, C.; Voigt, J.-U. The Echocardiographic Assessment of the Right Ventricle: What to Do in 2010? Eur. J. Echocardiogr. 2010, 11, 81–96. [Google Scholar] [CrossRef] [PubMed]
- Rudski, L.G.; Lai, W.W.; Afilalo, J.; Hua, L.; Handschumacher, M.D.; Chandrasekaran, K.; Solomon, S.D.; Louie, E.K.; Schiller, N.B. Guidelines for the Echocardiographic Assessment of the Right Heart in Adults: A Report from the American Society of Echocardiography Endorsed by the European Association of Echocardiography, a Registered Branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2010, 23, 685–713; quiz 786–788. [Google Scholar] [CrossRef]
- Hauser, A.M.; Dressendorfer, R.H.; Vos, M.; Hashimoto, T.; Gordon, S.; Timmis, G.C. Symmetric Cardiac Enlargement in Highly Trained Endurance Athletes: A Two-Dimensional Echocardiographic Study. Am. Heart J. 1985, 109, 1038–1044. [Google Scholar] [CrossRef]
- Ekblom, B.; Hermansen, L. Cardiac Output in Athletes. J. Appl. Physiol. 1968, 25, 619–625. [Google Scholar] [CrossRef]
- Pelliccia, A.; Spataro, A.; Caselli, G.; Maron, B.J. Absence of Left Ventricular Wall Thickening in Athletes Engaged in Intense Power Training. Am. J. Cardiol. 1993, 72, 1048–1054. [Google Scholar] [CrossRef]
- Pelliccia, A.; Caselli, S.; Sharma, S.; Basso, C.; Bax, J.J.; Corrado, D.; D’Andrea, A.; D’Ascenzi, F.; Di Paolo, F.M.; Edvardsen, T.; et al. European Association of Preventive Cardiology (EAPC) and European Association of Cardiovascular Imaging (EACVI) Joint Position Statement: Recommendations for the Indication and Interpretation of Cardiovascular Imaging in the Evaluation of the Athlete’s Heart. Eur. Heart J. 2018, 39, 1949–1969. [Google Scholar] [CrossRef]
- Lewis, E.J.H.; McKillop, A.; Banks, L. The Morganroth Hypothesis Revisited: Endurance Exercise Elicits Eccentric Hypertrophy of the Heart. J. Physiol. 2012, 590, 2833–2834. [Google Scholar] [CrossRef] [PubMed]
