Advanced Lung Cancer Inflammation Index as Predictor of All-Cause Mortality in ST-Elevation Myocardial Infarction Patients Undergoing Primary Percutaneous Coronary Intervention
Abstract
:1. Introduction
2. Materials and Methods
Statistical Analysis
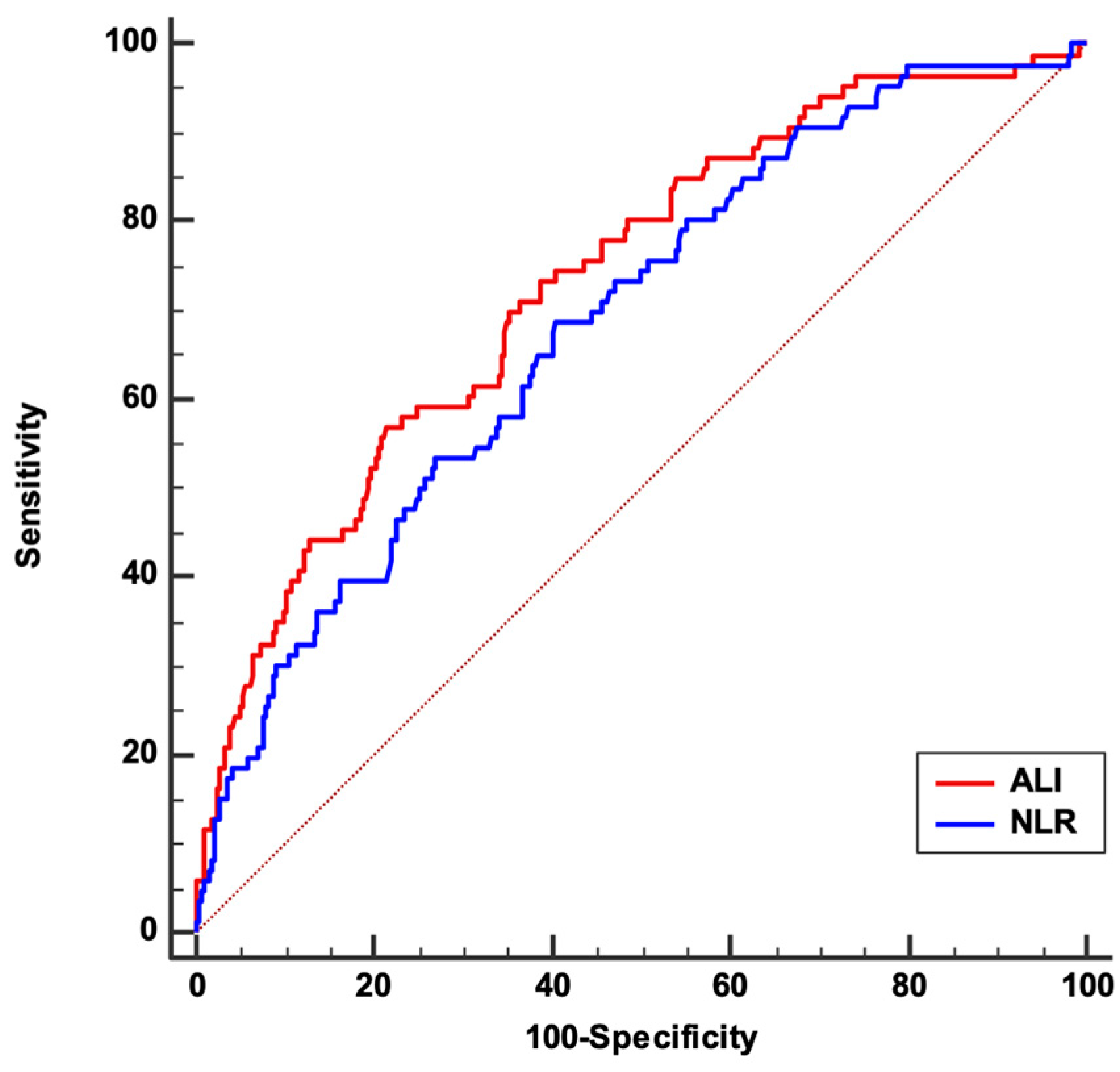
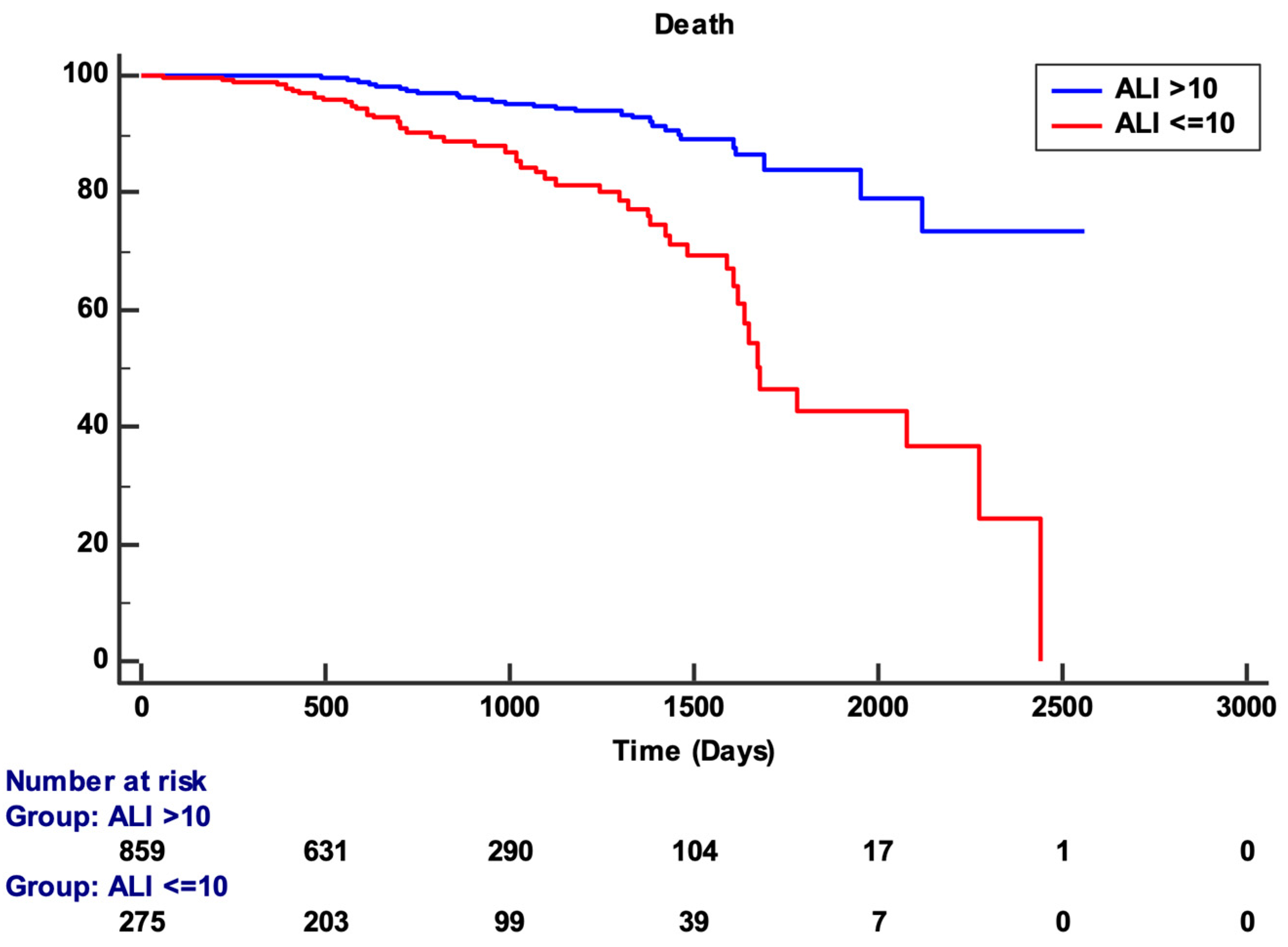
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Szummer, K.; Jernberg, T.; Wallentin, L. From Early Pharmacology to Recent Pharmacology Interventions in Acute Coronary Syndromes: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 74, 1618–1636. [Google Scholar] [CrossRef] [PubMed]
- Elendu, C.; Amaechi, D.C.; Elendu, T.C.; Omeludike, E.K.; Alakwe-Ojimba, C.E.; Obidigbo, B.; Akpovona, O.L.; Oros Sucari, Y.P.; Saggi, S.K.; Dang, K.; et al. Comprehensive Review of ST-Segment Elevation Myocardial Infarction: Understanding Pathophysiology, Diagnostic Strategies, and Current Treatment Approaches. Medicine 2023, 102, e35687. [Google Scholar] [CrossRef]
- Yamashita, Y.; Shiomi, H.; Morimoto, T.; Yaku, H.; Furukawa, Y.; Nakagawa, Y.; Ando, K.; Kadota, K.; Abe, M.; Nagao, K.; et al. Cardiac and Noncardiac Causes of Long-Term Mortality in ST-Segment-Elevation Acute Myocardial Infarction Patients Who Underwent Primary Percutaneous Coronary Intervention. Circ. Cardiovasc. Qual. Outcomes 2017, 10, e002790. [Google Scholar] [CrossRef]
- Garcia-Osuna, A.; Sans-Rosello, J.; Ferrero-Gregori, A.; Alquezar-Arbe, A.; Sionis, A.; Ordóñez-Llanos, J. Risk Assessment after ST-Segment Elevation Myocardial Infarction: Can Biomarkers Improve the Performance of Clinical Variables? J. Clin. Med. 2022, 11, 1266. [Google Scholar] [CrossRef]
- Li, Q.; Ma, X.; Shao, Q.; Yang, Z.; Wang, Y.; Gao, F.; Zhou, Y.; Yang, L.; Wang, Z. Prognostic Impact of Multiple Lymphocyte-Based Inflammatory Indices in Acute Coronary Syndrome Patients. Front. Cardiovasc. Med. 2022, 9, 811790. [Google Scholar] [CrossRef] [PubMed]
- Marchi, F.; Pylypiv, N.; Parlanti, A.; Storti, S.; Gaggini, M.; Paradossi, U.; Berti, S.; Vassalle, C. Systemic Immune-Inflammation Index and Systemic Inflammatory Response Index as Predictors of Mortality in ST-Elevation Myocardial Infarction. J. Clin. Med. 2024, 13, 1256. [Google Scholar] [CrossRef] [PubMed]
