Management of Acute Coronary Syndrome in Elderly Patients: A Narrative Review through Decisional Crossroads
Abstract
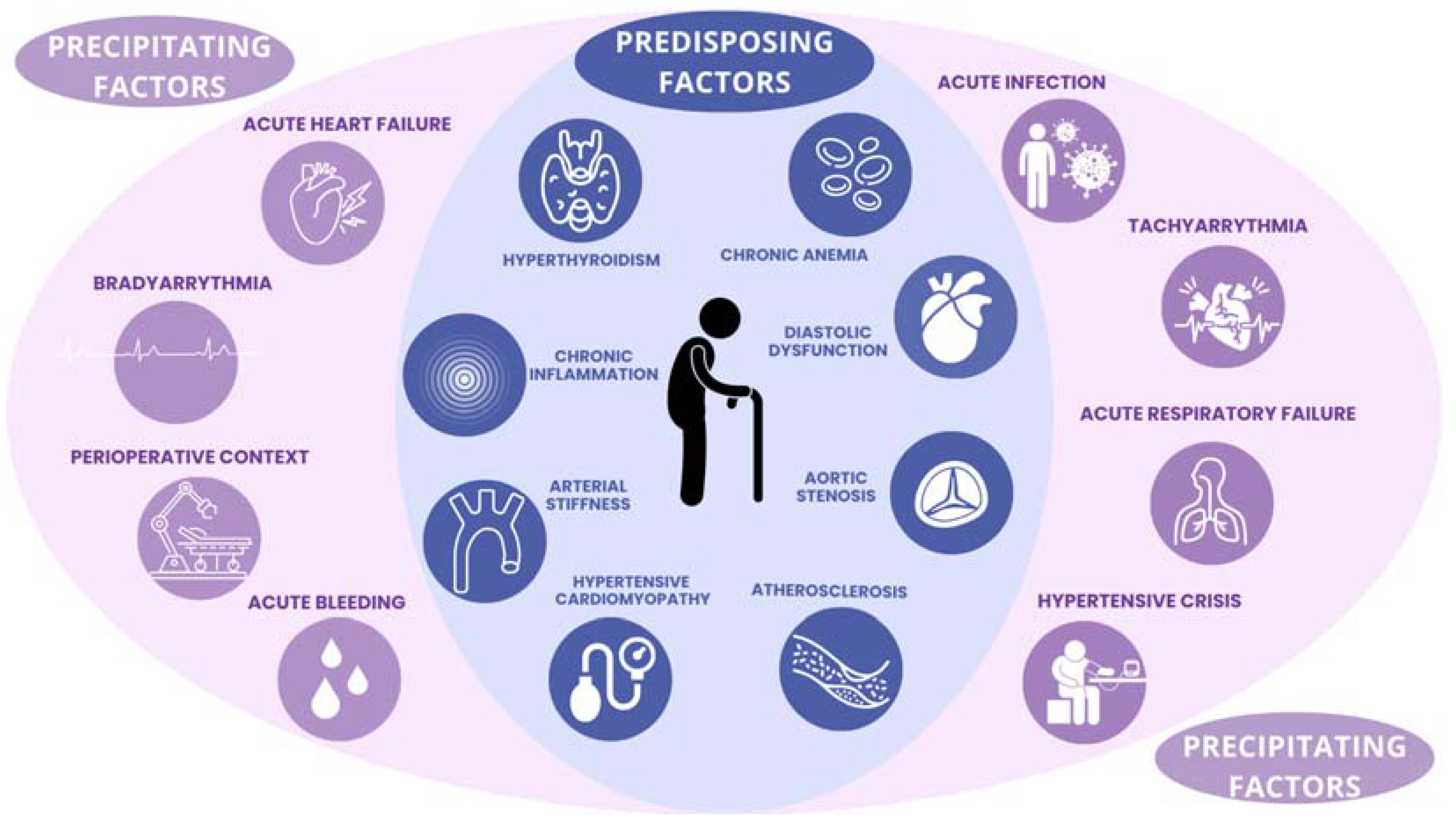
:1. Introduction
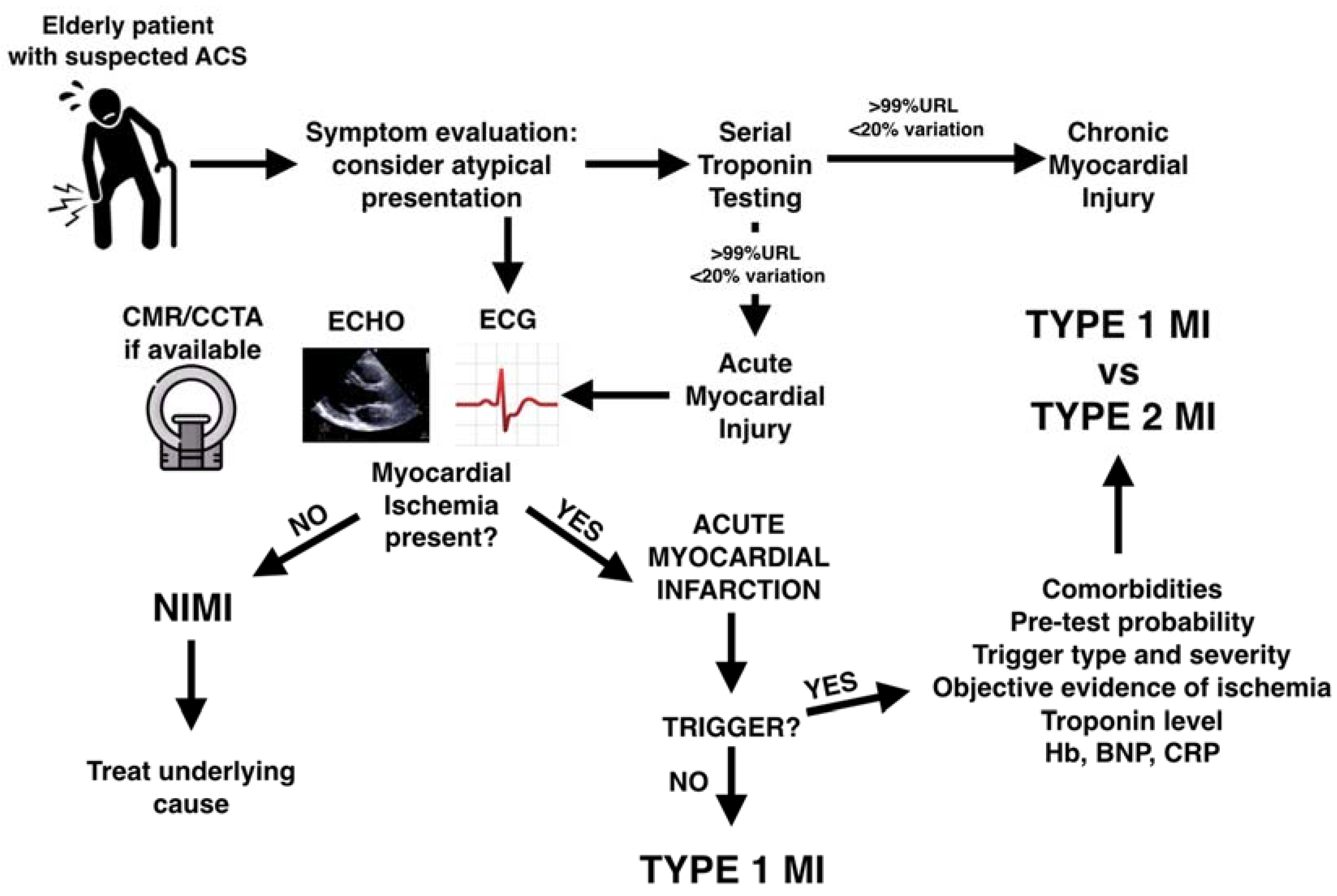
- Firstly, we focus on the interpretation of high troponin levels: from the diagnosis of MI vs. Non-Ischemic Myocardial Injury (NIMI) to the differentiation of atherothrombotic Type 1 MI (T1MI) from mismatch-related Type 2 MI (T2MI).