- Hellsten, Y.; Nyberg, M. Cardiovascular Adaptations to Exercise Training. Compr. Physiol. 2015, 6, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Casa, D.J.; Cheuvront, S.N.; Galloway, S.D.; Shirreffs, S.M. Fluid Needs for Training, Competition, and Recovery in Track-and-Field Athletes. Int. J. Sport Nutr. Exerc. Metab. 2019, 29, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Degens, H.; Stasiulis, A.; Skurvydas, A.; Statkeviciene, B.; Venckunas, T. Physiological Comparison between Non-Athletes, Endurance, Power and Team Athletes. Eur. J. Appl. Physiol. 2019, 119, 1377–1386. [Google Scholar] [CrossRef] [PubMed]
- Bandeira-Guimarães, M.; Blanco-Rambo, E.; Vieira, A.F.; Sáez de Asteasu, M.L.; Pinto, R.S.; Izquierdo, M.; Cadore, E.L. Chronic Effects of Different Intensities of Power Training on Neuromuscular Parameters in Older People: A Systematic Review with Meta-Analysis. Sports Med. Open 2023, 9, 98. [Google Scholar] [CrossRef]
- Liu, S.; Niu, Y.; Fu, L. Metabolic Adaptations to Exercise Training. J. Sci. Sport Exerc. 2020, 2, 1–6. [Google Scholar] [CrossRef]
- Malsagova, K.A.; Kopylov, A.T.; Stepanov, A.A.; Kulikova, L.I.; Izotov, A.A.; Yurku, K.A.; Balakin, E.I.; Pustovoyt, V.I.; Kaysheva, A.L. Metabolomic and Proteomic Profiling of Athletes Performing Physical Activity under Hypoxic Conditions. Sports 2024, 12, 72. [Google Scholar] [CrossRef]
- Authors/Task Force Members; Piepoli, M.F.; Hoes, A.W.; Agewall, S.; Albus, C.; Brotons, C.; Catapano, A.L.; Cooney, M.-T.; Corrà, U.; Cosyns, B.; et al. 2016 European Guidelines on Cardiovascular Disease Prevention in Clinical Practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (Constituted by Representatives of 10 Societies and by Invited Experts): Developed with the Special Contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur. J. Prev. Cardiol. 2016, 23, NP1–NP96. [Google Scholar] [CrossRef]