- Han, K.; Shi, D.; Yang, L.; Wang, Z.; Li, Y.; Gao, F.; Liu, Y.; Ma, X.; Zhou, Y. Prognostic Value of Systemic Inflammatory Response Index in Patients with Acute Coronary Syndrome Undergoing Percutaneous Coronary Intervention. Ann. Med. 2022, 54, 1667–1677. [Google Scholar] [CrossRef]
- Waksman, R.; Merdler, I.; Case, B.C.; Waksman, O.; Porto, I. Targeting Inflammation in Atherosclerosis: Overview, Strategy and Directions. EuroIntervention 2024, 20, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Ma, L. Inflammation in Coronary Artery Disease-Clinical Implications of Novel HDL-Cholesterol–Related Inflammatory Parameters as Predictors. Coron. Artery Dis. 2023, 34, 66–77. [Google Scholar] [CrossRef]
- Adam, A.M.; Rizvi, A.H.; Haq, A.; Naseem, R.; Rehan, A.; Shaikh, A.T.; Abbas, A.H.; Godil, A.; Ali, A.; Mallick, M.S.A.; et al. Prognostic Value of Blood Count Parameters in Patients with Acute Coronary Syndrome. Indian Heart J. 2018, 70, 233–240. [Google Scholar] [CrossRef]
- Roseiro, M.; Henriques, J.; Paredes, S.; Rocha, T.; Sousa, J. An Interpretable Machine Learning Approach to Estimate the Influence of Inflammation Biomarkers on Cardiovascular Risk Assessment. Comput. Methods Programs Biomed. 2023, 230, 107347. [Google Scholar] [CrossRef] [PubMed]
- Moggio, A.; Schunkert, H.; Kessler, T.; Sager, H.B. Quo Vadis? Immunodynamics of Myeloid Cells after Myocardial Infarction. Int. J. Mol. Sci. 2022, 23, 15814. [Google Scholar] [CrossRef] [PubMed]
- Peet, C.; Ivetic, A.; Bromage, D.I.; Shah, A.M. Cardiac Monocytes and Macrophages after Myocardial Infarction. Cardiovasc. Res. 2020, 116, 1101–1112. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Wei, C.; Liu, Y.; Sun, Q.; Tian, Y.; Wang, X.; Liu, J.; Zhang, Y.; Sun, L. The Prognostic Value of Hematologic Inflammatory Markers in Patients With Acute Coronary Syndrome Undergoing Percutaneous Coronary Intervention. Clin. Appl. Thromb. Off. J. Int. Acad. Clin. Appl. Thromb. 2022, 28, 10760296221146183. [Google Scholar] [CrossRef] [PubMed]