- We then address the evidence of invasive vs. conservative management and myocardial revascularization of culprit and non-culprit coronary lesions.
- Finally, we mention some specific issues of the medical treatment in this patient population.
2. Myocardial Infarction or Non-Ischemic Myocardial Injury?
2.1. Troponin
- The use of an age-adjusted cutoff was evaluated in a secondary analysis of the High-STEACS study with more than 45,000 patients. In patients ≥75 years, a modest improvement in specificity (82.6% versus 91.3%) and PPV (51.5% vs. 59.3%) was observed. However, this was coupled with a marked reduction in sensitivity when compared with the use of the guideline-recommended threshold (55.9% versus 81.6%) [26].
- The difference in relative and absolute troponin change at serial testing (troponin kinetics) has been identified as a potential discriminator between NIMI and MI. However, while patients with T1MI showed higher absolute and relative changes on serial sampling, T2MI and NIMI values were similar and overall discrimination was only marginally improved [30].
2.2. Non-Invasive Evaluation of Myocardial Ischemia
2.2.1. Symptoms
- On the one hand, ACS is an infrequent diagnosis in elderly patients presenting with chest pain. For example, in a nationwide study, only 3.7% of patients ≥80 years presenting with chest pain had an ACS [31].
2.2.2. ECG
2.2.3. Echocardiography
2.2.4. Cardiac Magnetic Resonance and Coronary Computed Tomography Angiography
3. Type 1 or Type 2 Myocardial Infarction?
3.1. T2MI Definition
3.2. Multivariable and Biomarker Scores
4. Invasive or Conservative Treatment?
- The After Eighty Study was, until recently, the largest RCT comparing an invasive vs. conservative approach in elderly patients with NSTEMI [80]. At 5.3 years, the invasive strategy was superior to the conservative strategy in the reduction in the composite endpoint of MI, urgent revascularization, stroke, and death, with a gain in event-free survival of 276 days. Such a result was secondary to a significant reduction in MI and urgent revascularization, while no effect was detectable on mortality.
- MOSCA FRAIL was a multicenter study of 167 NSTEMI patients with frailty (Clinical Frailty Scale score ≥ 4) and a mean age of 86 years [81]. The recent analysis of 5-year outcomes showed that a higher 1-year mortality in patients randomized to invasive treatment was followed by a later benefit.
5. Complete or Culprit-Only Revascularization after STEMI?
6. Complete or Culprit-Only Revascularization after NSTEMI?
- The risk profiles showed notable differences, as the SENIOR-RITA population consisted of frailer patients, with higher rates of comorbidities, cognitive impairments, a larger proportion of women, and lower GRACE scores compared to the FIRE-NSTEMI population.
- The timing of revascularization varied as well. In SENIOR-RITA, revascularization occurred after 5 days, while in FIRE-NSTEMI, it took place within 1 day. It is well established that earlier invasive strategies are linked to lower mortality in NSTEMI.
- Complete revascularization was achieved in 100% of patients in the experimental arm of FIRE-NSTEMI, compared to only 54% in SENIOR-RITA.
- The follow-up periods also differed, with FIRE-NSTEMI having a 1-year follow-up, whereas SENIOR-RITA extended to 4 years.
7. Medical Therapy
7.1. Dual AntiPlatelet Therapy
7.2. Lipid-Lowering Therapy
7.3. Blood Transfusion in Anemic Patients
7.4. Polypill
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pencina, M.J.; Navar, A.M.; Wojdyla, D.; Sanchez, R.J.; Khan, I.; Elassal, J.; D’Agostino, R.B.; Peterson, E.D.; Sniderman, A.D. Quantifying Importance of Major Risk Factors for Coronary Heart Disease. Circulation 2019, 139, 1603–1611. [Google Scholar] [CrossRef] [PubMed]
- Gerland, P.; Hertog, S.; Wheldon, M.C.; Kantorova, V.; Gu, D.; Gonnella, G.; Williams, I.; Zeifman, L.; Bay, G.; Castanheira, H.C.; et al. World Population Prospects 2022: Summary of Results; United Nations: San Francisco, CA, USA, 2022; pp. 3–12. [Google Scholar]
- Christensen, D.M.; Strange, J.E.; Phelps, M.; Schjerning, A.-M.; Sehested, T.S.G.; Gerds, T.; Gislason, G. Age- and Sex-Specific Trends in the Incidence of Myocardial Infarction in Denmark, 2005 to 2021. Atherosclerosis 2022, 346, 63–67. [Google Scholar] [CrossRef]
- Mensah, G.A.; Fuster, V.; Murray, C.J.L.; Roth, G.A. Global Burden of Cardiovascular Diseases and Risks Collaborators Global Burden of Cardiovascular Diseases and Risks, 1990–2022. J. Am. Coll. Cardiol. 2023, 82, 2350–2473. [Google Scholar] [CrossRef] [PubMed]
- Naghavi, M.; Abajobir, A.A.; Abbafati, C.; Abbas, K.M.; Abd-Allah, F.; Abera, S.F.; Aboyans, V.; Adetokunboh, O.; Afshin, A.; Agrawal, A.; et al. Global, Regional, and National Age-Sex Specific Mortality for 264 Causes of Death, 1980–2016: A Systematic Analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1151–1210. [Google Scholar] [CrossRef]
- Rittger, H.; Hochadel, M.; Behrens, S.; Hauptmann, K.-E.; Zahn, R.; Mudra, H.; Brachmann, J.; Senges, J.; Zeymer, U. Age-Related Differences in Diagnosis, Treatment and Outcome of Acute Coronary Syndromes: Results from the German ALKK Registry. EuroIntervention 2012, 7, 1197–1205. [Google Scholar] [CrossRef] [PubMed]
- De Luca, L.; Olivari, Z.; Bolognese, L.; Lucci, D.; Gonzini, L.; Di Chiara, A.; Casella, G.; Chiarella, F.; Boccanelli, A.; Di Pasquale, G.; et al. A Decade of Changes in Clinical Characteristics and Management of Elderly Patients with Non-ST Elevation Myocardial Infarction Admitted in Italian Cardiac Care Units. Open Heart 2014, 1, e000148. [Google Scholar] [CrossRef]
- Joseph, P.; Leong, D.; McKee, M.; Anand, S.S.; Schwalm, J.-D.; Teo, K.; Mente, A.; Yusuf, S. Reducing the Global Burden of Cardiovascular Disease, Part 1: The Epidemiology and Risk Factors. Circ. Res. 2017, 121, 677–694. [Google Scholar] [CrossRef]
- Madhavan, M.V.; Gersh, B.J.; Alexander, K.P.; Granger, C.B.; Stone, G.W. Coronary Artery Disease in Patients ≥80 Years of Age. J. Am. Coll. Cardiol. 2018, 71, 2015–2040. [Google Scholar] [CrossRef]