- Mosteller, R.D. Simplified Calculation of Body-Surface Area. N. Engl. J. Med. 1987, 317, 1098. [Google Scholar] [CrossRef]
- Drezner, J.A.; Sharma, S.; Baggish, A.; Papadakis, M.; Wilson, M.G.; Prutkin, J.M.; Gerche, A.L.; Ackerman, M.J.; Borjesson, M.; Salerno, J.C.; et al. International Criteria for Electrocardiographic Interpretation in Athletes: Consensus Statement. Br. J. Sports Med. 2017, 51, 704–731. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef] [PubMed]
- Devereux, R.B. Detection of Left Ventricular Hypertrophy by M-Mode Echocardiography. Anatomic Validation, Standardization, and Comparison to Other Methods. Hypertens. Dallas Tex 1979 1987, 9, II19-26. [Google Scholar] [CrossRef] [PubMed]
- Mor-Avi, V.; Lang, R.M.; Badano, L.P.; Belohlavek, M.; Cardim, N.M.; Derumeaux, G.; Galderisi, M.; Marwick, T.; Nagueh, S.F.; Sengupta, P.P.; et al. Current and Evolving Echocardiographic Techniques for the Quantitative Evaluation of Cardiac Mechanics: ASE/EAE Consensus Statement on Methodology and Indications Endorsed by the Japanese Society of Echocardiography. Eur. J. Echocardiogr. J. Work. Group Echocardiogr. Eur. Soc. Cardiol. 2011, 12, 167–205. [Google Scholar] [CrossRef] [PubMed]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F.; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef] [PubMed]
- Jozwiak, M.; Mercado, P.; Teboul, J.-L.; Benmalek, A.; Gimenez, J.; Dépret, F.; Richard, C.; Monnet, X. What Is the Lowest Change in Cardiac Output That Transthoracic Echocardiography Can Detect? Crit. Care Lond. Engl. 2019, 23, 116. [Google Scholar] [CrossRef]