- Qi, Q.; Zhuang, L.; Shen, Y.; Geng, Y.; Yu, S.; Chen, H.; Liu, L.; Meng, Z.; Wang, P.; Chen, Z. A Novel Systemic Inflammation Response Index (SIRI) for Predicting the Survival of Patients with Pancreatic Cancer after Chemotherapy. Cancer 2016, 122, 2158–2167. [Google Scholar] [CrossRef]
- Liu, J.; Liu, J.; Wang, J.; Yan, Z.; Liang, Q.; Wang, X.; Wang, Z.; Liu, M.; Luan, X. Prevalence and Impact of Malnutrition on Readmission among Hospitalized Patients with Heart Failure in China. ESC Heart Fail. 2022, 9, 4271–4279. [Google Scholar] [CrossRef]
- Esteban-Fernández, A.; Villar-Taibo, R.; Alejo, M.; Arroyo, D.; Bonilla Palomas, J.L.; Cachero, M.; Joaquin, C.; Méndez Bailón, M.; Pérez-Rivera, J.Á.; Romero-Vigara, J.C.; et al. Diagnosis and Management of Malnutrition in Patients with Heart Failure. J. Clin. Med. 2023, 12, 3320. [Google Scholar] [CrossRef]
- Anzaki, K.; Kanda, D.; Ikeda, Y.; Takumi, T.; Tokushige, A.; Ohmure, K.; Sonoda, T.; Arikawa, R.; Ohishi, M. Impact of Malnutrition on Prognosis and Coronary Artery Calcification in Patients with Stable Coronary Artery Disease. Curr. Probl. Cardiol. 2023, 48, 101185. [Google Scholar] [CrossRef]
- Tonet, E.; Campo, G.; Maietti, E.; Formiga, F.; Martinez-Sellés, M.; Pavasini, R.; Biscaglia, S.; Serenelli, M.; Sanchis, J.; Diez-Villanueva, P.; et al. Nutritional Status and All-Cause Mortality in Older Adults with Acute Coronary Syndrome. Clin. Nutr. Edinb. Scotl. 2020, 39, 1572–1579. [Google Scholar] [CrossRef]
- Yoo, S.H.; Kook, H.Y.; Hong, Y.J.; Kim, J.H.; Ahn, Y.; Jeong, M.H. Influence of Undernutrition at Admission on Clinical Outcomes in Patients with Acute Myocardial Infarction. J. Cardiol. 2017, 69, 555–560. [Google Scholar] [CrossRef]
- Raposeiras Roubín, S.; Abu Assi, E.; Cespón Fernandez, M.; Barreiro Pardal, C.; Lizancos Castro, A.; Parada, J.A.; Pérez, D.D.; Blanco Prieto, S.; Rossello, X.; Ibanez, B.; et al. Prevalence and Prognostic Significance of Malnutrition in Patients With Acute Coronary Syndrome. J. Am. Coll. Cardiol. 2020, 76, 828–840. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; He, L.; Zhou, Y.; Man, C. Predictive Value of Geriatric Nutritional Risk Index in Patients With Coronary Artery Disease: A Meta-Analysis. Front. Nutr. 2021, 8, 736884. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shen, J.; Hou, X.; Su, Y.; Jiao, Y.; Wang, J.; Liu, H.; Fu, Z. Geriatric Nutritional Risk Index Predicts All-Cause Mortality in the Oldest-Old Patients with Acute Coronary Syndrome: A 10-Year Cohort Study. Front. Nutr. 2023, 10, 1129978. [Google Scholar] [CrossRef]
