Hemoincompatibility in Hemodialysis-Related Therapies and Their Health Economic Perspectives
Abstract
:1. Introduction
2. Understanding Hemoincompatibility and Their Mechanisms
2.1. Contact Phase and Protein Deposition
2.2. Activation of the Clotting System, Including Platelet Activation
2.3. Activation of the Complement System
2.4. Activation of the Kallikrein–Kinin System
2.5. Activation of Blood Cells, NET Formation, Release of Mediators
2.6. Activation of Endothelial Cells and Release of Extracellular Microvesicles
2.7. Activation of Acute Phase Protein Synthesis and Inflammation
3. Clinical Outcomes and Health Implications
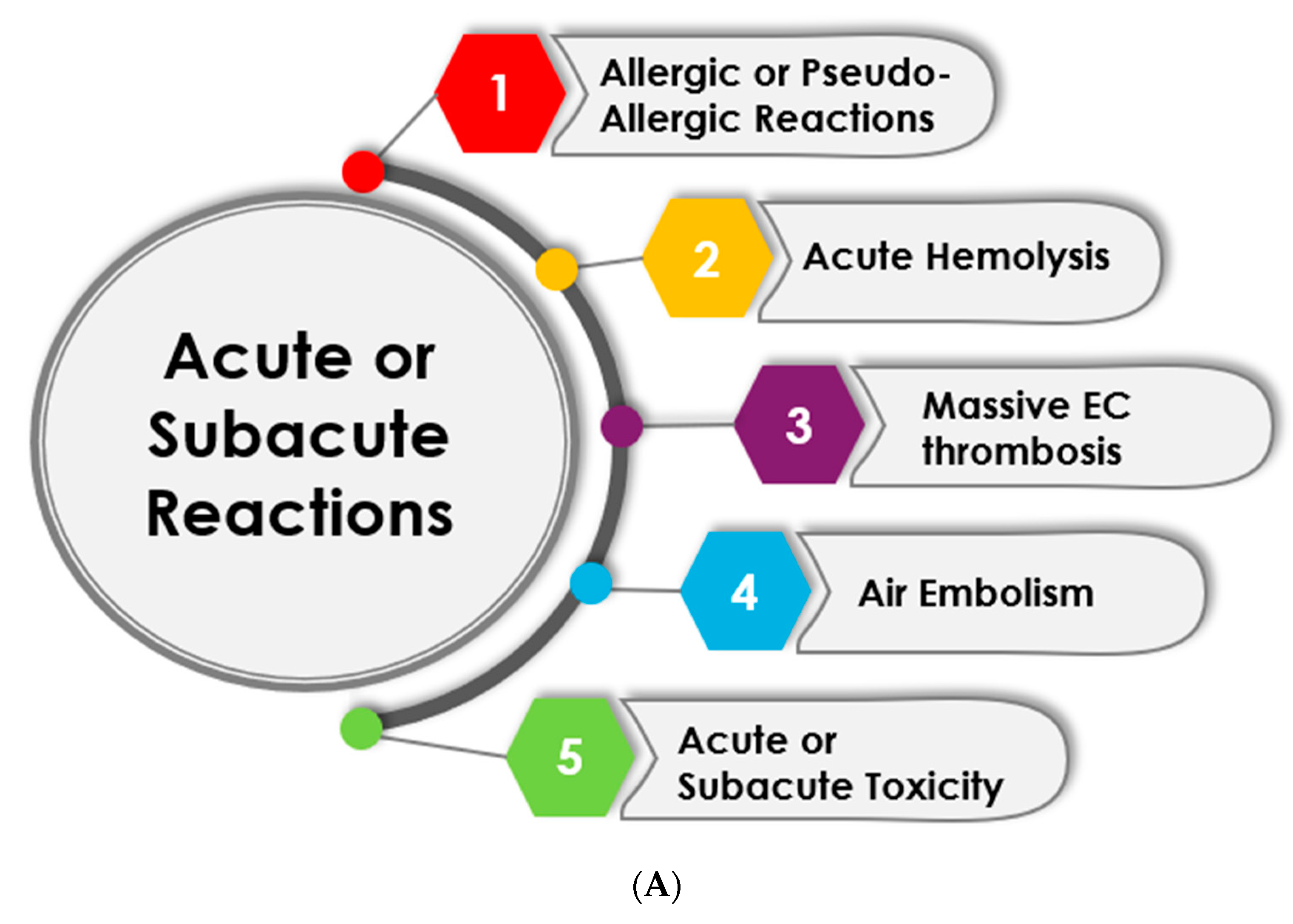
3.1. Acute and Subacute Reactions
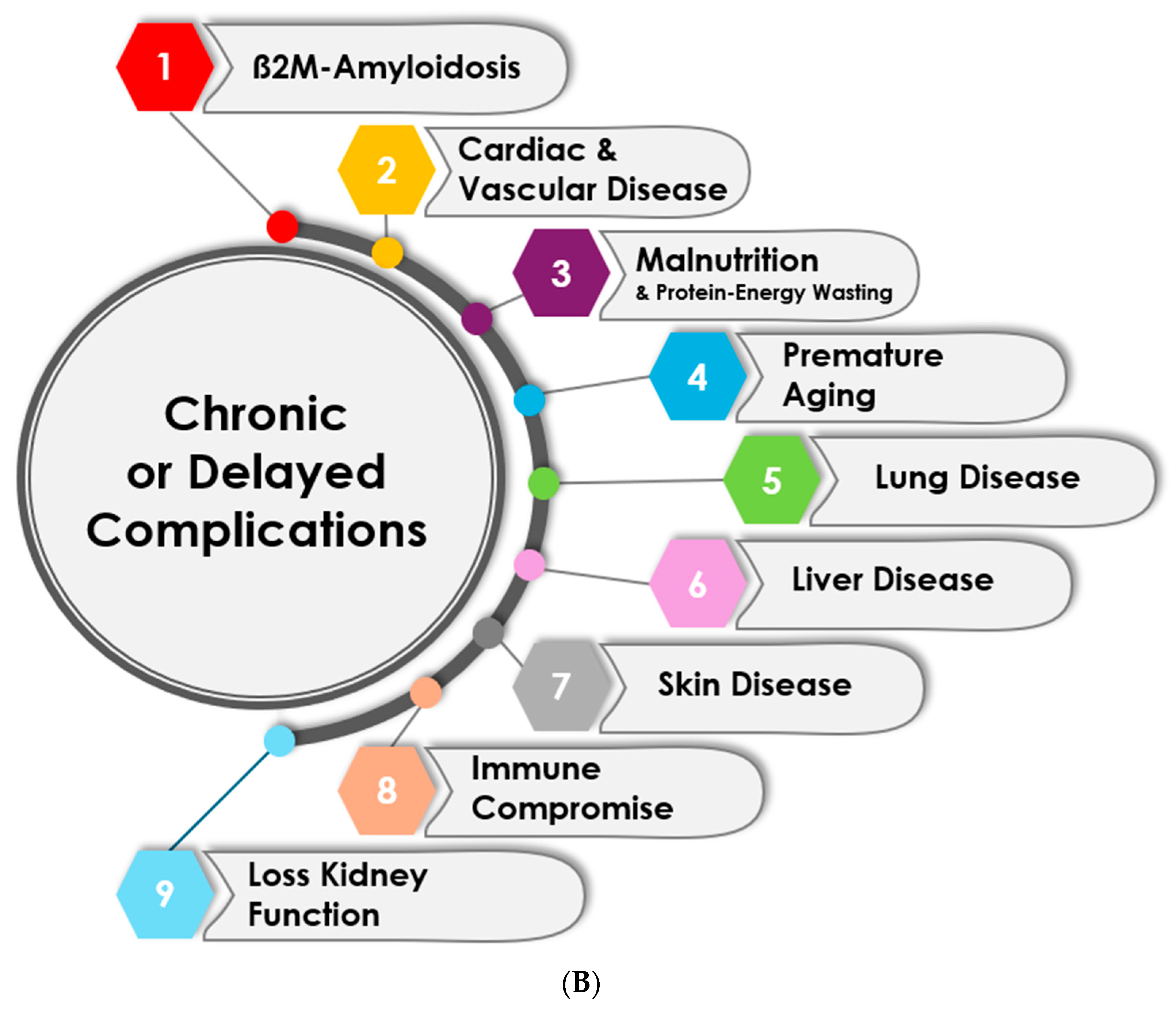
3.2. Chronic and Delayed Complications
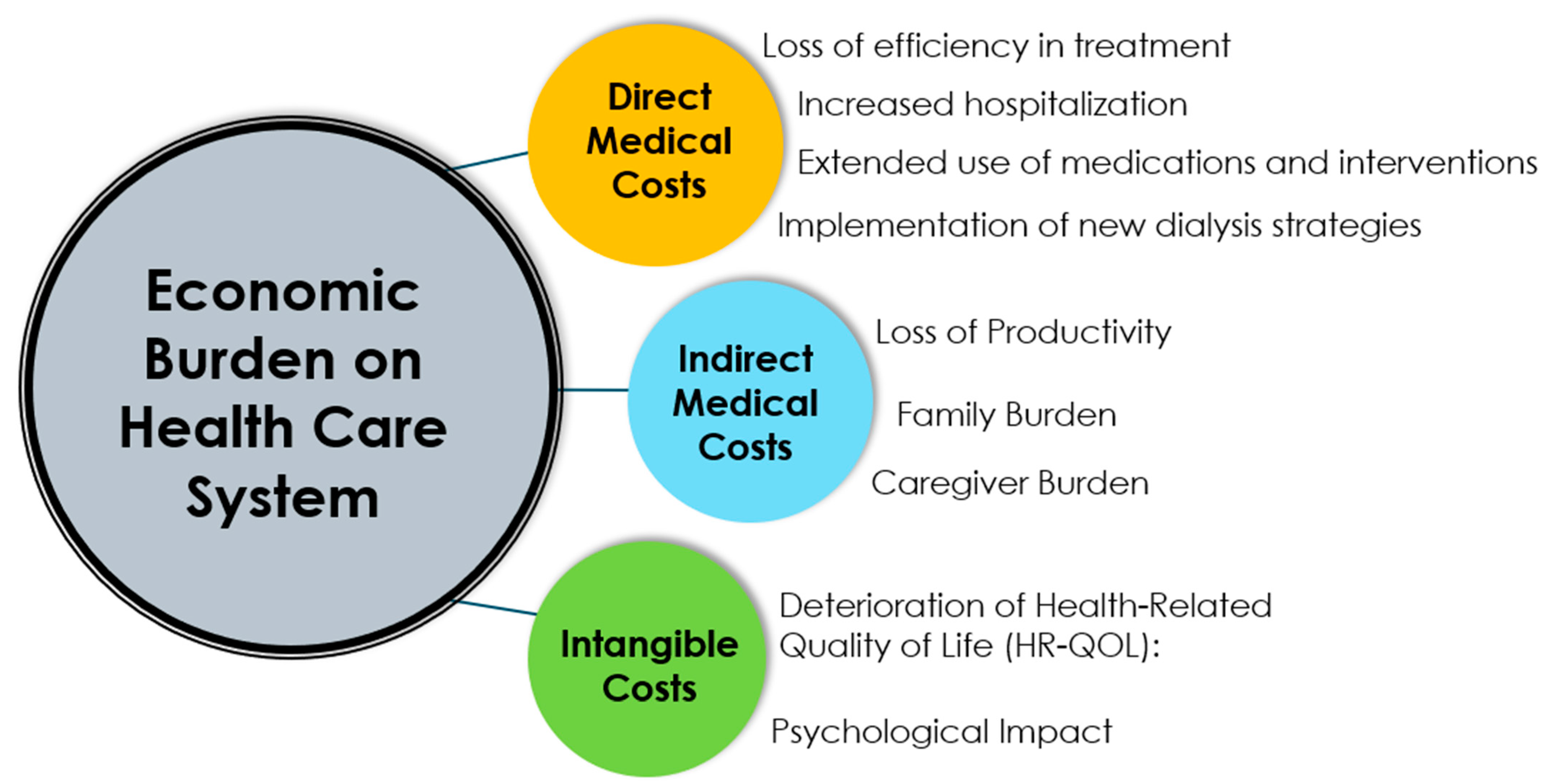
4. Economic Burden on Healthcare System
4.1. Direct Medical Costs
4.2. Indirect Medical Costs
4.3. Intangible Costs
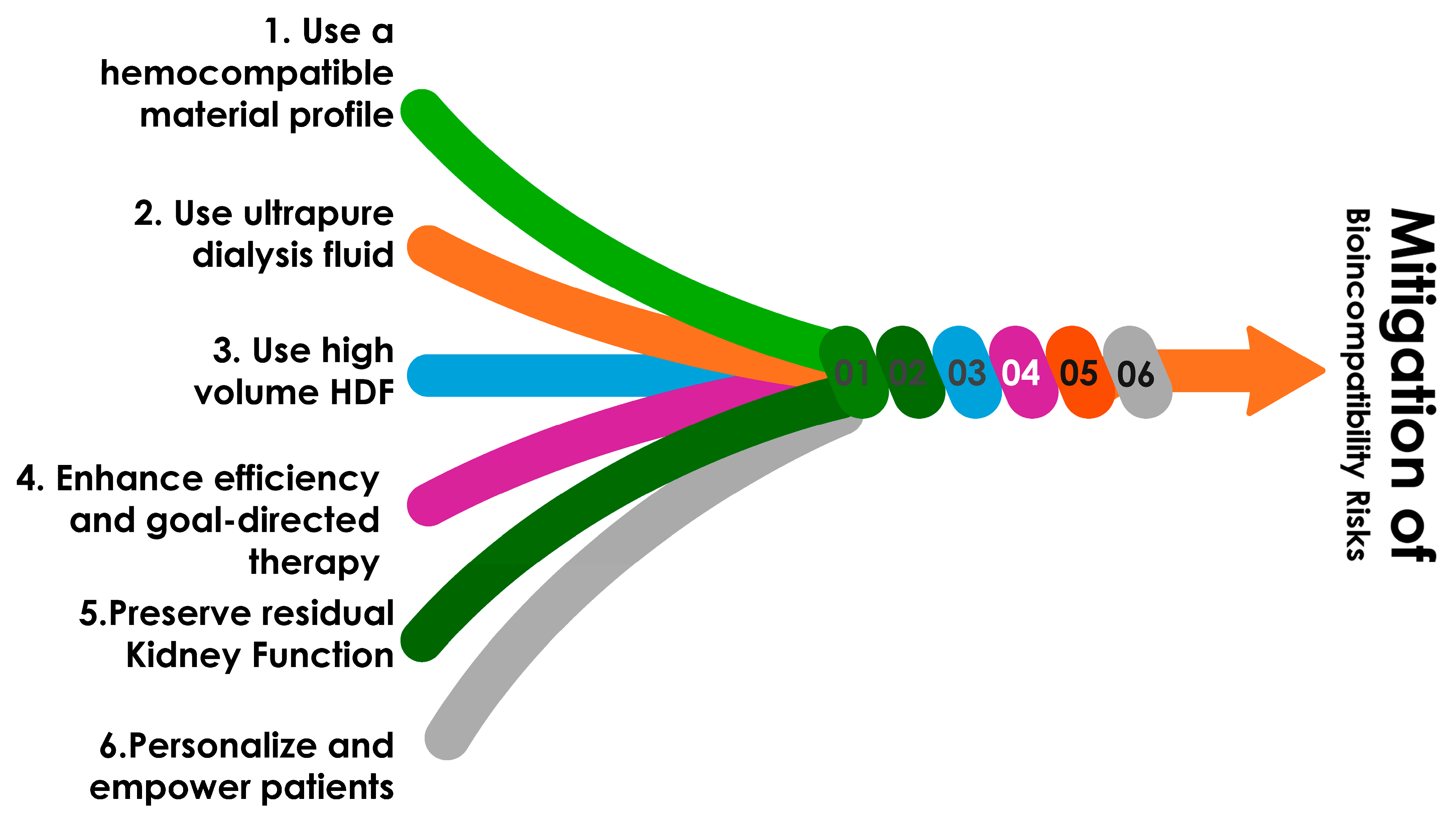
5. Strategies to Mitigate Economic Impact and Address Cost-Effectiveness
5.1. Use of More Biocompatible Extracorporeal Components
5.2. Use of Ultrapure Dialysis Fluid
5.3. Utilize Convective-Based Therapies Such as High-Volume Hemodiafiltration
5.4. Personalizing Treatment Prescriptions and Schedules
5.5. Enhancing Patient Monitoring and Early Risk Detection
5.6. Empowering Patients
5.7. Using Additional Medications When Necessary
5.8. Preserving Residual Kidney Function
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Himmelfarb, J.; Vanholder, R.; Mehrotra, R.; Tonelli, M. The current and future landscape of dialysis. Nat. Rev. Nephrol. 2020, 16, 573–585. [Google Scholar] [CrossRef] [PubMed]