- Dodd, K.S.; Saczynski, J.S.; Zhao, Y.; Goldberg, R.J.; Gurwitz, J.H. Exclusion of Older Adults and Women from Recent Trials of Acute Coronary Syndromes. J. Am. Geriatr. Soc. 2011, 59, 506–511. [Google Scholar] [CrossRef]
- Díez-Villanueva, P.; Jiménez-Méndez, C.; Bonanad, C.; García-Blas, S.; Pérez-Rivera, Á.; Allo, G.; García-Pardo, H.; Formiga, F.; Camafort, M.; Martínez-Sellés, M.; et al. Risk Factors and Cardiovascular Disease in the Elderly. Rev. Cardiovasc. Med. 2022, 23, 188. [Google Scholar] [CrossRef]
- Tian, F.; Chen, L.; Qian, Z.M.; Xia, H.; Zhang, Z.; Zhang, J.; Wang, C.; Vaughn, M.G.; Tabet, M.; Lin, H. Ranking Age-Specific Modifiable Risk Factors for Cardiovascular Disease and Mortality: Evidence from a Population-Based Longitudinal Study. eClinicalMedicine 2023, 64, 102230. [Google Scholar] [CrossRef] [PubMed]
- Paradossi, U.; De Caterina, A.R.; Trimarchi, G.; Pizzino, F.; Bastiani, L.; Dossi, F.; Raccis, M.; Bianchi, G.; Palmieri, C.; de Gregorio, C.; et al. The Enigma of the “Smoker’s Paradox”: Results from a Single-Center Registry of Patients with STEMI Undergoing Primary Percutaneous Coronary Intervention. Cardiovasc. Revascularization Med. 2024. [Google Scholar] [CrossRef] [PubMed]
- Beska, B.; Ratcovich, H.; Bagnall, A.; Burrell, A.; Edwards, R.; Egred, M.; Jordan, R.; Khan, A.; Mills, G.B.; Morrison, E.; et al. Angiographic and Procedural Characteristics in Frail Older Patients with Non-ST Elevation Acute Coronary Syndrome. Interv. Cardiol. Lond. Engl. 2023, 18, e04. [Google Scholar] [CrossRef]
- Gu, S.Z.; Qiu, W.; Batty, J.A.; Sinclair, H.; Veerasamy, M.; Brugaletta, S.; Neely, D.; Ford, G.; Calvert, P.A.; Mintz, G.S.; et al. Coronary Artery Lesion Phenotype in Frail Older Patients with Non-ST-Elevation Acute Coronary Syndrome Undergoing Invasive Care. EuroIntervention 2019, 15, e261–e268. [Google Scholar] [CrossRef]
- Nanna, M.G.; Chen, S.T.; Nelson, A.J.; Navar, A.M.; Peterson, E.D. Representation of Older Adults in Cardiovascular Disease Trials Since the Inclusion Across the Lifespan Policy. JAMA Intern. Med. 2020, 180, 1531–1533. [Google Scholar] [CrossRef]
- Ungar, A.; Cherubini, A.; Fratiglioni, L.; de la Fuente-Núñez, V.; Fried, L.P.; Krasovitsky, M.S.; Tinetti, M.E.; Officer, A.; Vellas, B.; Ferrucci, L. Carta of Florence Against Ageism: No Place for Ageism in Healthcare. J. Gerontol. A Biol. Sci. Med. Sci. 2024, 79, glad264. [Google Scholar] [CrossRef]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.-A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the Management of Acute Coronary Syndromes. Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef] [PubMed]
- Damluji, A.A.; Forman, D.E.; Wang, T.Y.; Chikwe, J.; Kunadian, V.; Rich, M.W.; Young, B.A.; Page, R.L.; DeVon, H.A.; Alexander, K.P.; et al. Management of Acute Coronary Syndrome in the Older Adult Population: A Scientific Statement From the American Heart Association. Circulation 2023, 147, e32–e62. [Google Scholar] [CrossRef]
- Núñez, J.E.; Núñez, E.; Fácila, L.; Bertomeu, V.; Llàcer, À.; Bodí, V.; Sanchis, J.; Sanjuán, R.; Blasco, M.L.; Consuegra, L.; et al. Prognostic Value of Charlson Comorbidity Index at 30 Days and 1 Year After Acute Myocardial Infarction. Rev. Esp. Cardiol. Engl. Ed. 2004, 57, 842–849. [Google Scholar] [CrossRef]
- Beska, B.; Mills, G.B.; Ratcovich, H.; Wilkinson, C.; Damluji, A.A.; Kunadian, V. Impact of Multimorbidity on Long-Term Outcomes in Older Adults with Non-ST Elevation Acute Coronary Syndrome in the North East of England: A Multi-Centre Cohort Study of Patients Undergoing Invasive Care. BMJ Open 2022, 12, e061830. [Google Scholar] [CrossRef]
- Capranzano, P.; Angiolillo, D.J. Antithrombotic Management of Elderly Patients With Coronary Artery Disease. JACC Cardiovasc. Interv. 2021, 14, 723–738. [Google Scholar] [CrossRef] [PubMed]
- Alonso Salinas, G.L.; Cepas-Guillén, P.; León, A.M.; Jiménez-Méndez, C.; Lozano-Vicario, L.; Martínez-Avial, M.; Díez-Villanueva, P. The Impact of Geriatric Conditions in Elderly Patients with Coronary Heart Disease: A State-of-the-Art Review. J. Clin. Med. 2024, 13, 1891. [Google Scholar] [CrossRef] [PubMed]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D. Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction Fourth Universal Definition of Myocardial Infarction (2018). Circulation 2018, 138, e618–e651. [Google Scholar] [CrossRef] [PubMed]
- Reiter, M.; Twerenbold, R.; Reichlin, T.; Haaf, P.; Peter, F.; Meissner, J.; Hochholzer, W.; Stelzig, C.; Freese, M.; Heinisch, C.; et al. Early Diagnosis of Acute Myocardial Infarction in the Elderly Using More Sensitive Cardiac Troponin Assays. Eur. Heart J. 2011, 32, 1379–1389. [Google Scholar] [CrossRef]
- Lowry, M.T.H.; Doudesis, D.; Wereski, R.; Kimenai, D.M.; Tuck, C.; Ferry, A.V.; Bularga, A.; Taggart, C.; Lee, K.K.; Chapman, A.R.; et al. Influence of Age on the Diagnosis of Myocardial Infarction. Circulation 2022, 146, 1135–1148. [Google Scholar] [CrossRef]
- Welsh, P.; Preiss, D.; Shah, A.S.V.; McAllister, D.; Briggs, A.; Boachie, C.; McConnachie, A.; Hayward, C.; Padmanabhan, S.; Welsh, C.; et al. Comparison between High-Sensitivity Cardiac Troponin T and Cardiac Troponin I in a Large General Population Cohort. Clin. Chem. 2018, 64, 1607–1616. [Google Scholar] [CrossRef]
- Mariathas, M.; Allan, R.; Ramamoorthy, S.; Olechowski, B.; Hinton, J.; Azor, M.; Nicholas, Z.; Calver, A.; Corbett, S.; Mahmoudi, M.; et al. True 99th Centile of High Sensitivity Cardiac Troponin for Hospital Patients: Prospective, Observational Cohort Study. BMJ 2019, 364, l729. [Google Scholar] [CrossRef]
- Olivieri, F.; Galeazzi, R.; Giavarina, D.; Testa, R.; Abbatecola, A.M.; Çeka, A.; Tamburrini, P.; Busco, F.; Lazzarini, R.; Monti, D.; et al. Aged-Related Increase of High Sensitive Troponin T and Its Implication in Acute Myocardial Infarction Diagnosis of Elderly Patients. Mech. Ageing Dev. 2012, 133, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Wereski, R.; Kimenai, D.M.; Taggart, C.; Doudesis, D.; Lee, K.K.; Lowry, M.T.H.; Bularga, A.; Lowe, D.J.; Fujisawa, T.; Apple, F.S.; et al. Cardiac Troponin Thresholds and Kinetics to Differentiate Myocardial Injury and Myocardial Infarction. Circulation 2021, 144, 528–538. [Google Scholar] [CrossRef]
