Impact of Intraoperative Blood Transfusion on Cerebral Injury in Pediatric Patients Undergoing Congenital Septal Heart Defect Surgery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Anesthetic Management
2.3. Characteristics of Cardiopulmonary Bypass
2.4. Applied Markers
2.5. Postoperative Delirium
2.6. Statistical Data Analysis
3. Results
4. Discussion
4.1. Dynamics of Cerebral Injury Markers
4.2. Dynamics of SIRS Markers
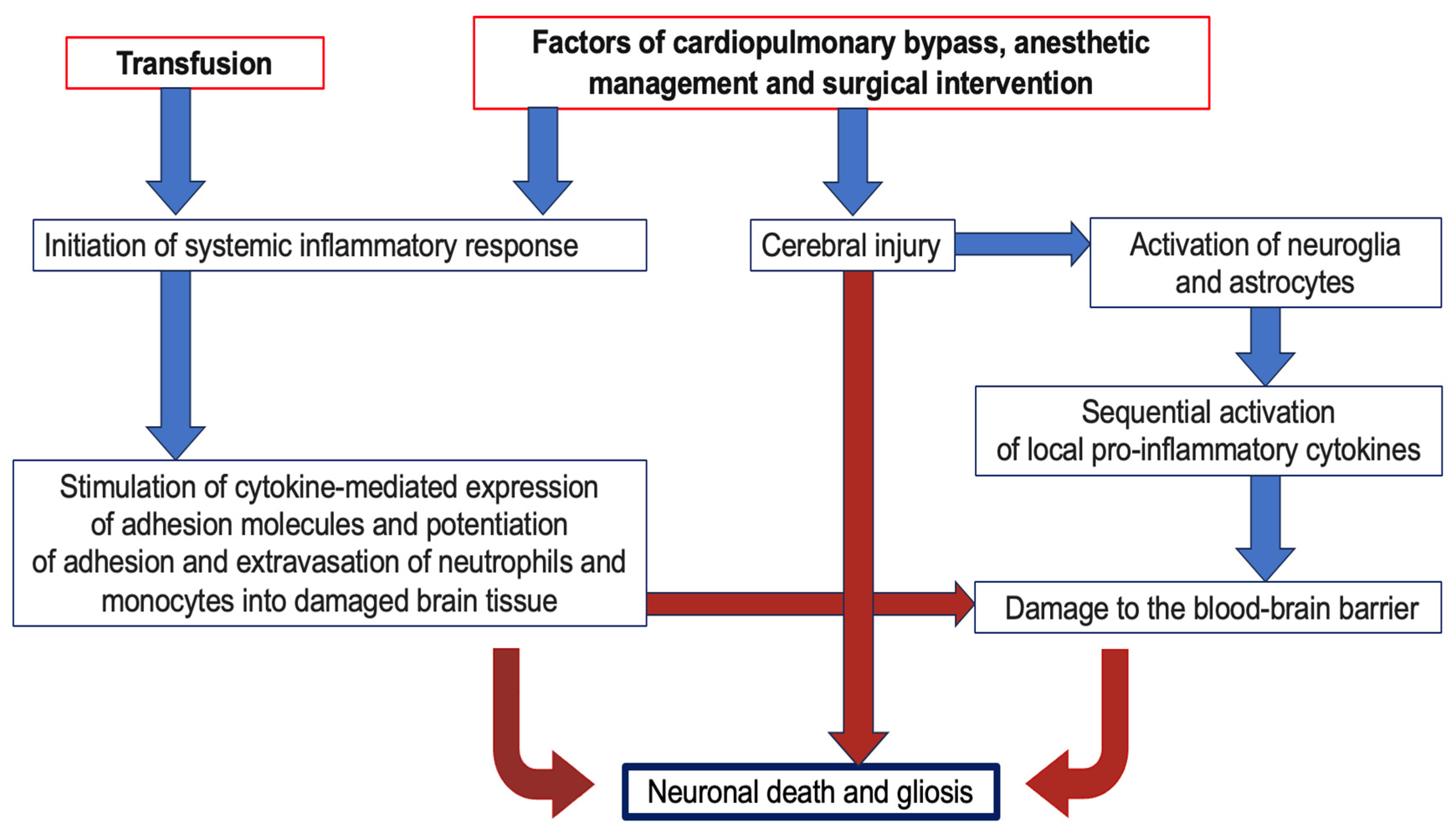
4.3. The Importance of Transfusion in the Development of Cerebral Damage
4.4. Advantage of Research
4.5. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Staveski, S.L.P.; Pickler, R.H.P.; Khoury, P.R.; Ollberding, N.J.; Donnellan, A.L.D.; Mauney, J.A.M.; Lincoln, P.A.A.; Baird, J.D.P.; Gilliland, F.L.D.; Merritt, A.D.M.; et al. Prevalence of ICU Delirium in Postoperative Pediatric Cardiac Surgery Patients. Pediatr. Crit. Care Med. 2021, 22, 68–78. [Google Scholar] [CrossRef]
- Patel, A.K.; Biagas, K.V.; Clarke, E.C.; Gerber, L.M.; Mauer, E.; Silver, G.; Chai, P.; Corda, R.; Traube, C. Delirium in Children After Cardiac Bypass Surgery. Pediatr. Crit. Care Med. 2017, 18, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, R.V.; Palmer, C.; Czaja, A.S.; Peyton, C.; Silver, G.; Traube, C.; Mourani, P.M.; Kaufman, J. Delirium is a Common and Early Finding in Patients in the Pediatric Cardiac Intensive Care Unit. J. Pediatr. 2018, 195, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Köditz, H.; Drouche, A.; Dennhardt, N.; Schmidt, M.; Schultz, M.; Schultz, B. Depth of anesthesia, temperature, and postoperative delirium in children and adolescents undergoing cardiac surgery. BMC Anesthesiol. 2023, 23, 148. [Google Scholar] [CrossRef]
- Chomat, M.R.; Said, A.S.; Mann, J.L.; Wallendorf, M.; Bickhaus, A.; Figueroa, M. Changes in Sedation Practices in Association with Delirium Screening in Infants After Cardiopulmonary Bypass. Pediatr. Cardiol. 2021, 42, 1334–1340. [Google Scholar] [CrossRef]
- Dechnik, A.; Traube, C. Delirium in hospitalised children. Lancet Child Adolesc. Health 2020, 4, 312–321. [Google Scholar] [CrossRef]
- Goldberg, T.E.; Chen, C.; Wang, Y.; Jung, E.; Swanson, A.; Ing, C.; Garcia, P.S.; Whittington, R.A.; Moitra, V. Association of Delirium With Long-term Cognitive Decline. JAMA Neurol. 2020, 77, 1373–1381. [Google Scholar] [CrossRef]
- Gunn, J.K.; Beca, J.; Hunt, R.W.; Goldsworthy, M.; Brizard, C.P.; Finucane, K.; Donath, S.; Shekerdemian, L.S. Perioperative risk factors for impaired neurodevelopment after cardiac surgery in early infancy. Arch. Dis. Child. 2016, 101, 1010–1016. [Google Scholar] [CrossRef]