- Miano, N.; Di Marco, M.; Alaimo, S.; Coppolino, G.; L’Episcopo, G.; Leggio, S.; Scicali, R.; Piro, S.; Purrello, F.; Di Pino, A. Controlling Nutritional Status (CONUT) Score as a Potential Prognostic Indicator of In-Hospital Mortality, Sepsis and Length of Stay in an Internal Medicine Department. Nutrients 2023, 15, 1554. [Google Scholar] [CrossRef] [PubMed]
- Basta, G.; Chatzianagnostou, K.; Paradossi, U.; Botto, N.; Del Turco, S.; Taddei, A.; Berti, S.; Mazzone, A. The Prognostic Impact of Objective Nutritional Indices in Elderly Patients with ST-Elevation Myocardial Infarction Undergoing Primary Coronary Intervention. Int. J. Cardiol. 2016, 221, 987–992. [Google Scholar] [CrossRef]
- Zengin, A.; Karataş, M.B.; Çanga, Y.; Durmuş, G.; Güzelburç, Ö.; Durak, F.; Emre, A. Prognostic Performance of Controlling Nutritional Status Score in Patients with ST Segment Elevation Myocardial Infarction Treated with Primary Percutaneous Coronary Intervention. Anatol. J. Cardiol. 2021, 26, 23–28. [Google Scholar] [CrossRef]
- Yuan, X.; Huang, B.; Wang, R.; Tie, H.; Luo, S. The Prognostic Value of Advanced Lung Cancer Inflammation Index (ALI) in Elderly Patients with Heart Failure. Front. Cardiovasc. Med. 2022, 9, 934551. [Google Scholar] [CrossRef]
- Jafri, S.H.; Shi, R.; Mills, G. Advance Lung Cancer Inflammation Index (ALI) at Diagnosis Is a Prognostic Marker in Patients with Metastatic Non-Small Cell Lung Cancer (NSCLC): A Retrospective Review. BMC Cancer 2013, 13, 158. [Google Scholar] [CrossRef]
- Yin, C.; Toiyama, Y.; Okugawa, Y.; Omura, Y.; Kusunoki, Y.; Kusunoki, K.; Imaoka, Y.; Yasuda, H.; Ohi, M.; Kusunoki, M. Clinical Significance of Advanced Lung Cancer Inflammation Index, a Nutritional and Inflammation Index, in Gastric Cancer Patients after Surgical Resection: A Propensity Score Matching Analysis. Clin. Nutr. Edinb. Scotl. 2021, 40, 1130–1136. [Google Scholar] [CrossRef]