- Webster, A.C.; Nagler, E.V.; Morton, R.L.; Masson, P. Chronic Kidney Disease. Lancet 2017, 389, 1238–1252. [Google Scholar] [CrossRef] [PubMed]
- Meyer, T.W.; Hostetter, T.H. Uremia. N. Engl. J. Med. 2007, 357, 1316–1325. [Google Scholar] [CrossRef] [PubMed]
- Himmelfarb, J. Hemodialysis complications. Am. J. Kidney Dis. 2005, 45, 1122–1131. [Google Scholar] [CrossRef] [PubMed]
- Maher, J.F.; Schreiner, G.E. Hazards and complications of dialysis. N. Engl. J. Med. 1965, 273, 370–377. [Google Scholar] [CrossRef]
- Brown, E.; Brown, E.A.; Parfrey, P.S. Complications of Long-Term Dialysis; Oxford University Press: Oxford, UK, 1999. [Google Scholar]
- Jaffer, I.H.; Weitz, J.I. The blood compatibility challenge. Part 1: Blood-contacting medical devices: The scope of the problem. Acta Biomater. 2019, 94, 2–10. [Google Scholar] [CrossRef]
- Kokubo, K.; Kurihara, Y.; Kobayashi, K.; Tsukao, H.; Kobayashi, H. Evaluation of the Biocompatibility of Dialysis Membranes. Blood Purif. 2015, 40, 293–297. [Google Scholar] [CrossRef]
- Weber, M.; Steinle, H.; Golombek, S.; Hann, L.; Schlensak, C.; Wendel, H.P.; Avci-Adali, M. Blood-Contacting Biomaterials: In Vitro Evaluation of the Hemocompatibility. Front. Bioeng. Biotechnol. 2018, 6, 99. [Google Scholar] [CrossRef]
- Mollahosseini, A.; Abdelrasoul, A. Novel insights in hemodialysis: Most recent theories on membrane hemocompatibility improvement. Biomed. Eng. Adv. 2022, 3, 100034. [Google Scholar] [CrossRef]
- Canaud, B.; Kooman, J.P.; Selby, N.M.; Taal, M.W.; Francis, S.; Maierhofer, A.; Kopperschmidt, P.; Collins, A.; Kotanko, P. Dialysis-Induced Cardiovascular and Multiorgan Morbidity. Kidney Int. Rep. 2020, 5, 1856–1869. [Google Scholar] [CrossRef]