- Hsia, R.Y.; Hale, Z.; Tabas, J.A. A National Study of the Prevalence of Life-Threatening Diagnoses in Patients with Chest Pain. JAMA Intern. Med. 2016, 176, 1029–1032. [Google Scholar] [CrossRef]
- Tisminetzky, M.; Gurwitz, J.H.; Miozzo, R.; Nunes, A.; Gore, J.M.; Lessard, D.; Yarzebski, J.; Granillo, E.; Goldberg, R.J. Age Differences in the Chief Complaint Associated With a First Acute Myocardial Infarction and Patient’s Care-Seeking Behavior. Am. J. Med. 2020, 133, e501–e507. [Google Scholar] [CrossRef] [PubMed]
- Gregoratos, G. Clinical Manifestations of Acute Myocardial Infarction in Older Patients. Am. J. Geriatr. Cardiol. 2001, 10, 345–347. [Google Scholar] [CrossRef] [PubMed]
- Carro, A.; Kaski, J.C. Myocardial Infarction in the Elderly. Aging Dis. 2011, 2, 116–137. [Google Scholar] [PubMed]
- Wang, A.Z.; Schaffer, J.T.; Holt, D.B.; Morgan, K.L.; Hunter, B.R. Troponin Testing and Coronary Syndrome in Geriatric Patients With Nonspecific Complaints: Are We Overtesting? Acad. Emerg. Med. Off. J. Soc. Acad. Emerg. Med. 2020, 27, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, Y.; Smith, S.W.; Sexter, A.; Schulz, K.; Apple, F.S. Use of Objective Evidence of Myocardial Ischemia to Facilitate the Diagnostic and Prognostic Distinction between Type 2 Myocardial Infarction and Myocardial Injury. Eur. Heart J. Acute Cardiovasc. Care 2020, 9, 62–69. [Google Scholar] [CrossRef]
- Knott, J.D.; De Michieli, L.; Ola, O.; Akula, A.; Mehta, R.A.; Hodge, D.O.; Tak, T.; Cagin, C.; Gulati, R.; Jaffe, A.S.; et al. Diagnosis and Prognosis of Type 2 Myocardial Infarction Using Objective Evidence of Acute Myocardial Ischemia: A Validation Study. Am. J. Med. 2023, 136, 687–693.e2. [Google Scholar] [CrossRef]
- Basile, G.; Cucinotta, M.D.; Figliomeni, P.; Lo Balbo, C.; Maltese, G.; Lasco, A. Electrocardiographic Changes in Centenarians: A Study on 42 Subjects and Comparison with the Literature. Gerontology 2012, 58, 216–220. [Google Scholar] [CrossRef]
- Martínez-Sellés, M.; García de la Villa, B.; Cruz-Jentoft, A.J.; Vidán, M.T.; Gil, P.; Cornide, L.; Ramos Cortés, M.; González Guerrero, J.L.; Barros Cerviño, S.M.; Díaz Castro, Ó.; et al. Centenarians and Their Hearts: A Prospective Registry with Comprehensive Geriatric Assessment, Electrocardiogram, Echocardiography, and Follow-Up. Am. Heart J. 2015, 169, 798–805.e2. [Google Scholar] [CrossRef]
- Friedman, A.; Chudow, J.; Merritt, Z.; Shulman, E.; Fisher, J.D.; Ferrick, K.J.; Krumerman, A. Electrocardiogram Abnormalities in Older Individuals by Race and Ethnicity. J. Electrocardiol. 2020, 63, 91–93. [Google Scholar] [CrossRef]
- Pope, J.H.; Ruthazer, R.; Kontos, M.C.; Beshansky, J.R.; Griffith, J.L.; Selker, H.P. The Impact of Electrocardiographic Left Ventricular Hypertrophy and Bundle Branch Block on the Triage and Outcome of ED Patients with a Suspected Acute Coronary Syndrome: A Multicenter Study. Am. J. Emerg. Med. 2004, 22, 156–163. [Google Scholar] [CrossRef]
- Strange, G.; Stewart, S.; Playford, D. Echocardiographic Wall Motion Abnormalities and Mortality Reported in 492,338 Individuals. Eur. Heart J. 2023, 44, ehad655.052. [Google Scholar] [CrossRef]
- Playford, D.; Stewart, S.; Harris, S.A.; Chan, Y.-K.; Strange, G. Pattern and Prognostic Impact of Regional Wall Motion Abnormalities in 255 697 Men and 236 641 Women Investigated with Echocardiography. J. Am. Heart Assoc. 2023, 12, e031243. [Google Scholar] [CrossRef] [PubMed]
- Writing Committee; Kittleson, M.M.; Ruberg, F.L.; Ambardekar, A.V.; Brannagan, T.H.; Cheng, R.K.; Clarke, J.O.; Dember, L.M.; Frantz, J.G.; Hershberger, R.E.; et al. 2023 ACC Expert Consensus Decision Pathway on Comprehensive Multidisciplinary Care for the Patient With Cardiac Amyloidosis: A Report of the American College of Cardiology Solution Set Oversight Committee. J. Am. Coll. Cardiol. 2023, 81, 1076–1126. [Google Scholar] [CrossRef]
- Ghadri, J.-R.; Wittstein, I.S.; Prasad, A.; Sharkey, S.; Dote, K.; Akashi, Y.J.; Cammann, V.L.; Crea, F.; Galiuto, L.; Desmet, W.; et al. International Expert Consensus Document on Takotsubo Syndrome (Part I): Clinical Characteristics, Diagnostic Criteria, and Pathophysiology. Eur. Heart J. 2018, 39, 2032–2046. [Google Scholar] [CrossRef]
- Diamond, J.A.; Phillips, R.A. Hypertensive Heart Disease. Hypertens. Res. 2005, 28, 191–202. [Google Scholar] [CrossRef]
- Trimarchi, G.; Carerj, S.; Di Bella, G.; Manganaro, R.; Pizzino, F.; Restelli, D.; Pelaggi, G.; Lofrumento, F.; Licordari, R.; Taverna, G.; et al. Clinical Applications of Myocardial Work in Echocardiography: A Comprehensive Review. J. Cardiovasc. Echogr. 2024, 34, 99–113. [Google Scholar] [CrossRef]
- Shanmuganathan, M.; Nikolaidou, C.; Burrage, M.K.; Borlotti, A.; Kotronias, R.; Scarsini, R.; Banerjee, A.; Terentes-Printzios, D.; Pitcher, A.; Gara, E.; et al. Cardiovascular Magnetic Resonance Before Invasive Coronary Angiography in Suspected Non–ST-Segment Elevation Myocardial Infarction. JACC Cardiovasc. Imaging 2024, 17, 1044–1058. [Google Scholar] [CrossRef] [PubMed]
- Trimarchi, G.; Teresi, L.; Licordari, R.; Pingitore, A.; Pizzino, F.; Grimaldi, P.; Calabrò, D.; Liotta, P.; Micari, A.; De Gregorio, C.; et al. Transient Left Ventricular Dysfunction from Cardiomyopathies to Myocardial Viability: When and Why Cardiac Function Recovers. Biomedicines 2024, 12, 1051. [Google Scholar] [CrossRef]
- Trimarchi, G.; Pizzino, F.; Paradossi, U.; Gueli, I.A.; Palazzini, M.; Gentile, P.; Di Spigno, F.; Ammirati, E.; Garascia, A.; Tedeschi, A.; et al. Charting the Unseen: How Non-Invasive Imaging Could Redefine Cardiovascular Prevention. J. Cardiovasc. Dev. Dis. 2024, 11, 245. [Google Scholar] [CrossRef]
- Onnis, C.; Muscogiuri, G.; Cademartiri, F.; Fanni, D.; Faa, G.; Gerosa, C.; Mannelli, L.; Suri, J.S.; Sironi, S.; Montisci, R.; et al. Non-Invasive Coronary Imaging in Elderly Population. Eur. J. Radiol. 2023, 162, 110794. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D. Fourth Universal Definition of Myocardial Infarction (2018). Glob. Heart 2018, 13, 305–338. [Google Scholar] [CrossRef] [PubMed]