- Shi, T.; Wang, Y.; Peng, Y.; Wang, M.; Zhou, Y.; Gu, W.; Li, Y.; Zou, J.; Zhu, N.; Chen, L. Advanced Lung Cancer Inflammation Index Combined with Geriatric Nutritional Risk Index Predict All-Cause Mortality in Heart Failure Patients. BMC Cardiovasc. Disord. 2023, 23, 565. [Google Scholar] [CrossRef]
- Gong, M.; Sasmita, B.R.; Zhu, Y.; Chen, S.; Wang, Y.; Xiang, Z.; Jiang, Y.; Luo, S.; Huang, B. Prognostic Value of the Advanced Lung Cancer Inflammation Index Ratio in Patients with Acute Myocardial Infarction Complicated by Cardiogenic Shock: A Cohort Study. Rev. Cardiovasc. Med. 2024, 25, 267. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Tang, W.; Yang, C.; Liu, X.; Huang, J. The Prognostic Value of Advanced Lung Cancer Inflammation Index in Elderly Patients with Acute Coronary Syndrome Undergoing Percutaneous Coronary Intervention. Int. Heart. J. 2024, 65, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.-A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the Management of Acute Coronary Syndromes. Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef] [PubMed]
- Paradossi, U.; De Caterina, A.R.; Trimarchi, G.; Pizzino, F.; Bastiani, L.; Dossi, F.; Raccis, M.; Bianchi, G.; Palmieri, C.; de Gregorio, C.; et al. The Enigma of the ‘Smoker’s Paradox’: Results from a Single-Center Registry of Patients with STEMI Undergoing Primary Percutaneous Coronary Intervention. Cardiovasc. Revasc. Med. 2024. [Google Scholar] [CrossRef] [PubMed]
- Tardif, J.-C.; Kouz, S.; Waters, D.D.; Bertrand, O.F.; Diaz, R.; Maggioni, A.P.; Pinto, F.J.; Ibrahim, R.; Gamra, H.; Kiwan, G.S.; et al. Efficacy and Safety of Low-Dose Colchicine after Myocardial Infarction. N. Engl. J. Med. 2019, 381, 2497–2505. [Google Scholar] [CrossRef]
- Ridker, P.M. The Time to Initiate Anti-Inflammatory Therapy for Patients with Chronic Coronary Atherosclerosis Has Arrived. Circulation 2023, 148, 1071–1073. [Google Scholar] [CrossRef]
- Libby, P. Inflammation and the Pathogenesis of Atherosclerosis. Vascul. Pharmacol. 2024, 154, 107255. [Google Scholar] [CrossRef]
- Ait-Oufella, H.; Libby, P. Inflammation and Atherosclerosis: Prospects for Clinical Trials. Arterioscler. Thromb. Vasc. Biol. 2024, 44, 1899–1905. [Google Scholar] [CrossRef]