- Skinner, S.C.; Derebail, V.K.; Poulton, C.J.; Bunch, D.C.; Roy-Chaudhury, P.; Key, N.S. Hemodialysis-Related Complement and Contact Pathway Activation and Cardiovascular Risk: A Narrative Review. Kidney Med. 2021, 3, 607–618. [Google Scholar] [CrossRef] [PubMed]
- Klinkmann, H.; Davison, A.M. The biocompatibility puzzle—Partly solved, partly enigmatic. Nephrol. Dial. Transplant. 1994, 9 (Suppl. 2), 184–186. [Google Scholar] [PubMed]
- Klinkmann, H.; Falkenhagen, D.; Stefoni, S.; Bonomini, V. Biocompatibility: A systems approach. Contrib. Nephrol. 1989, 70, 213–226. [Google Scholar] [PubMed]
- Klinkmann, H.; Ivanovich, P.; Falkenhagen, D. Biocompatibility: The need for a systems approach. Nephrol. Dial. Transplant. 1993, 8 (Suppl. 2), 40–42. [Google Scholar] [CrossRef]
- Westphalen, H.; Abdelrasoul, A.; Shoker, A. Protein adsorption phenomena in hemodialysis membranes: Mechanisms, influences of clinical practices, modeling, and challenges. Colloid. Interface Sci. Commun. 2021, 40, 100348. [Google Scholar] [CrossRef]
- Westphalen, H.; Saadati, S.; Eduok, U.; Abdelrasoul, A.; Shoker, A.; Choi, P.; Doan, H.; Ein-Mozaffari, F. Case studies of clinical hemodialysis membranes: Influences of membrane morphology and biocompatibility on uremic blood-membrane interactions and inflammatory biomarkers. Sci. Rep. 2020, 10, 14808. [Google Scholar] [CrossRef]
- Hakim, R.M. Clinical implications of hemodialysis membrane biocompatibility. Kidney Int. 1993, 44, 484–494. [Google Scholar] [CrossRef]
- Bonomini, M.; Piscitani, L.; Di Liberato, L.; Sirolli, V. Biocompatibility of Surface-Modified Membranes for Chronic Hemodialysis Therapy. Biomedicines 2022, 10, 844. [Google Scholar] [CrossRef]
- Pérez-García, R.; Rodríguez-Benítez, P.O. Why and how to monitor bacterial contamination of dialysate? Nephrol. Dial. Transplant. 2000, 15, 760–764. [Google Scholar] [CrossRef]