- Lindahl, B.; Mills, N.L. A New Clinical Classification of Acute Myocardial Infarction. Nat. Med. 2023, 29, 2200–2205. [Google Scholar] [CrossRef] [PubMed]
- de Lemos, J.A.; Newby, L.K.; Mills, N.L. A Proposal for Modest Revision of the Definition of Type 1 and Type 2 Myocardial Infarction. Circulation 2019, 140, 1773–1775. [Google Scholar] [CrossRef] [PubMed]
- DeFilippis, A.P.; Chapman, A.R.; Mills, N.L.; de Lemos, J.A.; Arbab-Zadeh, A.; Newby, L.K.; Morrow, D.A. Assessment and Treatment of Patients With Type 2 Myocardial Infarction and Acute Nonischemic Myocardial Injury. Circulation 2019, 140, 1661–1678. [Google Scholar] [CrossRef] [PubMed]
- Raphael, C.E.; Roger, V.L.; Sandoval, Y.; Singh, M.; Bell, M.; Lerman, A.; Rihal, C.S.; Gersh, B.J.; Lewis, B.; Lennon, R.J.; et al. Incidence, Trends, and Outcomes of Type 2 Myocardial Infarction in a Community Cohort. Circulation 2020, 141, 454–463. [Google Scholar] [CrossRef]
- Sarkisian, L.; Saaby, L.; Poulsen, T.S.; Gerke, O.; Jangaard, N.; Hosbond, S.; Diederichsen, A.C.P.; Thygesen, K.; Mickley, H. Clinical Characteristics and Outcomes of Patients with Myocardial Infarction, Myocardial Injury, and Nonelevated Troponins. Am. J. Med. 2016, 129, 446.e5–446.e21. [Google Scholar] [CrossRef]
- McCarthy, C.; Murphy, S.; Cohen, J.A.; Rehman, S.; Jones-O’Connor, M.; Olshan, D.S.; Singh, A.; Vaduganathan, M.; Januzzi, J.L.; Wasfy, J.H. Misclassification of Myocardial Injury as Myocardial Infarction: Implications for Assessing Outcomes in Value-Based Programs. JAMA Cardiol. 2019, 4, 460–464. [Google Scholar] [CrossRef]
- Shang, Z. Use of Delphi in Health Sciences Research: A Narrative Review. Medicine 2023, 102, e32829. [Google Scholar] [CrossRef]
- Taggart, C.; Ferry, A.; Chapman, A.R.; Bularga, A.; Wereski, R.J.; Boeddinghaus, J.; Eggers, K.; Thygesen, K.; Lindahl, B.; Mills, N.L. Consensus on the Diagnosis and Management of Patients with Type 2 Myocardial Infarction: An International Delphi Study. Eur. Heart J. 2023, 44 (Suppl. 2), ehad655.1502. [Google Scholar] [CrossRef]
- Putot, A.; Putot, S.; Chagué, F.; Cottin, Y.; Zeller, M.; Manckoundia, P. New Horizons in Type 2 Myocardial Infarction: Pathogenesis, Assessment and Management of an Emerging Geriatric Disease. Age Ageing 2022, 51, afac085. [Google Scholar] [CrossRef]
- Putot, A.; Jeanmichel, M.; Chague, F.; Manckoundia, P.; Cottin, Y.; Zeller, M. Type 2 Myocardial Infarction: A Geriatric Population-Based Model of Pathogenesis. Aging Dis. 2020, 11, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Neumann, J.T.; Sörensen, N.A.; Rübsamen, N.; Ojeda, F.; Renné, T.; Qaderi, V.; Teltrop, E.; Kramer, S.; Quantius, L.; Zeller, T.; et al. Discrimination of Patients with Type 2 Myocardial Infarction. Eur. Heart J. 2017, 38, 3514–3520. [Google Scholar] [CrossRef] [PubMed]
- Nestelberger, T.; Lopez-Ayala, P.; Boeddinghaus, J.; Strebel, I.; Rubini Gimenez, M.; Huber, I.; Wildi, K.; Wussler, D.; Koechlin, L.; Prepoudis, A.; et al. External Validation and Extension of a Clinical Score for the Discrimination of Type 2 Myocardial Infarction. J. Clin. Med. 2021, 10, 1264. [Google Scholar] [CrossRef]
- Goetze, J.P.; Bruneau, B.G.; Ramos, H.R.; Ogawa, T.; de Bold, M.K.; de Bold, A.J. Cardiac Natriuretic Peptides. Nat. Rev. Cardiol. 2020, 17, 698–717. [Google Scholar] [CrossRef]
- Nowak, R.M.; Jacobsen, G.; Christenson, R.H.; Moyer, M.; Hudson, M.; McCord, J. Differentiating Type 1 and 2 Acute Myocardial Infarctions Using the N-Terminal pro B-Type Natriuretic Peptide/Cardiac Troponin T Ratio. Am. J. Emerg. Med. 2018, 36, 1849–1854. [Google Scholar] [CrossRef]
- Pepys, M.B.; Hirschfield, G.M. C-Reactive Protein: A Critical Update. J. Clin. Investig. 2003, 111, 1805–1812. [Google Scholar] [CrossRef]
- Putot, A.; Jeanmichel, M.; Chagué, F.; Avondo, A.; Ray, P.; Manckoundia, P.; Zeller, M.; Cottin, Y. Type 1 or Type 2 Myocardial Infarction in Patients with a History of Coronary Artery Disease: Data from the Emergency Department. J. Clin. Med. 2019, 8, 2100. [Google Scholar] [CrossRef]
- Kassem, M.; Ayala, P.L.; Andric-Cancarevic, T.; Tajsic, M.; Vargas, K.G.; Bendik, D.; Kaufmann, C.; Wojta, J.; Mueller, C.; Huber, K. Copeptin for the Differentiation of Type 1 versus Type 2 Myocardial Infarction or Myocardial Injury. Int. J. Cardiol. 2024, 403, 131879. [Google Scholar] [CrossRef] [PubMed]
- Nestelberger, T.; Boeddinghaus, J.; Lopez-Ayala, P.; Kaier, T.E.; Marber, M.; Gysin, V.; Koechlin, L.; Sanchez, A.Y.; Giménez, M.R.; Wussler, D.; et al. Cardiovascular Biomarkers in the Early Discrimination of Type 2 Myocardial Infarction. JAMA Cardiol. 2021, 6, 771–780. [Google Scholar] [CrossRef]
- Neumann, J.T.; Toprak, B. Machines Running for Phenotyping of Myocardial Injury. JACC Adv. 2024, 3, 101012. [Google Scholar] [CrossRef]
- Bueno, H.; Betriu, A.; Heras, M.; Alonso, J.J.; Cequier, A.; García, E.J.; López-Sendón, J.L.; Macaya, C.; Hernández-Antolín, R.; TRIANA Investigators Primary Angioplasty, vs. Fibrinolysis in Very Old Patients with Acute Myocardial Infarction: TRIANA (TRatamiento Del Infarto Agudo de Miocardio eN Ancianos) Randomized Trial and Pooled Analysis with Previous Studies. Eur. Heart J. 2011, 32, 51–60. [Google Scholar] [CrossRef] [PubMed]
- de Boer, M.-J.; Ottervanger, J.P.; Van’t Hof, A.W.J.; Hoorntje, J.C.A.; Suryapranata, H.; Zijlstra, F.; Zwolle Myocardial Infarction Study Group. Final Benefit of Primary Percutaneous Coronary Intervention for ST-Elevation Myocardial Infarction in Older Patients: Long-Term Results of a Randomised Trial. Neth. Heart J. 2022, 30, 567–571. [Google Scholar] [CrossRef] [PubMed]
- Cepas-Guillén, P.L.; Borrego-Rodriguez, J.; Flores-Umanzor, E.; Echarte-Morales, J.; Fernandez-Valledor, A.; Menendez-Suarez, P.; Vazquez, S.; Alonso, N.; Ortiz, J.T.; Regueiro, A.; et al. Outcomes of Nonagenarians With ST Elevation Myocardial Infarction. Am. J. Cardiol. 2020, 125, 11–18. [Google Scholar] [CrossRef]