- Shumilah, A.M.; Othman, A.M.; Al-Madhagi, A.K. Accuracy of Neutrophil to Lymphocyte and Monocyte to Lymphocyte Ratios as New Inflammatory Markers in Acute Coronary Syndrome. BMC Cardiovasc. Disord. 2021, 21, 422. [Google Scholar] [CrossRef]
- Ha, E.T.; Yee, A.; Peterson, S.J.; Kobayashi, Y.; Sacchi, T.; Parikh, M.; Brener, S.J. Neutrophil-to-Lymphocyte Ratio and Prognosis in Patients Undergoing Percutaneous Coronary Intervention for Acute Coronary Syndrome. Cardiovasc. Revasc. Med. Mol. Interv. 2024, 60, 29–34. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, J.; Cao, D.; Han, L. Correlation of Neutrophil-to-Lymphocyte Ratio with the Prognosis of Non-ST-Segment Elevation in Patients with Acute Coronary Syndrome Undergoing Selective Percutaneous Coronary Intervention. J. Int. Med. Res. 2020, 48, 300060520959510. [Google Scholar] [CrossRef] [PubMed]
- Shahsanaei, F.; Abbaszadeh, S.; Behrooj, S.; Rahimi Petrudi, N.; Ramezani, B. The Value of Neutrophil-to-Lymphocyte Ratio in Predicting Severity of Coronary Involvement and Long-Term Outcome of Percutaneous Coronary Intervention in Patients with Acute Coronary Syndrome: A Systematic Review and Meta-Analysis. Egypt. Heart J. EHJ Off. Bull. Egypt. Soc. Cardiol. 2024, 76, 39. [Google Scholar] [CrossRef]
- Alzahrani, S.H.; Alamri, S.H. Prevalence of Malnutrition and Associated Factors among Hospitalized Elderly Patients in King Abdulaziz University Hospital, Jeddah, Saudi Arabia. BMC Geriatr. 2017, 17, 136. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-R.; Wang, L.-L.; Zhang, B.; Liu, X.-Y.; Li, Z.-W.; Kang, B.; Yuan, C.; Wei, Z.-Q.; Peng, D. The Advanced Lung Cancer Inflammation Index Is a Prognostic Factor for Gastrointestinal Cancer Patients Undergoing Surgery: A Systematic Review and Meta-Analysis. World J. Surg. Oncol. 2023, 21, 81. [Google Scholar] [CrossRef]
- Wang, X.; Wei, C.; Fan, W.; Sun, L.; Zhang, Y.; Sun, Q.; Liu, Y.; Liu, J. Advanced Lung Cancer Inflammation Index for Predicting Prognostic Risk for Patients with Acute Coronary Syndrome Undergoing Percutaneous Coronary Intervention. J. Inflamm. Res. 2023, 16, 3631–3641. [Google Scholar] [CrossRef]