- Pluim, B.M.; Zwinderman, A.H.; Van Der Laarse, A.; Van Der Wall, E.E. The Athlete’s Heart: A Meta-Analysis of Cardiac Structure and Function. Circulation 2000, 101, 336–344. [Google Scholar] [CrossRef]
- Vinereanu, D.; Florescu, N.; Sculthorpe, N.; Tweddel, A.C.; Stephens, M.R.; Fraser, A.G. Left Ventricular Long-Axis Diastolic Function Is Augmented in the Hearts of Endurance-Trained Compared with Strength-Trained Athletes. Clin. Sci. 2002, 103, 249–257. [Google Scholar] [CrossRef]
- Utomi, V.; Oxborough, D.; Whyte, G.P.; Somauroo, J.; Sharma, S.; Shave, R.; Atkinson, G.; George, K. Systematic Review and Meta-Analysis of Training Mode, Imaging Modality and Body Size Influences on the Morphology and Function of the Male Athlete’s Heart. Heart 2013, 99, 1727–1733. [Google Scholar] [CrossRef]
- Morganroth, J. Comparative Left Ventricular Dimensions in Trained Athletes. Ann. Intern. Med. 1975, 82, 521. [Google Scholar] [CrossRef]
- Coates, A.M.; Cheung, C.P.; Currie, K.D.; King, T.J.; Mountjoy, M.L.; Burr, J.F. Cardiac Remodeling in Elite Aquatic Sport Athletes. Clin. J. Sport Med. Off. J. Can. Acad. Sport Med. 2022, 32, e485–e491. [Google Scholar] [CrossRef]
- Faiss, R.; Saugy, J.; Zollinger, A.; Robinson, N.; Schuetz, F.; Saugy, M.; Garnier, P.-Y. Prevalence Estimate of Blood Doping in Elite Track and Field Athletes During Two Major International Events. Front. Physiol. 2020, 11, 160. [Google Scholar] [CrossRef] [PubMed]
- Sottas, P.-E.; Robinson, N.; Fischetto, G.; Dollé, G.; Alonso, J.M.; Saugy, M. Prevalence of Blood Doping in Samples Collected from Elite Track and Field Athletes. Clin. Chem. 2011, 57, 762–769. [Google Scholar] [CrossRef] [PubMed]
| N = 140 | 100–200 mt | 400 mt | 800–1500–3000 mt | 5000–10,000 mt–Marathon | |
|---|---|---|---|---|---|
| N, (%) | 46 (32.9) | 34 (24.3) | 25 (17.9) | 35 (25) | |