- Numasawa, Y.; Inohara, T.; Ishii, H.; Yamaji, K.; Kohsaka, S.; Sawano, M.; Kodaira, M.; Uemura, S.; Kadota, K.; Amano, T.; et al. Comparison of Outcomes After Percutaneous Coronary Intervention in Elderly Patients, Including 10 628 Nonagenarians: Insights From a Japanese Nationwide Registry (J-PCI Registry). J. Am. Heart Assoc. 2019, 8, e011183. [Google Scholar] [CrossRef] [PubMed]
- Damman, P.; Clayton, T.; Wallentin, L.; Lagerqvist, B.; Fox, K.A.A.; Hirsch, A.; Windhausen, F.; Swahn, E.; Pocock, S.J.; Tijssen, J.G.P.; et al. Effects of Age on Long-Term Outcomes after a Routine Invasive or Selective Invasive Strategy in Patients Presenting with Non-ST Segment Elevation Acute Coronary Syndromes: A Collaborative Analysis of Individual Data from the FRISC II—ICTUS—RITA-3 (FIR) Trials. Heart Br. Card. Soc. 2012, 98, 207–213. [Google Scholar] [CrossRef]
- Kotanidis, C.P.; Mills, G.B.; Bendz, B.; Berg, E.S.; Hildick-Smith, D.; Hirlekar, G.; Milasinovic, D.; Morici, N.; Myat, A.; Tegn, N.; et al. Invasive vs. Conservative Management of Older Patients with Non-ST-Elevation Acute Coronary Syndrome: Individual Patient Data Meta-Analysis. Eur. Heart J. 2024, 45, 2052–2062. [Google Scholar] [CrossRef]
- Sanchis, J.; Bueno, H.; García-Blas, S.; Alegre, O.; Martí, D.; Martínez-Sellés, M.; Domínguez-Pérez, L.; Díez-Villanueva, P.; Barrabés, J.A.; Marín, F.; et al. Invasive Treatment Strategy in Adults With Frailty and Non-ST-Segment Elevation Myocardial Infarction: A Secondary Analysis of a Randomized Clinical Trial. JAMA Netw. Open 2024, 7, e240809. [Google Scholar] [CrossRef]
- Berg, E.S.; Tegn, N.K.; Abdelnoor, M.; Røysland, K.; Ryalen, P.C.; Aaberge, L.; Eek, C.; Øie, E.; Juliebø, V.; Gjertsen, E.; et al. Long-Term Outcomes of Invasive vs Conservative Strategies for Older Patients With Non-ST-Segment Elevation Acute Coronary Syndromes. J. Am. Coll. Cardiol. 2023, 82, 2021–2030. [Google Scholar] [CrossRef]
- Tegn, N.; Abdelnoor, M.; Aaberge, L.; Endresen, K.; Smith, P.; Aakhus, S.; Gjertsen, E.; Dahl-Hofseth, O.; Ranhoff, A.H.; Gullestad, L.; et al. Invasive versus Conservative Strategy in Patients Aged 80 Years or Older with Non-ST-Elevation Myocardial Infarction or Unstable Angina Pectoris (After Eighty Study): An Open-Label Randomised Controlled Trial. Lancet Lond. Engl. 2016, 387, 1057–1065. [Google Scholar] [CrossRef]
- Sanchis, J.; Bueno, H.; Miñana, G.; Guerrero, C.; Martí, D.; Martínez-Sellés, M.; Domínguez-Pérez, L.; Díez-Villanueva, P.; Barrabés, J.A.; Marín, F.; et al. Effect of Routine Invasive vs Conservative Strategy in Older Adults With Frailty and Non–ST-Segment Elevation Acute Myocardial Infarction: A Randomized Clinical Trial. JAMA Intern. Med. 2023, 183, 407–415. [Google Scholar] [CrossRef]
- Kunadian, V.; Mossop, H.; Shields, C.; Bardgett, M.; Watts, P.; Teare, M.D.; Pritchard, J.; Adams-Hall, J.; Runnett, C.; Ripley, D.P.; et al. Invasive Treatment Strategy for Older Patients with Myocardial Infarction. N. Engl. J. Med. 2024. [Google Scholar] [CrossRef] [PubMed]
- Savonitto, S.; Cavallini, C.; Petronio, A.S.; Murena, E.; Antonicelli, R.; Sacco, A.; Steffenino, G.; Bonechi, F.; Mossuti, E.; Manari, A.; et al. Early aggressive versus initially conservative treatment in elderly patients with non-ST-segment elevation acute coronary syndrome: A randomized controlled trial. JACC Cardiovasc. Interv. 2012, 5, 906–916. [Google Scholar] [CrossRef] [PubMed]
- Sanchis, J.; Núñez, E.; Barrabés, J.A.; Marín, F.; Consuegra-Sánchez, L.; Ventura, S.; Valero, E.; Roqué, M.; Bayés-Genís, A.; Del Blanco, B.G.; et al. Randomized comparison between the invasive and conservative strategies in comorbid elderly patients with non-ST elevation myocardial infarction. Eur. J. Intern. Med. 2016, 35, 89–94. [Google Scholar] [CrossRef]
- Hirlekar, G.; Libungan, B.; Karlsson, T.; Bäck, M.; Herlitz, J.; Albertsson, P. Percutaneous coronary intervention in the very elderly with NSTE-ACS: The randomized 80+study. Scand. Cardiovasc. J. 2020, 54, 315–321. [Google Scholar] [CrossRef]
- De Belder, A.; Myat, A.; Blaxill, J.; Haworth, P.; O’Kane, P.D.; Hatrick, R.; Aggarwal, R.; Davie, A.; Smith, W.; Gerber, R.; et al. Revascularisation or medical therapy in elderly patients with acute anginal syndromes: The RINCAL randomised trial. EuroIntervention 2021, 17, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Smits, P.C.; Abdel-Wahab, M.; Neumann, F.-J.; Boxma-de Klerk, B.M.; Lunde, K.; Schotborgh, C.E.; Piroth, Z.; Horak, D.; Wlodarczak, A.; Ong, P.J.; et al. Fractional Flow Reserve-Guided Multivessel Angioplasty in Myocardial Infarction. N. Engl. J. Med. 2017, 376, 1234–1244. [Google Scholar] [CrossRef]
- Wald, D.S.; Morris, J.K.; Wald, N.J.; Chase, A.J.; Edwards, R.J.; Hughes, L.O.; Berry, C.; Oldroyd, K.G.; PRAMI Investigators. Randomized Trial of Preventive Angioplasty in Myocardial Infarction. N. Engl. J. Med. 2013, 369, 1115–1123. [Google Scholar] [CrossRef]
- Gershlick, A.H.; Khan, J.N.; Kelly, D.J.; Greenwood, J.P.; Sasikaran, T.; Curzen, N.; Blackman, D.J.; Dalby, M.; Fairbrother, K.L.; Banya, W.; et al. Randomized Trial of Complete versus Lesion-Only Revascularization in Patients Undergoing Primary Percutaneous Coronary Intervention for STEMI and Multivessel Disease: The CvLPRIT Trial. J. Am. Coll. Cardiol. 2015, 65, 963–972. [Google Scholar] [CrossRef]
- Mehta, S.R.; Wood, D.A.; Storey, R.F.; Mehran, R.; Bainey, K.R.; Nguyen, H.; Meeks, B.; Di Pasquale, G.; López-Sendón, J.; Faxon, D.P.; et al. Complete Revascularization with Multivessel PCI for Myocardial Infarction. N. Engl. J. Med. 2019, 381, 1411–1421. [Google Scholar] [CrossRef]
- Engstrøm, T.; Kelbæk, H.; Helqvist, S.; Høfsten, D.E.; Kløvgaard, L.; Holmvang, L.; Jørgensen, E.; Pedersen, F.; Saunamäki, K.; Clemmensen, P.; et al. Complete Revascularisation versus Treatment of the Culprit Lesion Only in Patients with ST-Segment Elevation Myocardial Infarction and Multivessel Disease (DANAMI-3—PRIMULTI): An Open-Label, Randomised Controlled Trial. Lancet 2015, 386, 665–671. [Google Scholar] [CrossRef]