- Konuş, A.H.; Özderya, A.; Çırakoğlu, Ö.F.; Sayın, M.R.; Yerlikaya, M.G. The Relationship between Advanced Lung Cancer Inflammation Index and High SYNTAX Score in Patients with Non-ST-Elevation Myocardial Infarction. Adv. Interv. Cardiol. W Kardiologii Interwencyjnej 2024, 20, 277–284. [Google Scholar] [CrossRef]
- Ahmed, N.; Carberry, J.; Teng, V.; Carrick, D.; Berry, C. Risk Assessment in Patients with an Acute ST-Elevation Myocardial Infarction. J. Comp. Eff. Res. 2016, 5, 581–593. [Google Scholar] [CrossRef]
- Banahene, N.O.; Sinha, T.; Shaikh, S.; Zin, A.K.; Khreis, K.; Chaudhari, S.S.; Wei, C.R.; Palleti, S.K. Effect of Elevated Neutrophil-to-Lymphocyte Ratio on Adverse Outcomes in Patients With Myocardial Infarction: A Systematic Review and Meta-Analysis. Cureus 2024, 16, e61647. [Google Scholar] [CrossRef]
| Total Population, N = 1171 | Survival Group, N = 1085 | Death Group, N = 86 | p | |
|---|---|---|---|---|
| Age (years) | 65 ± 12 | 65 ± 12 | 77 ± 11 | <0.001 |
| Gender (female) | 311 (24.2%) | 261 (24.1%) | 30 (34.9%) | 0.03 |
| BMI, kg/m2 | 27.4 ± 8.2 | 27.5 ± 8.5 | 25.2 ± 3.6 | 0.01 |
| Smoking | 520 (44.4%) | 502 (46.3%) | 18 (20.9%) | <0.001 |
| Hypertension | 669 (57.1%) | 610 (56.2%) | 59 (68.6%) | 0.03 |
| Diabetes | 228 (19.5%) | 203 (18.7%) | 25 (29.1%) | 0.02 |
| Dyslipidemia | 481 (41.1% | 452 (41.6%) | 29 (33.7%) | 0.17 |
| CAD familial history | 362 (30.9%) | 344 (31.7%) | 18 (20.9%) | 0.51 |
| Anterior MI | 507 (43.3%) | 466 (42.9%) | 41 (47.7%) | 0.4 |
| No-IRA critical stenosis | 309 (26.4%) | 279 (25.7%) | 30 (34.8%) | 0.9 |
| Prior MI | 129 (11%) | 110 (10%) | 19 (22%) | 0.001 |
| Neutrophils (103/μL) | 8 (6.2–10.4) | 8 (6.1–10.3) | 9.3 (7–12.1) | 0.001 |
| Lymphocytes (103/μL) | 1.5 (1–2) | 1.5 (1–2) | 1.1 (0.8–1.6) | <0.001 |
| Albumin, g/dL | 3.6 ± 0.4 | 3.6 ± 0.4 | 3.3 ± 0.5 | <0.001 |
| LVEF (%) | 45 ± 10 | 45.6 ± 9 | 38.3 ± 11 | <0.001 |
| NLR | 5.7 (3.5–9) | 5.6 (3.5–8.5) | 8.4 (5.5–14.1) | <0.001 |
| ALI | 17 (10–27) | 17.7(11–27.8) | 9.6 (5.7–15.8) | <0.001 |
| Univariate | Multivariate | ||||
|---|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | ||
| Age (years) | 1.1 (1.07–1.12) | <0.001 | Age (years) | 1.1 (1.05–1.11) | <0.001 |