- Houben, A.; Ghamari, S.; Fischer, A.; Neumann, C.; Baehner, T.; Ellerkmann, R.K. Pediatric emergence delirium is linked to increased early postoperative negative behavior within two weeks after adenoidectomy: An observational study. Braz. J. Anesthesiol. 2024, 74, 744114. [Google Scholar] [CrossRef]
- Koch, S.; Stegherr, A.-M.; Rupp, L.; Kruppa, J.; Prager, C.; Kramer, S.; Fahlenkamp, A.; Spies, C. Emergence delirium in children is not related to intraoperative burst suppression—Prospective, observational electrography study. BMC Anesthesiol. 2019, 19, 146. [Google Scholar] [CrossRef]
- Hansen, T.G. Anesthesia-related neurotoxicity and the developing animal brain is not a significant problem in children. Paediatr. Anaesth. 2015, 25, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Jevtovic-Todorovic, V. General Anesthetics and Neurotoxicity: How Much Do We Know? Anesthesiol. Clin. 2016, 34, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Dahmani, S.; Stany, I.; Brasher, C.; Lejeune, C.; Bruneau, B.; Wood, C.; Nivoche, Y.; Constant, I.; Murat, I. Pharmacological prevention of sevoflurane- and des- flurane-related emergence agitation in children: A meta-analysis of published studies. Br. J. Anaesth. 2010, 104, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Hogue, C.W., Jr.; Palin, C.A.; Arrowsmith, J.E. Cardiopulmonary bypass management and neurologic outcomes: An evidence- based appraisal of current practices. Anesth. Analg. 2006, 103, 21–37. [Google Scholar] [CrossRef]
- Hori, D.; Brown, C.; Ono, M.; Rappold, T.; Sieber, F.; Gottschalk, A.; Neufeld, K.J.; Gottesman, R.; Adachi, H.; Hogue, C.W. Arterial pressure above the upper cerebral autoregulation limit during cardiopulmonary bypass is associated with postoperative delirium. Br. J. Anaesth. 2014, 113, 1009–1017. [Google Scholar] [CrossRef]
- Hirata, Y. Cardiopulmonary bypass for pediatric cardiac surgery. Gen. Thorac. Cardiovasc. Surg. 2018, 66, 65–70. [Google Scholar] [CrossRef]
- Toomasian, C.J.; Aiello, S.R.; Drumright, B.L.; Major, T.C.; Bartlett, R.H.; Toomasian, J.M. The effect of air exposure on leucocyte and cytokine activation in an in-vitro model of cardiotomy suction. Perfusion 2018, 33, 538–545. [Google Scholar] [CrossRef]
- Myers, G.J.; Wegner, J. Endothelial Glycocalyx and Cardiopulmonary Bypass. J. ExtraCorporeal Technol. 2017, 49, 174–181. [Google Scholar] [CrossRef]
- Pozhilenkova, E.A.; Lopatina, O.L.; Komleva, Y.K.; Salmin, V.V.; Salmina, A.B. Blood-brain barrier-supported neurogenesis in healthy and diseased brain. Nat. Rev. Neurosci. 2017, 28, 397–415. [Google Scholar] [CrossRef]
- Cerejeira, J.; Firmino, H.; Vaz-Serra, A.; Mukaetova-Ladinska, E.B. The neuroinflammatory hypothesis of delirium. Acta Neuropathol. 2010, 119, 737–754. [Google Scholar] [CrossRef]