- Böhm, F.; Mogensen, B.; Engstrøm, T.; Stankovic, G.; Srdanovic, I.; Lønborg, J.; Zwackman, S.; Hamid, M.; Kellerth, T.; Lauermann, J.; et al. FFR-Guided Complete or Culprit-Only PCI in Patients with Myocardial Infarction. N. Engl. J. Med. 2024, 390, 1481–1492. [Google Scholar] [CrossRef] [PubMed]
- Campo, G.; Böhm, F.; Engstrøm, T.; Smits, P.C.; Elgendy, I.Y.; McCann, G.; Wood, D.; Serenelli, M.; James, S.; Høfsten, D.E.; et al. Complete vs. Culprit-Only Revascularization in Older Patients with ST-Segment Elevation Myocardial Infarction: An Individual Patient Meta-Analysis. Circulation 2024. [Google Scholar] [CrossRef] [PubMed]
- Balbi, M.M.; Scarparo, P.; Tovar, M.N.; Masdjedi, K.; Daemen, J.; Den Dekker, W.; Ligthart, J.; Witberg, K.; Cummins, P.; Wilschut, J.; et al. Culprit Lesion Detection in Patients Presenting with Non-ST Elevation Acute Coronary Syndrome and Multivessel Disease. Cardiovasc. Revasc. Med. 2022, 35, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Heitner, J.F.; Senthilkumar, A.; Harrison, J.K.; Klem, I.; Sketch, M.H.; Ivanov, A.; Hamo, C.; Van Assche, L.; White, J.; Washam, J.; et al. Identifying the Infarct-Related Artery in Patients With Non–ST-Segment–Elevation Myocardial Infarction: Insights From Cardiac Magnetic Resonance Imaging. Circ. Cardiovasc. Interv. 2019, 12, e007305. [Google Scholar] [CrossRef]
- He, L.; Qin, Y.; Xu, Y.; Hu, S.; Wang, Y.; Zeng, M.; Feng, X.; Liu, Q.; Syed, I.; Demuyakor, A.; et al. Predictors of Non-Stenting Strategy for Acute Coronary Syndrome Caused by Plaque Erosion: Four-Year Outcomes of the EROSION Study. EuroIntervention 2021, 17, 497–505. [Google Scholar] [CrossRef]
- Rathod, K.S.; Koganti, S.; Jain, A.K.; Astroulakis, Z.; Lim, P.; Rakhit, R.; Kalra, S.S.; Dalby, M.C.; O’Mahony, C.; Malik, I.S.; et al. Complete Versus Culprit-Only Lesion Intervention in Patients With Acute Coronary Syndromes. J. Am. Coll. Cardiol. 2018, 72, 1989–1999. [Google Scholar] [CrossRef]
- Wang, T.Y.; McCoy, L.A.; Bhatt, D.L.; Rao, S.V.; Roe, M.T.; Resnic, F.S.; Cavender, M.A.; Messenger, J.C.; Peterson, E.D. Multivessel vs Culprit-Only Percutaneous Coronary Intervention among Patients 65 Years or Older with Acute Myocardial Infarction. Am. Heart J. 2016, 172, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Biscaglia, S.; Guiducci, V.; Escaned, J.; Moreno, R.; Lanzilotti, V.; Santarelli, A.; Cerrato, E.; Sacchetta, G.; Jurado-Roman, A.; Menozzi, A.; et al. Complete or Culprit-Only PCI in Older Patients with Myocardial Infarction. N. Engl. J. Med. 2023, 389, 889–898. [Google Scholar] [CrossRef]
- Cocco, M.; Campo, G.; Guiducci, V.; Casella, G.; Cavazza, C.; Cerrato, E.; Sacchetta, G.; Moreno, R.; Menozzi, A.; Amat Santos, I.; et al. Complete vs Culprit-Only Revascularization in Older Patients With Myocardial Infarction With or Without ST-Segment Elevation. J. Am. Coll. Cardiol. 2024. [Google Scholar] [CrossRef]
- Angiolillo, D.J.; Galli, M.; Collet, J.-P.; Kastrati, A.; O’Donoghue, M.L. Antiplatelet Therapy after Percutaneous Coronary Intervention. EuroIntervention J. Eur. Collab. Work. Group Interv. Cardiol. Eur. Soc. Cardiol. 2022, 17, e1371–e1396. [Google Scholar] [CrossRef]
- Piccolo, R.; Magnani, G.; Ariotti, S.; Gargiulo, G.; Marino, M.; Santucci, A.; Franzone, A.; Tebaldi, M.; Heg, D.; Windecker, S.; et al. Ischaemic and Bleeding Outcomes in Elderly Patients Undergoing a Prolonged versus Shortened Duration of Dual Antiplatelet Therapy after Percutaneous Coronary Intervention: Insights from the PRODIGY Randomised Trial. EuroIntervention 2017, 13, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Giustino, G.; Chieffo, A.; Palmerini, T.; Valgimigli, M.; Feres, F.; Abizaid, A.; Costa, R.A.; Hong, M.-K.; Kim, B.-K.; Jang, Y.; et al. Efficacy and Safety of Dual Antiplatelet Therapy After Complex PCI. J. Am. Coll. Cardiol. 2016, 68, 1851–1864. [Google Scholar] [CrossRef]
- Park, D.Y.; Hu, J.-R.; Jamil, Y.; Kelsey, M.D.; Jones, W.S.; Frampton, J.; Kochar, A.; Aronow, W.S.; Damluji, A.A.; Nanna, M.G. Shorter Dual Antiplatelet Therapy for Older Adults After Percutaneous Coronary Intervention: A Systematic Review and Network Meta-Analysis. JAMA Netw. Open 2024, 7, e244000. [Google Scholar] [CrossRef]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the Management of Dyslipidaemias: Lipid Modification to Reduce Cardiovascular Risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef]
- Cholesterol Treatment Trialists’ Collaboration. Efficacy and Safety of Statin Therapy in Older People: A Meta-Analysis of Individual Participant Data from 28 Randomised Controlled Trials. Lancet 2019, 393, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Fayol, A.; Schiele, F.; Ferrières, J.; Puymirat, E.; Bataille, V.; Tea, V.; Chamandi, C.; Albert, F.; Lemesle, G.; Cayla, G.; et al. Association of Use and Dose of Lipid-Lowering Therapy Post Acute Myocardial Infarction With 5-Year Survival in Older Adults. Circ. Cardiovasc. Qual. Outcomes 2024, 17, e010685. [Google Scholar] [CrossRef]
- Thompson, P.D.; Panza, G.; Zaleski, A.; Taylor, B. Statin-Associated Side Effects. J. Am. Coll. Cardiol. 2016, 67, 2395–2410. [Google Scholar] [CrossRef]
- Sabatine, M.S.; Morrow, D.A.; Giugliano, R.P.; Burton, P.B.J.; Murphy, S.A.; McCabe, C.H.; Gibson, C.M.; Braunwald, E. Association of Hemoglobin Levels with Clinical Outcomes in Acute Coronary Syndromes. Circulation 2005, 111, 2042–2049. [Google Scholar] [CrossRef] [PubMed]
- Cooper, H.A.; Rao, S.V.; Greenberg, M.D.; Rumsey, M.P.; McKenzie, M.; Alcorn, K.W.; Panza, J.A. Conservative versus Liberal Red Cell Transfusion in Acute Myocardial Infarction (the CRIT Randomized Pilot Study). Am. J. Cardiol. 2011, 108, 1108–1111. [Google Scholar] [CrossRef]
- Putot, A.; Zeller, M.; Perrin, S.; Beer, J.-C.; Ravisy, J.; Guenancia, C.; Robert, R.; Manckoundia, P.; Cottin, Y. Blood Transfusion in Elderly Patients with Acute Myocardial Infarction: Data from the RICO Survey. Am. J. Med. 2018, 131, 422–429.e4. [Google Scholar] [CrossRef]
- Carson, J.L.; Brooks, M.M.; Hébert, P.C.; Goodman, S.G.; Bertolet, M.; Glynn, S.A.; Chaitman, B.R.; Simon, T.; Lopes, R.D.; Goldsweig, A.M.; et al. Restrictive or Liberal Transfusion Strategy in Myocardial Infarction and Anemia. N. Engl. J. Med. 2023, 389, 2446–2456. [Google Scholar] [CrossRef] [PubMed]