- Canaud, B.; Stephens, M.P.; Nikam, M.; Etter, M.; Collins, A. Multitargeted interventions to reduce dialysis- induced systemic stress. Clin. Kidney J. 2021, 14 (Suppl. 4), i72–i84. [Google Scholar] [CrossRef]
- Bowry, S.K.; Kircelli, F.; Himmele, R.; Nigwekar, S.U. Blood-incompatibility in haemodialysis: Alleviating inflammation and effects of coagulation. Clin. Kidney J. 2021, 14 (Suppl. 4), i59–i71. [Google Scholar] [CrossRef] [PubMed]
- Brash, J.L.; Horbett, T.A.; Latour, R.A.; Tengvall, P. The blood compatibility challenge. Part 2: Protein adsorption phenomena governing blood reactivity. Acta Biomater. 2019, 94, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Nesbitt, W.S.; Mangin, P.; Salem, H.H.; Jackson, S.P. The impact of blood rheology on the molecular and cellular events underlying arterial thrombosis. J. Mol. Med. 2006, 84, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Suranyi, M.; Chow, J.S. Review: Anticoagulation for haemodialysis. Nephrology 2010, 15, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Jaffer, I.H.; Fredenburgh, J.C.; Hirsh, J.; Weitz, J.I. Medical device-induced thrombosis: What causes it and how can we prevent it? J. Thromb. Haemost. 2015, 13 (Suppl. 1), S72–S81. [Google Scholar] [CrossRef]
- Penzes, K.; Hurjak, B.; Katona, E.; Becs, G.; Balla, J.; Muszbek, L. Terminal Phase Components of the Clotting Cascade in Patients with End-Stage Renal Disease Undergoing Hemodiafiltration or Hemodialysis Treatment. Int. J. Mol. Sci. 2020, 21, 8426. [Google Scholar] [CrossRef]
- Gritters-van den Oever, M.; Schoorl, M.; Schoorl, M.; Bartels, P.C.; Grooteman, M.P.; Nubé, M.J. The role of the extracorporeal circuit in the trapping and degranulation of platelets. Blood Purif. 2009, 28, 253–259. [Google Scholar] [CrossRef]