| Gender (female) | 2 (1.3–3.2) | 0.002 | Gender (female) | 1.4 (0.9–2.3) | 0.1 |
| Smoking | 0.3 (0.2–0.5) | <0.001 | Smoking | 1 (0.6–1.9) | 0.9 |
| Hypertension | 1.6 (1–2.5) | 0.04 | Hypertension | 1 (0.6–1.7) | 0.9 |
| Diabetes | 1.7 (1–2.6) | 0.03 | Diabetes | 1.4 (0.8–2.2) | 0.2 |
| Prior MI | 2.1 (1.3–3.5) | 0.004 | Prior MI | 2.4 (1.4–4.2) | 0.001 |
| LVEF | 0.94 (0.92–0.96) | <0.001 | LVEF | 0.97 (0.95–0.99) | 0.01 |
| ALI | 0.93 (0.90–0.95) | <0.001 | ALI | 0.95 (0.92–0.97) | <0.001 |
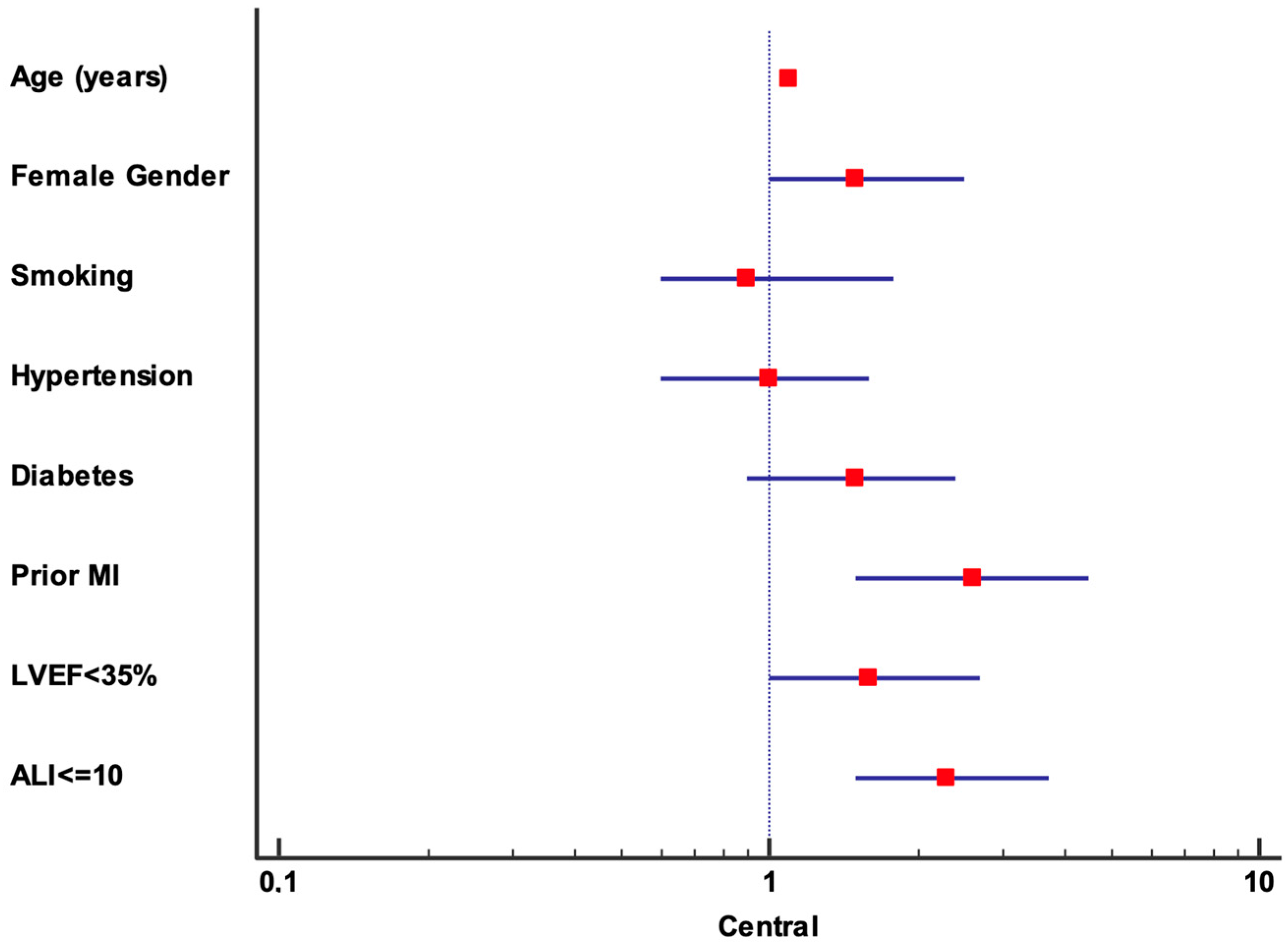
| Multivariate Analysis | ||
|---|---|---|
| HR (95% CI) | p | |
| Age (years) | 1.1 (1.1–1.11) | <0.001 |
| Gender (female) | 1.5 (1–2.5) | 0.1 |
| Smoking | 0.9 (0.6–1.8) | 0.9 |
| Hypertension | 1 (0.6–1.6) | 0.9 |
| Diabetes | 1.5 (0.9–2.4) | 0.2 |
| Prior MI | 2.6 (1.5–4.5) | 0.001 |
| LVEF < 35% | 1.6 (1–2.7) | 0.48 |
| ALI ≤ 10 | 2.3 (1.5–3.7) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trimarchi, G.; Pizzino, F.; Lilli, A.; De Caterina, A.R.; Esposito, A.; Dalmiani, S.; Mazzone, A.; Di Bella, G.; Berti, S.; Paradossi, U. Advanced Lung Cancer Inflammation Index as Predictor of All-Cause Mortality in ST-Elevation Myocardial Infarction Patients Undergoing Primary Percutaneous Coronary Intervention. J. Clin. Med. 2024, 13, 6059. https://doi.org/10.3390/jcm13206059
Trimarchi G, Pizzino F, Lilli A, De Caterina AR, Esposito A, Dalmiani S, Mazzone A, Di Bella G, Berti S, Paradossi U. Advanced Lung Cancer Inflammation Index as Predictor of All-Cause Mortality in ST-Elevation Myocardial Infarction Patients Undergoing Primary Percutaneous Coronary Intervention. Journal of Clinical Medicine. 2024; 13(20):6059. https://doi.org/10.3390/jcm13206059
Chicago/Turabian StyleTrimarchi, Giancarlo, Fausto Pizzino, Alessio Lilli, Alberto Ranieri De Caterina, Augusto Esposito, Stefano Dalmiani, Annamaria Mazzone, Gianluca Di Bella, Sergio Berti, and Umberto Paradossi. 2024. "Advanced Lung Cancer Inflammation Index as Predictor of All-Cause Mortality in ST-Elevation Myocardial Infarction Patients Undergoing Primary Percutaneous Coronary Intervention" Journal of Clinical Medicine 13, no. 20: 6059. https://doi.org/10.3390/jcm13206059