- Sun, Y.; Koyama, Y.; Shimada, S. Inflammation From Peripheral Organs to the Brain: How Does Systemic Inflammation Cause Neuroinflammation? Front. Aging Neurosci. 2022, 14, 903455. [Google Scholar] [CrossRef] [PubMed]
- Linnerbauer, M.; Wheeler, M.A.; Quintana, F.J. Astrocyte Crosstalk in CNS Inflammation. Neuron 2020, 108, 608–622. [Google Scholar] [CrossRef] [PubMed]
- Kress, B.T.; Iliff, J.J.; Xia, M.; Wang, M.; Wei, H.S.; Zeppenfeld, D.; Xie, L.; Kang, H.; Xu, Q.; Liew, J.A.; et al. Impairment of paravascular clearance pathways in the aging brain. Ann. Neurol. 2014, 76, 845–861. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.A.; Nikolic, A.; Onorati, F.; Alston, R.P. 2019 EACTS/EACTA/EBCP guidelines on cardiopulmonary bypass in in adult cardiac surgery: A tool to better clinical practice. Eur. J. Cardio-Thorac. Surg. 2020, 57, 207–209. [Google Scholar] [CrossRef]
- Ferraris, V.A.; Ballert, E.Q.; Mahan, A. The relationship between intraoperative blood transfusion and postoperative systemic inflammatory response syndrome. Am. J. Surg. 2013, 205, 457–465. [Google Scholar] [CrossRef]
- Wittenmeier, E.; Piekarski, F.; Steinbicker, A.U. Blood Product Transfusions for Children in the Perioperative Period and for Critically Ill Children. Dtsch. Arztebl. Int. 2024, 121, 58–65. [Google Scholar] [CrossRef]
- Moncharmont, P. Adverse transfusion reactions in transfused children. Transfus. Clin. Biol. 2019, 26, 329–335. [Google Scholar] [CrossRef]
- Malati, Z.A.; Pourfathollah, A.A.; Dabbaghi, R.; Balagholi, S.; Javan, M.R. Evaluation of a New Method of Leukocyte Extractions from the Leukoreduction Filter. Indian. J. Hematol. Blood Transfus. 2023, 39, 478–486. [Google Scholar] [CrossRef]
- Botwinski, C.A. Systemic inflammatory response syndrome. Neonatal Netw. 2001, 20, 21–28. [Google Scholar] [CrossRef]
- Smok, B.; Domagalski, K.; Pawłowska, M. Diagnostic and Prognostic Value of IL-6 and sTREM-1 in SIRS and Sepsis in Children. Mediat. Inflamm. 2020, 2020, 8201585. [Google Scholar] [CrossRef]
- Wiberg, S.; Holmgaard, F.; Zetterberg, H.; Nilsson, J.C.; Kjaergaard, J.; Wanscher, M.; Langkilde, A.R.; Hassager, C.; Rasmussen, L.S.; Blennow, K.; et al. Biomarkers of Cerebral Injury for Prediction of Postoperative Cognitive Dysfunction in Patients Undergoing Cardiac Surgery. J. Cardiothorac. Vasc. Anesth. 2022, 36, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Beer, C.; Blacker, D.; Bynevelt, M.; Hankey, G.J.; Puddey, I.B. Systemic markers of inflammation are independently associated with S100B concentration: Results of an observational study in subjects with acute ischaemic stroke. J. Neuroinflammation 2010, 7, 71. [Google Scholar] [CrossRef] [PubMed]