- Lucchi, L.; Ligabue, G.; Marietta, M.; Delnevo, A.; Malagoli, M.; Perrone, S.; Stipo, L.; Grandi, F.; Albertazzi, A. Activation of coagulation during hemodialysis: Effect of blood lines alone and whole extracorporeal circuit. Artif. Organs. 2006, 30, 106–110. [Google Scholar] [CrossRef]
- Schoorl, M.; Schoorl, M.; Nubé, M.J.; Bartels, P.C. Platelet depletion, platelet activation and coagulation during treatment with hemodialysis. Scand. J. Clin. Lab. Investig. 2011, 71, 240–247. [Google Scholar] [CrossRef]
- Canaud, B.; Mion, C.; Arujo, A.; N’Guyen, Q.V.; Paleyrac, G.; Hemmendinger, S.; Cazenave, J.P. Prostacyclin (epoprostenol) as the sole antithrombotic agent in postdilutional hemofiltration. Nephron 1988, 48, 206–212. [Google Scholar] [CrossRef]
- Poppelaars, F.; Faria, B.; Gaya da Costa, M.; Franssen, C.F.; Van Son, W.J.; Berger, S.P.; Daha, M.R.; Seelen, M.A. The Complement System in Dialysis: A Forgotten Story? Front. Immunol. 2018, 9, 71. [Google Scholar] [CrossRef] [PubMed]
- de Borst, M.H. The Complement System in Hemodialysis Patients: Getting to the Heart of the Matter. Nephron 2016, 132, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Craddock, P.R.; Fehr, J.; Brigham, K.L.; Kronenberg, R.S.; Jacob, H.S. Complement and leukocyte-mediated pulmonary dysfunction in hemodialysis. N. Engl. J. Med. 1977, 296, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Davenport, A. New Dialysis Technology and Biocompatible Materials. Contrib. Nephrol. 2017, 189, 130–136. [Google Scholar] [PubMed]
- Davenport, A. The changing face of dialyzer membranes and dialyzers. Semin. Dial. 2023; ahead of print. [Google Scholar]