- Bansilal, S.; Castellano, J.M.; Garrido, E.; Wei, H.G.; Freeman, A.; Spettell, C.; Garcia-Alonso, F.; Lizano, I.; Arnold, R.J.G.; Rajda, J.; et al. Assessing the Impact of Medication Adherence on Long-Term Cardiovascular Outcomes. J. Am. Coll. Cardiol. 2016, 68, 789–801. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Spertus, J.A.; Chan, P.S. Transfusion Strategy in Myocardial Infarction and Anemia. N. Engl. J. Med. 2024, 390, 960–962. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, R.; Khan, H.; Heydon, E.; Shroufi, A.; Fahimi, S.; Moore, C.; Stricker, B.; Mendis, S.; Hofman, A.; Mant, J.; et al. Adherence to Cardiovascular Therapy: A Meta-Analysis of Prevalence and Clinical Consequences. Eur. Heart J. 2013, 34, 2940–2948. [Google Scholar] [CrossRef]
- Castellano, J.M.; Pocock, S.J.; Bhatt, D.L.; Quesada, A.J.; Owen, R.; Fernandez-Ortiz, A.; Sanchez, P.L.; Marin Ortuño, F.; Vazquez Rodriguez, J.M.; Domingo-Fernández, A.; et al. Polypill Strategy in Secondary Cardiovascular Prevention. N. Engl. J. Med. 2022, 387, 967–977. [Google Scholar] [CrossRef]
| Trial | Enrollment | Population | Total Participants | Age (Median/Mean) | Sex (Women) | Comorbidities | Time of Angiography | Primary Outcome | Secondary Outcomes |
|---|---|---|---|---|---|---|---|---|---|
| Italian Elderly ACS Savonitto et al., 2012 [83] | 2008–2010 Italy | NSTEACS ≥75 years | 313 | 82 | 50% | DM2: 36.4% COPD: NA CKD: 45% Hb: 13.15 g/dL Prior Stroke: 7.9% PAD: NA Frailty: NA | 1 day § | Composite of all-cause mortality, non-fatal MI, CV rehospitalization for CV causes, disabling stroke, severe bleeding at 12 months: 27.9% invasive group vs. 34.6% conservative group; p = 0.26 | MI: 11% invasive group vs. 17% conservative group; p = 0.27 |
| After Eighty Tegn et al., 2016 [80] | 2010–2014 Norway | NSTEACS ≥80 years | 457 | 85 | 51% | DM2: 17% COPD: 9% CKD: NA Hb: NA Prior Stroke: NA PAD: 10.5% Frailty: NA | 3 days §§ | Composite of MI, urgent revascularization, stroke, and death at median 1.53 years: 41% invasive group vs. 61% conservative group; p = 0.0001 | MI: 17% invasive group vs. 30% conservative group; p = 0.001. Need for urgent revascularization: 2% invasive group vs. 11% conservative group; p = 0.001. Death from any cause: 25% invasive group vs. 27% conservative group; p = 0.53. |
| MOSCA Sanchis et al., 2016 [84] | 2012–2014 Spain | NSTEMI ≥70 years | 106 | 82 | 47% | DM2: 46% COPD: 31% CKD: 61% HB < 11 g/dL: 50% Prior stroke: NA PAD: 42% Frailty: NA | NA | Composite of all-cause mortality, recurrent MI, and readmission for revascularization or AHF at median 2.5 years: 67 patients in the invasive group vs. 56 patients in the conservative group; p = 0.877 | All-cause mortality: 42% invasive group vs. 48% conservative group (95% CI 0.387–1.225) |
| 80 + Study Hirlekar et al., 2020 [85] | 2009–2017 Sweden | NSTEACS ≥80 years | 186 | 84 | 42% | DM2: 19.3% COPD: NA CKD: 69% Hb: NA Prior Stroke: 13% PAD: 4,8% Frailty *: 15% | NA | Composite of MI, urgent revascularization, stroke, all-cause mortality, and recurrent hospitalization due to AF or HF at 12 months: 34.4% in invasive group vs. 37.4% in conservative group; p = 0.66 | All-cause mortality: 11% in invasive group vs. 15.2% in conservative group, p = 0.40. Death and/or myocardial infarction at 12 months: 22.2% in invasive group vs. 28.9% in conservative group, p = 0.31. |
| RINCAL De Belder et al., 2021 [86] | 2014–2018 United Kingdom | NSTEMI ≥80 years | 250 | 85 | 47% | DM2: 20.9% COPD: 12.5% GFR: NA Hb: NA Prior Stroke: NA PAD: 3.2% Frailty: NA | Mean 2 days § | Composite of non-fatal MI and all-cause mortality at 12 months: 18.5% invasive group vs. 22.2% conservative group; p = 0.39 | No angina at 3 months: 85.9% invasive group vs 66.4% conservative group; p = 0.001. No angina at 12 months: 78% invasive group vs. 71.3% conservative group; p = 0.25 |
| MOSCA FRAIL Sanchis et al., 2023 [81] | 2017–2021 Spain | NSTEMI ≥70 years | 167 | 86 | 53% | DM2: 55.6% COPD: NA Creatinine (mean): 1.35 mg/dL Hb: 12.4 mg/dL Prior Stroke: 17.9% PAD: 11% Frailty: mean value CFS 5.1 | NA | Number of days alive and out of hospital: 284 days invasive group vs. 312 days conservative group; p = 0.12 | Readmission due to all cardiac causes: 45.3% invasive group vs. 38.2% conservative group. Readmission due to bleeding: 8.9% invasive group vs. 2.9% conservative group (95% CI, 1.7–129; p = 0.02). |
| SENIOR RITA Kunadian et al., 2024 [82] | 2016–2023 UK | NSTEMI ≥75 years | 1518 | 82 | 45% | DM2: 30.6% COPD: 15.3% CKD: 20.7% Anemia: 50% Prior stroke: 15% PAD: 7.7% Frailty: median value CFS 3, FFIs 32% were frail | Median 2 days § 5 days §§ | CV death or non-fatal MI: 25.6% invasive group vs. 26.3% conservative group; p = 0.53. | Death from any cause or non-fatal MI: 42.4% invasive group vs. 42% conservative group; HR = 0.97; 95% CI 0.83– 1.13. Revascularization: 3.9% invasive group vs. 13.7% conservative group; HR = 0.26, 95% CI, 0.17–0.39. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verardi, R.; Iannopollo, G.; Casolari, G.; Nobile, G.; Capecchi, A.; Bruno, M.; Lanzilotti, V.; Casella, G. Management of Acute Coronary Syndrome in Elderly Patients: A Narrative Review through Decisional Crossroads. J. Clin. Med. 2024, 13, 6034. https://doi.org/10.3390/jcm13206034
Verardi R, Iannopollo G, Casolari G, Nobile G, Capecchi A, Bruno M, Lanzilotti V, Casella G. Management of Acute Coronary Syndrome in Elderly Patients: A Narrative Review through Decisional Crossroads. Journal of Clinical Medicine. 2024; 13(20):6034. https://doi.org/10.3390/jcm13206034
Chicago/Turabian StyleVerardi, Roberto, Gianmarco Iannopollo, Giulia Casolari, Giampiero Nobile, Alessandro Capecchi, Matteo Bruno, Valerio Lanzilotti, and Gianni Casella. 2024. "Management of Acute Coronary Syndrome in Elderly Patients: A Narrative Review through Decisional Crossroads" Journal of Clinical Medicine 13, no. 20: 6034. https://doi.org/10.3390/jcm13206034