- Lasek-Bal, A.; Jedrzejowska-Szypulka, H.; Student, S.; Warsz-Wianecka, A.; Zareba, K.; Puz, P.; Bal, W.; Pawletko, K.; Lewin-Kowalik, J. The importance of selected markers of inflammation and blood-brain barrier damage for short- term ischemic stroke prognosis. J. Physiol. Pharmacol. 2019, 70, 209–217. [Google Scholar] [CrossRef]
- Kuhn, J.E.; Pareja Zabala, M.C.; Chavez, M.M.; Almodóvar, M.; Mulinari, L.A.; Sainathan, S.; de Rivero Vaccari, J.P.; Wang, K.K.; Muñoz Pareja, J.C. Utility of Brain Injury Biomarkers in Children With Congenital Heart Disease Undergoing Cardiac Surgery. Pediatr. Neurol. 2023, 148, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Silver, G.; Kearney, J.; Traube, C.; Hertzig, M. Delirium screening anchored in child development: The Cornell Assessment for Pediatric Delirium. Palliat. Support. Care 2014, 13, 1005–1011. [Google Scholar] [CrossRef]
- Sessler, C.N.; Gosnell, M.S.; Grap, M.J.; Brophy, G.M.; O’Neal, P.V.; Keane, K.A.; Tesoro, E.P.; Elswick, R.K. The Richmond Agitation-Sedation Scale: Validity and reliability in adult intensive care unit patients. Am. J. Respir. Crit. Care Med. 2002, 166, 1338–1344. [Google Scholar] [CrossRef]
- Kain, Z.N.; Mayes, L.C.; Cicchetti, D.V.; Bagnall, A.L.; Finley, J.D.; Hofstadter, M.B. The Yale Preoperative Anxiety Scale: How does it compare with a “gold standard”? Anesth. Analg. 1997, 85, 783–788. [Google Scholar] [CrossRef]
- Yuan, S.M. S100 and S100β: Biomarkers of cerebral damage in cardiac surgery with or without the use of cardiopulmonary bypass. Rev. Bras. Cir. Cardiovasc. 2014, 29, 630–641. [Google Scholar] [CrossRef]
- Rabinowicz, A.L.; Correale, J.; Boutros, R.B.; Couldwell, W.T.; Henderson, C.W.; DeGiorgio, C.M. Neuronspecific enolase is increased after single seizures during inpatient video/EEG monitoring. Epilepsia 1996, 37, 122–125. [Google Scholar] [CrossRef]
- Pekny, M.; Wilhelmsson, U.; Pekna, M. The dual role of astrocyte activation and reactive gliosis. Neurosci. Lett. 2014, 565, 30–38. [Google Scholar] [CrossRef]
- Brenner, M.; Messing, A. Regulation of GFAP Expression. ASN Neuro 2021, 13, 1759091420981206. [Google Scholar] [CrossRef] [PubMed]
- Barbu, M.; Jónsson, K.; Zetterberg, H.; Blennow, K.; Kolsrud, O.; Ricksten, S.E.; Dellgren, G.; Björk, K.; Jeppsson, A. Serum biomarkers of brain injury after uncomplicated cardiac surgery: Secondary analysis from a randomized trial. Acta Anaesthesiol. Scand. 2022, 66, 447–453. [Google Scholar] [CrossRef] [PubMed]