- Daugirdas, J.T.; Ing, T.S. First-use reactions during hemodialysis: A definition of subtypes. Kidney Int. Suppl. 1988, 24, S37–S43. [Google Scholar]
- Canaud, B. Recent advances in dialysis membranes. Curr. Opin. Nephrol. Hypertens. 2021, 30, 613–622. [Google Scholar] [CrossRef]
- Basile, C.; Davenport, A.; Mitra, S.; Pal, A.; Stamatialis, D.; Chrysochou, C.; Kirmizis, D. Frontiers in hemodialysis: Innovations and technological advances. Artif. Organs. 2021, 45, 175–182. [Google Scholar] [CrossRef]
- Poppelaars, F.; Gaya da Costa, M.; Faria, B.; Berger, S.P.; Assa, S.; Daha, M.R.; Medina Pestana, J.O.; Van Son, W.J.; Franssen, C.F.; Seelen, M.A. Intradialytic Complement Activation Precedes the Development of Cardiovascular Events in Hemodialysis Patients. Front. Immunol. 2018, 9, 2070. [Google Scholar] [CrossRef]
- Ronco, C.; Clark, W.R. Haemodialysis membranes. Nat. Rev. Nephrol. 2018, 14, 394–410. [Google Scholar] [CrossRef]
- Renaux, J.L.; Thomas, M.; Crost, T.; Loughraieb, N.; Vantard, G. Activation of the kallikrein-kinin system in hemodialysis: Role of membrane electronegativity, blood dilution, and pH. Kidney Int. 1999, 55, 1097–1103. [Google Scholar] [CrossRef]
- Désormeaux, A.; Moreau, M.E.; Lepage, Y.; Chanard, J.; Adam, A. The effect of electronegativity and angiotensin-converting enzyme inhibition on the kinin-forming capacity of polyacrylonitrile dialysis membranes. Biomaterials 2008, 29, 1139–1146. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, R.M.; Fink, E.; Schaefer, L.; Barkhausen, R.; Kulzer, P.; Heidland, A. Role of bradykinin in anaphylactoid reactions during hemodialysis with AN69 dialyzers. Am. J. Nephrol. 1993, 13, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Krieter, D.H.; Grude, M.; Lemke, H.D.; Fink, E.; Bönner, G.; Schölkens, B.A.; Schulz, E.; Müller, G.A. Anaphylactoid reactions during hemodialysis in sheep are ACE inhibitor dose-dependent and mediated by bradykinin. Kidney Int. 1998, 53, 1026–1035. [Google Scholar] [CrossRef] [PubMed]