| Male, n (%) | 26 (56.5) | 14 (41.8) | 13 (52) | 20 (57.1) | 0.511 |
| Age, years | 24.8 ± 3.6 | 25.6 ± 3.7 | 26 ± 3.4 | 29 ± 5.1 | 0.0001 |
| Afro-Caribbean, n (%) | 12 (26.1) | 6 (17.6) | 8 (32) | 6 (17.1) | 0.459 |
| Familiarity for CVD, n (%) | 9 (19.6) | 6 (17.6) | 2 (8) | 9 (25.7) | 0.388 |
| Weight, kg | 69.8 ± 11.5 | 64.3 ± 10.8 | 58.9 ± 9.3 | 55.9 ± 9.3 | <0.0001 |
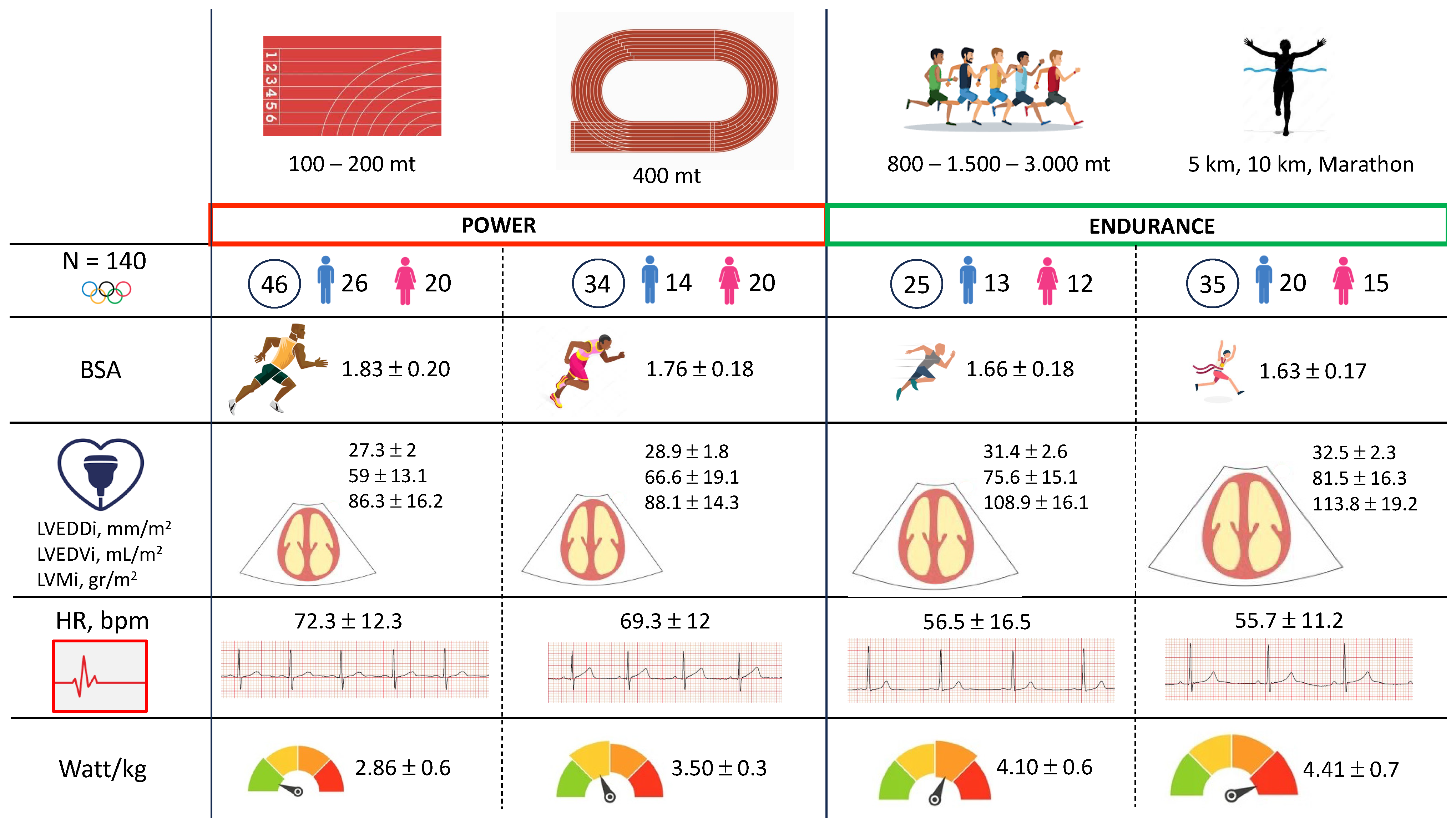
| BSA | 1.83 ± 0.20 | 1.76 ± 0.18 | 1.66 ± 0.18 | 1.63 ± 0.17 | <0.0001 |
| BMI, kg/m2 | 21.9 ± 3.1 | 20.7 ± 2.2 | 19.5 ± 1.5 | 19 ± 1.8 | <0.0001 |
| Fat mass, % | 11.4 ± 4.9 | 12.1 ± 4.4 | 12 ± 4.8 | 10.8 ± 4.9 | 0.677 |
| Training hours per week | 19.3 ± 6.2 | 18 ± 5.2 | 19.1 ± 5.7 | 22.6 ± 7.5 | 0.078 |
| N = 140 | 100–200 mt | 400 mt | 800–1500–3000 mt | 5000–10,000 mt–Marathon | |
|---|---|---|---|---|---|
| N, (%) | 46 (32.9) | 34 (24.3) | 25 (17.9) | 35 (25) | |
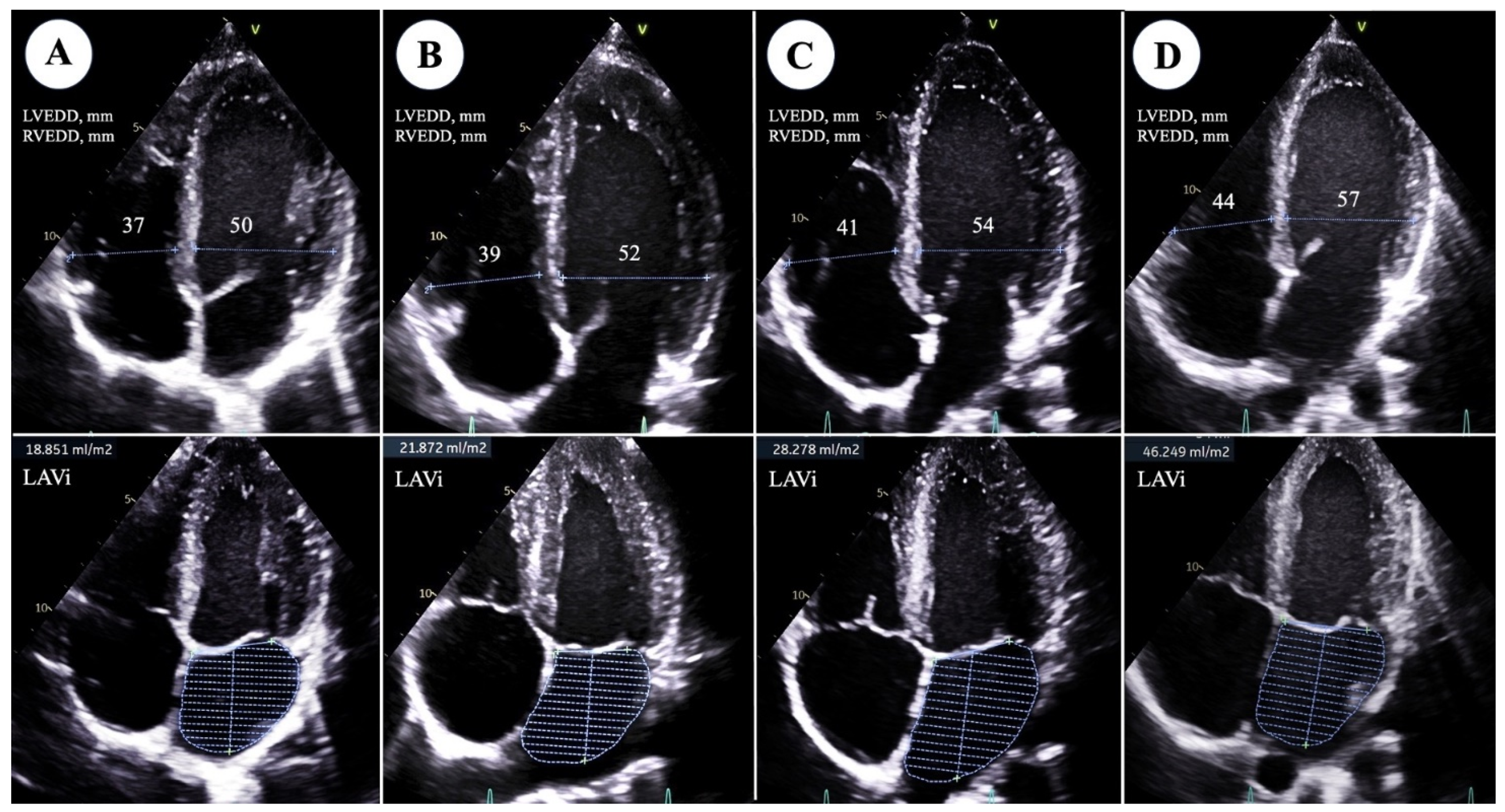
| LVEDDi, mm/m2 | 27.3 ± 2 | 28.9 ± 1.8 | 31.4 ± 2.6 | 32.5 ± 2.3 | <0.0001 |
| LVESDi, mm/m2 | 17.2 ± 1.9 | 17.8 ± 1.5 | 19.7 ± 1.9 | 19.5 ± 2.1 | <0.0001 |
| LVEDVi, mL/m2 | 59 ± 13.1 | 66.6 ± 19.1 | 75.6 ± 15.1 | 81.5 ± 16.3 | <0.0001 |
| LVESVi, mL/m2 | 21.4 ± 6.4 | 23.8 ± 8.7 | 26.7 ± 6.1 | 28.1 ± 8 | 0.0008 |
| IVS, mm | 9.3 ± 1 | 8.9 ± 1.1 | 9.6 ± 0.8 | 9.6 ± 1.1 | 0.008 |
| PWT, mm | 8.7 ± 1 | 8.6 ± 1 | 9.3 ± 0.9 | 9.3 ± 1 | 0.003 |
| LVMi, g/m2 | 86.3 ± 16.2 | 88.1 ± 14.3 | 108.9 ± 16.1 | 113.8 ± 19.2 | <0.0001 |
| EF, % | 64.3 ± 5.6 | 64.6 ± 6.1 | 63.9 ± 4.9 | 65.8 ± 5.4 | 0.587 |
| LAD, mm | 32.9 ± 3.5 | 33.5 ± 3.6 | 35.6 ± 3.4 | 36.3 ± 3.3 | <0.0001 |
| LAVi, mL/m3 | 18.1 ± 6 | 17 ± 3.1 | 22.4 ± 5.4 | 25.1 ± 9.3 | <0.0001 |
| AR, mm | 29.1 ± 3.5 | 29.1 ± 3.5 | 29.2 ± 3.3 | 29.6 ± 2.6 | 0.930 |