- DiMeglio, M.; Furey, W.; Hajj, J.; Lindekens, J.; Patel, S.; Acker, M.; Bavaria, J.; Szeto, W.Y.; Atluri, P.; Haber, M.; et al. Observational study of long-term persistent elevation of neurodegeneration markers after cardiac surgery. Sci. Rep. 2019, 9, 7177. [Google Scholar] [CrossRef] [PubMed]
- Yazdi, A.S.; Ghoreschi, K. The Interleukin-1 Family. Adv. Exp. Med. Biol. 2016, 941, 21–29. [Google Scholar] [CrossRef]
- for the PEGASUS Investigative Team; Kertai, M.D.; Ji, Y.; Li, Y.-J.; Mathew, J.P.; Daubert, J.P.; Podgoreanu, M.V. Interleukin-1β gene variants are associated with QTc interval prolongation following cardiac surgery: A prospective observational study. Can. J. Anaesth. 2016, 63, 397–410. [Google Scholar] [CrossRef]
- Ai, A.L.; Hall, D.; Bolling, S.F. Interleukin-6 and hospital length of stay after open-heart surgery. Biol. Psychiatry Psychopharmacol. 2012, 14, 79–82. [Google Scholar]
- Gu, J.; Hu, J.; Zhang, H.W.; Xiao, Z.H.; Fang, Z.; Qian, H.; Zhong, M.H.; Guo, Y.Q.; Zhang, E.Y.; Shi, Y.K.; et al. Time-dependent changes of plasma inflammatory biomarkers in type A aortic dissection patients with out optimal medical management. J. Cardiothorac. Surg. 2015, 10, 3. [Google Scholar] [CrossRef]
- Yuan, S.M. Interleukin-6 and cardiac operations. Eur. Cytokine Netw. 2018, 29, 1–15. [Google Scholar] [CrossRef]
- Allen, M.L.; Hoschtitzky, J.A.; Peters, M.J.; Elliott, M.; Goldman, A.; James, I.; Klein, N.J. Interleukin-10 and its role in clinical immunoparalysis following pediatric cardiac surgery. Crit. Care Med. 2006, 34, 2658–2665. [Google Scholar] [CrossRef]
- Kawamura, T.; Wakusawa, R.; Inada, K. Interleukin-10 and interleukin-1 receptor antagonists increase during cardiac surgery. Can. J. Anaesth. 1997, 44, 38–42. [Google Scholar] [CrossRef]
- Gorjipour, F.; Totonchi, Z.; Gholampour Dehaki, M. Serum levels of interleukin-6, interleukin-8, interleukin-10, and tumor necrosis factor-α, renal function biochemical parameters and clinical outcomes in pediatric cardiopulmonary bypass surgery. Perfusion 2019, 34, 651–659. [Google Scholar] [CrossRef] [PubMed]
- de Fontnouvelle, C.A.; Greenberg, J.H.; Thiessen-Philbrook, H.R.; Zappitelli, M.; Roth, J.; Kerr, K.F.; Devarajan, P.; Shlipak, M.; Coca, S.; Parikh, C.R.; et al. TRIBE-AKI Consortium. Interleukin-8 and Tumor Necrosis Factor Predict Acute Kidney Injury After Pediatric Cardiac Surgery. Ann. Thorac. Surg. 2017, 104, 2072–2079. [Google Scholar] [CrossRef] [PubMed]
- Delaney, M.; Stark, P.C.; Suh, M.; Triulzi, D.J.; Hess, J.R.; Steiner, M.E.; Stowell, C.P.; Sloan, S.R. The Impact of Blood Component Ratios on Clinical Outcomes and Survival. Anesth. Analg. 2017, 124, 1777–1782. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, K.; Harsh, S.; Amarjit, K. Effects of Prestorage Leukoreduction on the Rate of Febrile Nonhemolytic Transfusion Reactions to Red Blood Cells in a Tertiary Care Hospital. Ann. Med. Health Sci. Res. 2015, 5, 185–188. [Google Scholar] [CrossRef]