- Tielemans, C.; Madhoun, P.; Lenaers, M.; Schandene, L.; Goldman, M.; Vanherweghem, J.L. Anaphylactoid reactions during hemodialysis on AN69 membranes in patients receiving ACE inhibitors. Kidney Int. 1990, 38, 982–984. [Google Scholar] [CrossRef]
- Canaud, B.; Cristol, J.; Morena, M.; Leray-Moragues, H.; Bosc, J.; Vaussenat, F. Imbalance of oxidants and antioxidants in haemodialysis patients. Blood Purif. 1999, 17, 99–106. [Google Scholar] [CrossRef]
- Morena, M.; Cristol, J.P.; Senécal, L.; Leray-Moragues, H.; Krieter, D.; Canaud, B. Oxidative stress in hemodialysis patients: Is NADPH oxidase complex the culprit? Kidney Int. Suppl. 2002, 61, 109–114. [Google Scholar] [CrossRef]
- Schouten, W.E.; Grooteman, M.P.; van Houte, A.J.; Schoorl, M.; van Limbeek, J.; Nubé, M.J. Effects of dialyser and dialysate on the acute phase reaction in clinical bicarbonate dialysis. Nephrol. Dial. Transplant. 2000, 15, 379–384. [Google Scholar] [CrossRef]
- Campo, S.; Lacquaniti, A.; Trombetta, D.; Smeriglio, A.; Monardo, P. Immune System Dysfunction and Inflammation in Hemodialysis Patients: Two Sides of the Same Coin. J. Clin. Med. 2022, 11, 3759. [Google Scholar] [CrossRef]
- Cristol, J.P.; Thierry, A.R.; Bargnoux, A.S.; Morena-Carrere, M.; Canaud, B. What is the role of the neutrophil extracellular traps in the cardiovascular disease burden associated with hemodialysis bioincompatibility? Front. Med. 2023, 10, 1268748. [Google Scholar] [CrossRef]