| AA, mm | 25.3 ± 2.9 | 25.7 ± 2 | 25.9 ± 1.8 | 27.4 ± 3.1 | 0.042 |
| E wave, cm/sec | 82.7 ± 16.1 | 86.4 ± 15.2 | 82.2 ± 12.3 | 84.1 ± 13.4 | 0.663 |
| A wave, cm/sec | 48.6 ± 10.8 | 45.3 ± 11.7 | 42.4 ± 8.5 | 43.6 ± 10.5 | 0.082 |
| E/A | 1.77 ± 0.5 | 2.03 ± 0.6 | 2 ± 0.4 | 2.03 ± 0.5 | 0.083 |
| E’, m/sec | 12.5 ± 2.4 | 12.4 ± 2.5 | 11.9 ± 1.7 | 11.7 ± 2.1 | 0.408 |
| A’, m/sec | 6.3 ± 1.3 | 6.1 ± 1 | 5.9 ± 1.2 | 6.4 ± 1.4 | 0.497 |
| S’, m/sec | 7.8 ± 1.3 | 7.8 ± 1.3 | 7.6 ± 1.4 | 8.1 ± 1.2 | 0.568 |
| E/E’ | 6.79 ± 1.5 | 7.2 ± 2 | 7 ± 1.1 | 7.3 ± 1.6 | 0.431 |
| PASP, mmHg | 22.2 ± 3.2 | 23.1 ± 3.4 | 21.1 ± 4.7 | 24 ± 4.9 | 0.049 |
| TAPSE, mm | 25.3 ± 2.8 | 26.5 ± 3 | 26.3 ± 4 | 26.4 ± 4.5 | 0.430 |
| RVEDA, mm2 | 21.1 ± 5 | 22.3 ± 5.3 | 24.2 ± 6.4 | 23 ± 4.4 | 0.204 |
| RVESA, mm2 | 11.8 ± 3 | 12.7 ± 3.1 | 12.8 ± 3.6 | 11.8 ± 2.3 | 0.502 |
| FAC, % | 43.8 ± 6.1 | 43.1 ± 8 | 47.1 ± 5.4 | 48.7 ± 7.3 | 0.008 |
| RVOTi LAX, mm2 | 15.3 ± 2.1 | 16.2 ± 2.3 | 18.2 ± 2.8 | 18.4 ± 2.5 | <0.0001 |
| RVOTi SAX, mm2 | 14.8 ± 1.9 | 15.3 ± 2.1 | 17.6 ± 2.5 | 17.6 ± 3.9 | <0.0001 |
| RVEDDi, mm2 | 18.7 ± 1.3 | 22.3 ± 1.2 | 23.6 ± 1.1 | 23.8 ± 0.4 | 0.049 |
| RAA, mm2 | 6.9 ± 1.4 | 8.1 ± 1.6 | 12.7 ± 3 | 11.6 ± 2.1 | 0.003 |
| N = 140 | 100–200 mt | 400 mt | 800–1500–3000 mt | 5000–10,000 mt–Marathon | |
|---|---|---|---|---|---|
| N, (%) | 46 (32.9) | 34 (24.3) | 25 (17.9) | 35 (25) | |
| NG, n (%) | 41 (89.1) | 31 (91.2) | 12 (48) | 11 (31.4) | <0.0001 |
| EH, n (%) | 5 (10.9) | 3 (8.8) | 13 (52) | 23 (65.7) | <0.0001 |
| CR, n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | NS |
| CH, n (%) | 0 (0) | 0 (0) | 0 (0) | 1 (2.9) | NS |
| N = 140 | 100–200 mt | 400 mt | 800–1500–3000 mt | 5000–10,000 mt–Marathon | |
|---|---|---|---|---|---|
| N, (%) | 46 (32.9) | 34 (24.3) | 25 (17.9) | 35 (25) | |