- Stolla, M.; Refaai, M.A.; Heal, J.M.; Spinelli, S.L.; Garraud, O.; Phipps, R.P.; Blumberg, N. Platelet transfusion—The new immunology of an old therapy. Front. Immunol. 2015, 6, 28. [Google Scholar] [CrossRef]
- Aloui, C.; Prigent, A.; Tariket, S.; Sut, C.; Fagan, J.; Cognasse, F.; Chakroun, T.; Garraud, O.; Laradi, S. Levels of human platelet-derived soluble CD40 ligand depend on haplotypes of CD40LG-CD40-ITGA2. Sci. Rep. 2016, 6, 24715. [Google Scholar] [CrossRef]
- Naguib, A.N.; Winch, P.D.; Tobias, J.D.; Simsic, J.; Hersey, D.; Nicol, K.; Preston, T.; Gomez, D.; McConnell, P.; Galantowicz, M. A single-center strategy to minimize blood transfusion in neonates and children undergoing cardiac surgery. Paediatr. Anaesth. J. 2015, 25, 477–486. [Google Scholar] [CrossRef]
- Rosa, S.D.; Bristot Mde, L.; Topanotti, M.F.; Tomasi, C.D.; Felisberto, F.; Vuolo, F.S.; Petronilho, F.; Pizzol, F.D.; Ritter, C. Effect of red blood cell transfusion on parameters of inflammation and oxidative stress in critically ill patients. Rev. Bras. Ter. Intensiv. 2011, 23, 30–35. (In English) (In Portuguese) [Google Scholar] [CrossRef] [PubMed]
- Ivkin, A.A.; Grigoriev, E.; Sinitskaya, A.V. Refraining from Packed Red Blood Cells in Cardiopulmonary Bypass Priming as a Method of Neuroprotection in Pediatric Cardiac Surgery. J. Clin. Med. 2023, 12, 1465. [Google Scholar] [CrossRef]
| Marker | Stages | ||
|---|---|---|---|
| Before the Operation | After the Completion of Cardiopulmonary Bypass | 16 h after Surgery | |
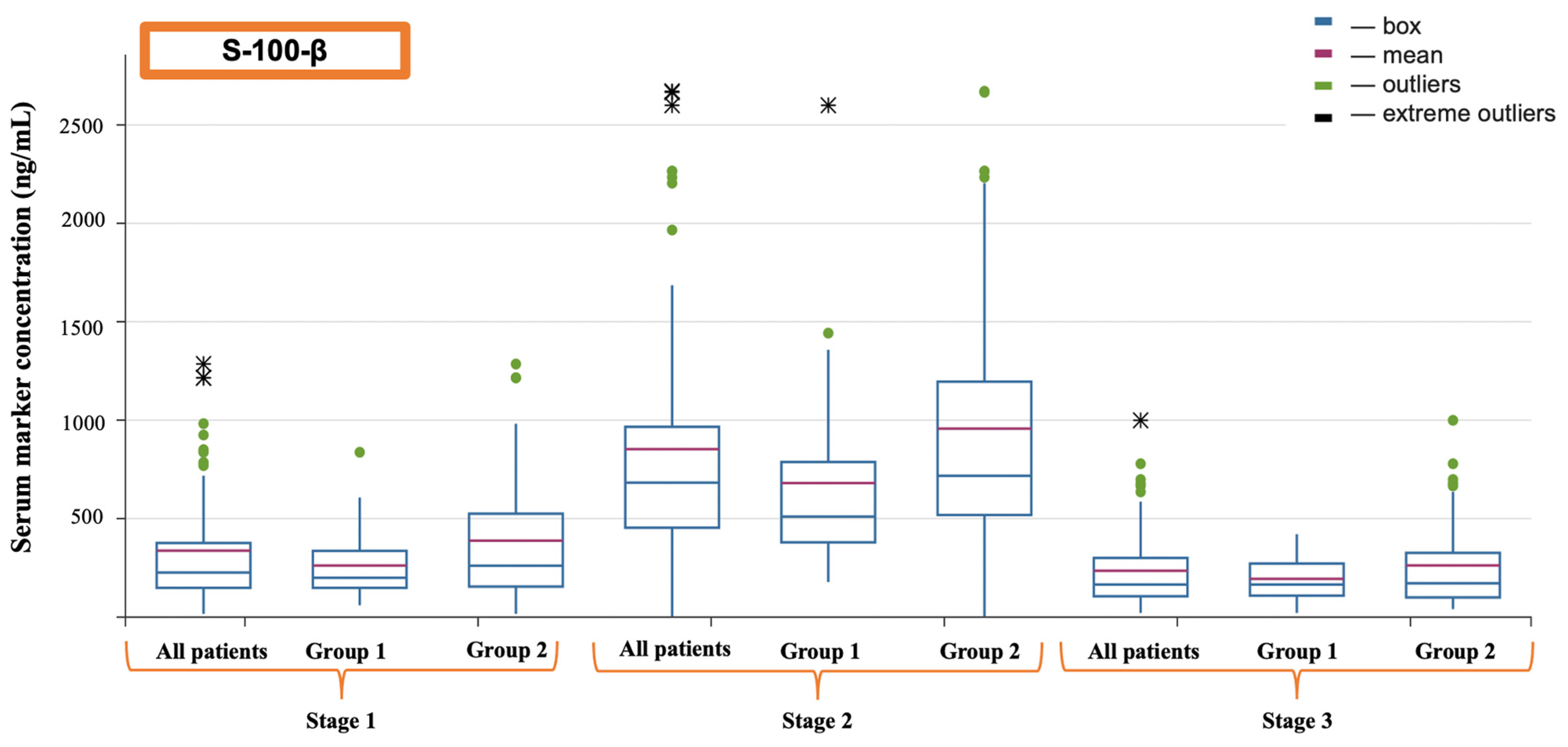
| S-100-β (ng/mL) | 226.10 (145.00–376.10) | 682.00 (453.50–965.65) | 171.40 (110.12–314.91) |
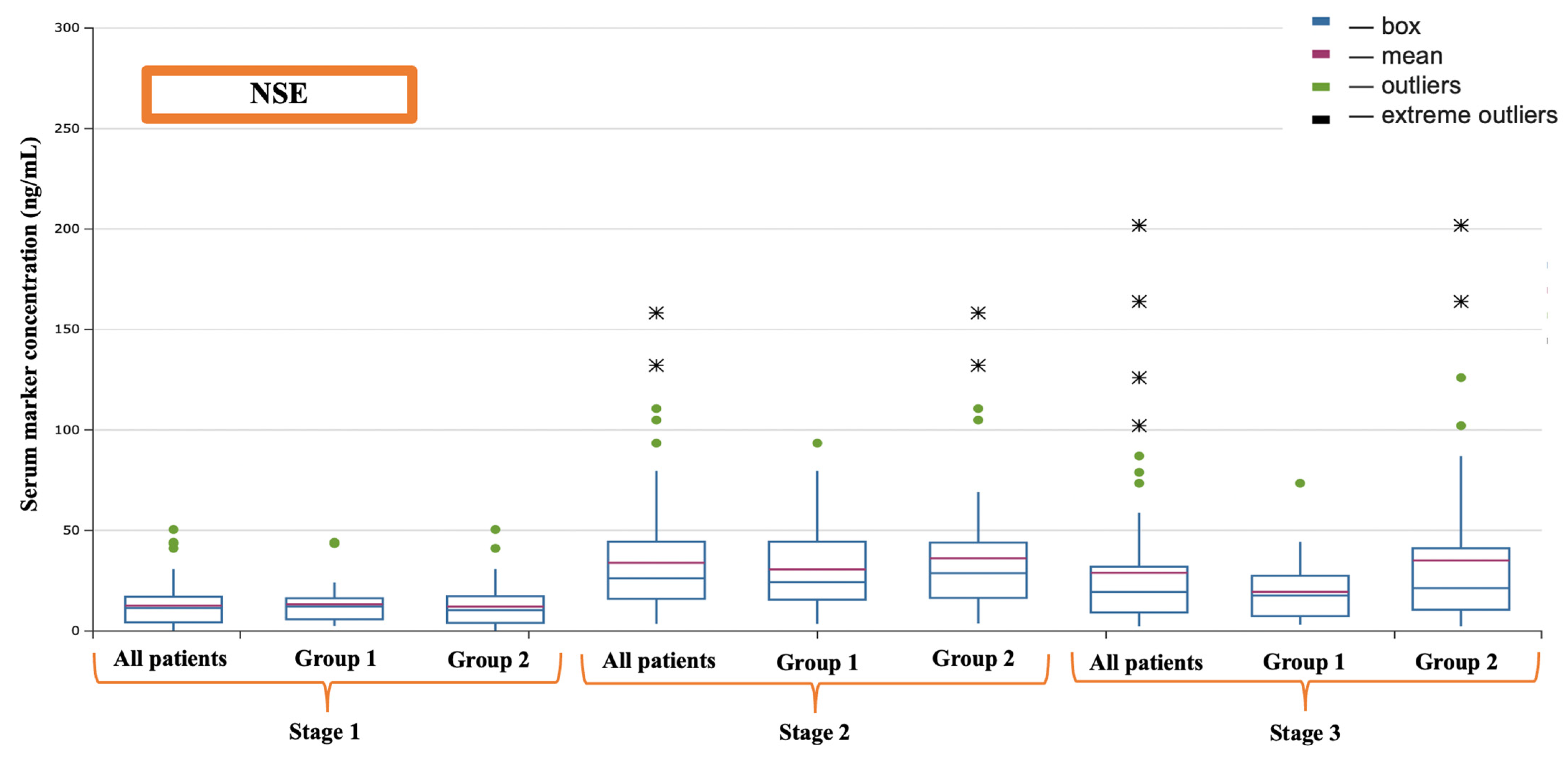
| NSE (ng/mL) | 11.42 (4.33–17.10) | 23.30 (11.19–39.64) | 19.67 (9.15–31.94) |
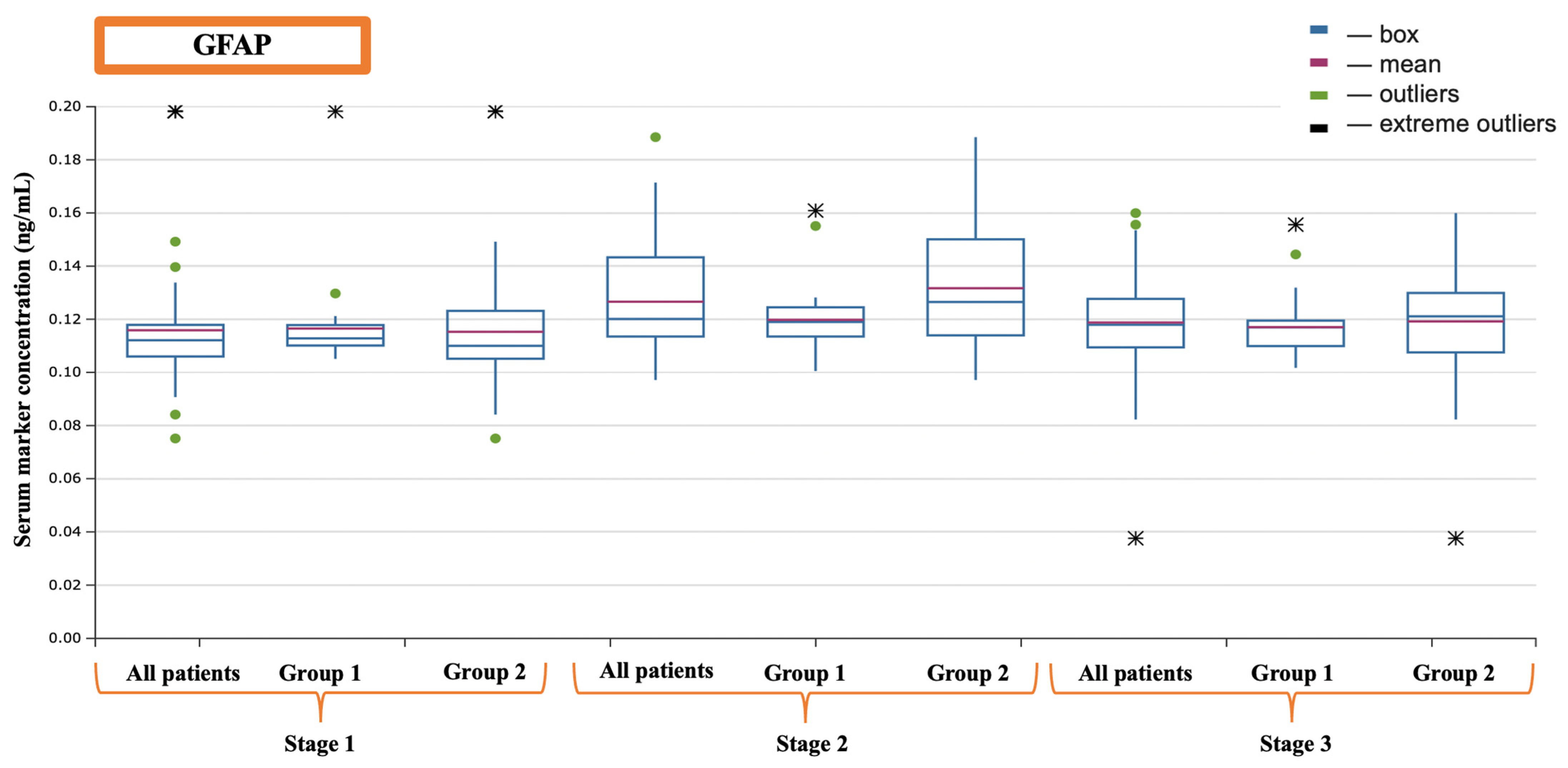
| GFAP (ng/mL) | 0.1121 (0.1061–0.1212) | 0.1215 (0.1135–0.1433) | 0.1181 (0.1095–0.1231) |
| IL-1b (pg/mL) | 3.11 (2.55–3.67) | 3.50 (3.01–4.40) | 3.17 (2.66–4.01) |
| IL-6 (pg/mL) | 2.10 (0.93–2.55) | 10.75 (3.38–23.61) | 16.18 (3.10–27.18) |
| IL-10 (pg/mL) | 0.79 (0.62–1.28) | 7.65 (3.03–13.60) | 0.94 (0.70–1.79) |
| TNF-α (pg/mL) | 1.14 (0.86–1.29) | 1.33 (1.10–2.10) | 1.13 (0.97–1.45) |
| Investigated Characteristic | Group 1 (n = 30) | Group 2 (n = 48) | p |
|---|---|---|---|
| Male, n | 11 | 20 | 0.6607 |
| Female, n | 19 | 28 | |
| Age (months) | 15 (12–28) | 11 (8–18) | 0.0064 |
| Body weight (kg) | 10.3 (8–12) | 8 (5.5–9.6) | 0.0008 |
| Height (cm) | 81 (75.75–90.5) | 71 (60.75–78.5) | 0.0002 |
| Diagnosis, n (%) | 0.1053 | ||
| ASD, n | 20 | 23 | |
| VSD, n | 10 | 25 | |
| Surgical approach, n (%) | 0.3723 | ||
| Median sternotomy | 19 | 35 | |
| Side sternotomy | 11 | 13 | |
| CPB duration (min.) | 43 (38–50) | 50 (40–63) | 0.0450 |
| Duration of aortic clamping (min.) | 27 (21–33) | 31 (25.5–42.5) | 0.0640 |
| Laboratory indicators | |||
| Hemoglobin before the operation (g/L) | 123 (115–128) | 113 (108.5–117.5) | 0.00003 |
| Hemoglobin level during the CPB (g/L) | 87 (81–91.5) | 89 (85–98.5) | 0.0528 |
| Hemoglobin level at the end of the operation (g/L) | 105 (100–110.5) | 123 (112–134) | 0.00001 |
| Venous blood saturation during the CPB (%) | 71 (67–73.25) | 69.5 (65–73.5) | 0.1990 |
| Venous blood saturation at the end of the operation (%) | 71 (68.5–75) | 70 (65–77) | 0.1891 |
| Blood lactate during the CPB (mmol/L) | 1.3 (1.1–1.55) | 1.3 (1.15–1.4) | 0.3459 |
| Blood lactate at the end of the operation (mmol/L) | 1.3 (1.15–1.5) | 1.3 (1.0–1.5) | 0.2602 |
| Monitoring indicators | |||
| rSO2 indicators before the operation (%) | 77 (72–80) | 75 (70–78) | 0.0568 |
| rSO2 indicators during the CPB (%) | 79 (77–81.25) | 77 (74–82) | 0.0835 |
| rSO2 indicators at the end of the operation (%) | 78 (75–82.5) | 77 (74–80) | 0.0894 |
| Inotropic drugs | |||
| Number of patients with inotropic drugs | 11 | 27 | 0.0923 |
| Echocardiographic data | |||
| Ejection fraction (%) | 65 (63–67) | 65 (62–67) | 0.49 |
| End-systolic size of left ventricle (mm) | 13 (11–16) | 14 (10.5–16.5) | 0.47 |
| End-diastolic size of left ventricle (mm) | 23 (19.5–30.5) | 24 (19.5–30.5) | 0.69 |
| Left atrial size (mm) | 17 (14–20) | 18 (14.75–19) | 0.43 |
| Marker | Stage | Group 1 (n = 30) | Group 2 (n = 48) | p |
|---|---|---|---|---|
| S-100-β (ng/mL) | Before the operation | 186.90 (141.70–336.00) | 260.80 (154.85–459.71) | 0.084 |
| After the completion of cardiopulmonary bypass | 509.90 (379.30–871.70) | 717.10 (517.90–1195.33) | 0.024 | |
| 16 h after surgery | 165.40 (141.00–271.90) | 183.45 (106.06–347.65) | 0.247 | |
| NSE (ng/mL) | Before the operation | 11.89 (5.90–16.31) | 10.36 (4.03–17.34) | 0.245 |
| After the completion of cardiopulmonary bypass | 17.55 (11.19–26.41) | 34.05 (17.06–44.90) | 0.023 | |
| 16 h after surgery | 17.63 (7.43–21.66) | 22.23 (10.60–41.17) | 0.044 | |
| GFAP (ng/mL) | Before the operation | 0.1128 (0.1101–0.1178) | 0.1102 (0.1052–0.1232) | 0.134 |
| After the completion of cardiopulmonary bypass | 0.1190 (0.1135–0.1245) | 0.1231 (0.1138–0.1493) | 0.035 | |
| 16 h after surgery | 0.1156 (0.1099–0.1193) | 0.1211 (0.1083–0.1299) | 0.062 | |
| IL-1b (pg/mL) | Before the operation | 2.97 (2.52–3.66) | 3.17 (2.56–3.70) | 0.436 |