| Rest HR, bpm | 72.3 ± 12.3 | 69.3 ± 12 | 56.5 ± 16.5 | 55.7 ± 11.2 | <0.0001 |
| Peak HR, bpm | 166.6 ± 9.5 | 167 ± 8.8 | 158.5 ± 8.5 | 157.5 ± 13.9 | <0.0001 |
| Rest SBP, mmHg | 108.7 ± 10 | 105.2 ± 12.1 | 105 ± 12.2 | 106.2 ± 15.1 | 0.619 |
| Rest DPB, mmHg | 67.1 ± 7.4 | 67.8 ± 7.6 | 69.5 ± 7.1 | 66.5 ± 8 | 0.546 |
| Peak SBP, mmHg | 173.3 ± 17.9 | 171.7 ± 19.8 | 176.2 ± 19.7 | 170.3 ± 23.4 | 0.722 |
| Peak DPB, mmHg | 71.8 ± 6.2 | 72.7 ± 8.3 | 71.6 ± 7.4 | 74.6 ± 8.3 | 0.365 |
| Watt max | 205.1 ± 45.4 | 224.6 ± 43.9 | 239.6 ± 39.1 | 245.6 ± 43.4 | 0.0004 |
| Watt max/kg | 2.86 ± 0.6 | 3.50 ± 0.3 | 4.1 ± 0.6 | 4.41 ± 0.7 | <0.0001 |
| SVEB, n (%) | 0 (0) | 3 (8.8) | 1 (4) | 1 (2.8) | 0.227 |
| VEB, n (%) | 5 (10.9) | 5 (14.7) | 4 (16) | 5 (14.3) | 0.560 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Gioia, G.; Ferrera, A.; Vespasiano, F.; Maestrini, V.; Monosilio, S.; Lemme, E.; Serdoz, A.; Mango, F.; Casciani, E.; Pelliccia, A.; et al. Insight on Exercise-Induced Heart Remodeling in Different Track and Field Disciplines. J. Clin. Med. 2024, 13, 6027. https://doi.org/10.3390/jcm13206027
Di Gioia G, Ferrera A, Vespasiano F, Maestrini V, Monosilio S, Lemme E, Serdoz A, Mango F, Casciani E, Pelliccia A, et al. Insight on Exercise-Induced Heart Remodeling in Different Track and Field Disciplines. Journal of Clinical Medicine. 2024; 13(20):6027. https://doi.org/10.3390/jcm13206027
Chicago/Turabian StyleDi Gioia, Giuseppe, Armando Ferrera, Francesca Vespasiano, Viviana Maestrini, Sara Monosilio, Erika Lemme, Andrea Serdoz, Federica Mango, Emanuele Casciani, Antonio Pelliccia, and et al. 2024. "Insight on Exercise-Induced Heart Remodeling in Different Track and Field Disciplines" Journal of Clinical Medicine 13, no. 20: 6027. https://doi.org/10.3390/jcm13206027