| After the completion of cardiopulmonary bypass | 3.13 (2.88–3.71) | 3.88 (3.23–4.63) | 0.011 | |
| 16 h after surgery | 3.04 (2.64–3.49) | 3.3 (2.71–4.06) | 0.060 | |
| IL-6 (pg/mL) | Before the operation | 2.14 (1.08–2.47) | 2.11 (0.89–2.63) | 0.451 |
| After the completion of cardiopulmonary bypass | 12.28 (3.06–17.41) | 9.07 (3.43–27.16) | 0.231 | |
| 16 h after surgery | 11.92 (2.21–18.88) | 18.97 (4.45–38.25) | 0.021 | |
| IL-10 (pg/mL) | Before the operation | 0.66 (0.59–1.04) | 0.88 (0.62–1.29) | 0.11 |
| After the completion of cardiopulmonary bypass | 6.32 (2.71–12.20) | 8.29 (3.55–15.95) | 0.123 | |
| 16 h after surgery | 0.82 (0.62–1.46) | 1.03 (0.45–1.89) | 0.018 | |
| TNF-α (pg/mL) | Before the operation | 1.19 (0.93–1.28) | 1.04 (0.83–1.28) | 0.126 |
| After the completion of cardiopulmonary bypass | 1.26 (1.04–1.34) | 1.52 (1.15–12.22) | 0.011 | |
| 16 h after surgery | 1.11 (0.98–1.24) | 1.18 (1.01–1.95) | 0.104 |
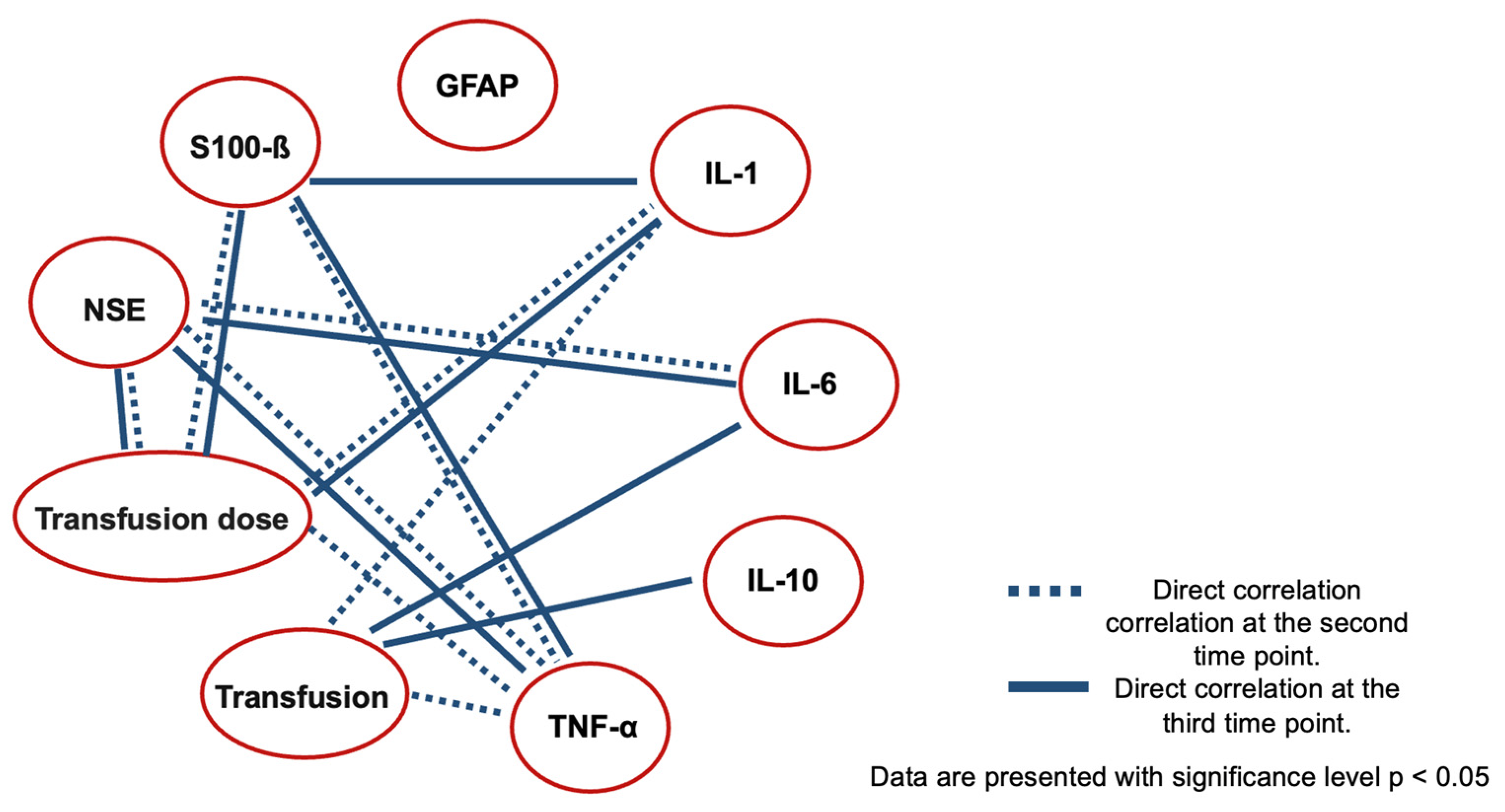
| Correlation of Markers at the Second Stage | |||
|---|---|---|---|
| S-100-β Protein | NSE | GFAP | |
| IL-1b | 0.14176 (p = 0.21569) | −0.14474 (p = 0.20611) | 0.03819 (p = 0.73990) |
| IL-6 | 0.09605 (p = 0.40284) | 0.35 (p = 0.00206) * | −0.04037 (p = 0.72566) |
| IL-10 | −0.00108 (p = 0.99250) | 0.05147 (p = 0.65450) | 0.02675 (p = 0.81616) |
| TNF-α | 0.23 (p = 0.04762) * | 0.30 (p = 0.0071) * | 0.06155 (p = 0.59244) |
| Correlation of markers at the third stage | |||
| S-100-β protein | NSE | GFAP | |
| IL-1b | 0.32 (p = 0.00474) * | −0.15412 (p = 0.17789) | 0.15316 (p = 0.18066) |
| IL-6 | 0.03485 (p = 0.76193) | 0.50 (p = 0.00001) * | 0.11572 (p = 0.31302) |
| IL-10 | 0.12187 (p = 0.28782) | −0.04421 (p = 0.70072) | 0.10806 (p = 0.34632) |
| TNF-α | 0.28 (p = 0.00474) * | 0.43 (p = 0.00010) * | 0.21933 (p = 0.05530) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivkin, A.; Grigoriev, E.; Mikhailova, A. Impact of Intraoperative Blood Transfusion on Cerebral Injury in Pediatric Patients Undergoing Congenital Septal Heart Defect Surgery. J. Clin. Med. 2024, 13, 6050. https://doi.org/10.3390/jcm13206050
Ivkin A, Grigoriev E, Mikhailova A. Impact of Intraoperative Blood Transfusion on Cerebral Injury in Pediatric Patients Undergoing Congenital Septal Heart Defect Surgery. Journal of Clinical Medicine. 2024; 13(20):6050. https://doi.org/10.3390/jcm13206050
Chicago/Turabian StyleIvkin, Artem, Evgeny Grigoriev, and Alena Mikhailova. 2024. "Impact of Intraoperative Blood Transfusion on Cerebral Injury in Pediatric Patients Undergoing Congenital Septal Heart Defect Surgery" Journal of Clinical Medicine 13, no. 20: 6050. https://doi.org/10.3390/jcm13206050