- Korabecna, M.; Tesar, V. NETosis provides the link between activation of neutrophils on hemodialysis membrane and comorbidities in dialyzed patients. Inflamm. Res. 2017, 66, 369–378. [Google Scholar] [CrossRef]
- Bellien, J.; Fréguin-Bouilland, C.; Joannidès, R.; Hanoy, M.; Rémy-Jouet, I.; Monteil, C.; Iacob, M.; Martin, L.; Renet, S.; Vendeville, C.; et al. High-efficiency on- line haemodiafiltration improves conduit artery endothelial function compared with high-flux haemodialysis in end-stage renal disease patients. Nephrol. Dial. Transplant. 2014, 29, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Ruzicka, M.; Xiao, F.; Abujrad, H.; Al-Rewashdy, Y.; Tang, V.A.; Langlois, M.A.; Sorisky, A.; Ooi, T.C.; Burger, D. Effect of hemodialysis on extracellular vesicles and circulating submicron particles. BMC Nephrol. 2019, 20, 294. [Google Scholar] [CrossRef] [PubMed]
- Jalal, D.; Renner, B.; Laskowski, J.; Stites, E.; Cooper, J.; Valente, K.; You, Z.; Perrenoud, L.; Le Quintrec, M.; Muhamed, I.; et al. Endothelial Microparticles and Systemic Complement Activation in Patients With Chronic Kidney Disease. J. Am. Heart Assoc. 2018, 7, e007818. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Liu, H.; Liu, Y.; Jin, J.; He, Q.; Lin, B. The role of extracellular vesicles in vascular calcification in chronic kidney disease. Front. Med. 2022, 9, 997554. [Google Scholar] [CrossRef] [PubMed]
- Cavallari, C.; Dellepiane, S.; Fonsato, V.; Medica, D.; Marengo, M.; Migliori, M.; Quercia, A.D.; Pitino, A.; Formica, M.; Panichi, V.; et al. Online Hemodiafiltration Inhibits Inflammation-Related Endothelial Dysfunction and Vascular Calcification of Uremic Patients Modulating miR-223 Expression in Plasma Extracellular Vesicles. J. Immunol. 2019, 202, 2372–2383. [Google Scholar] [CrossRef]
- Jia, P.; Jin, W.; Teng, J.; Zhang, H.; Zou, J.; Liu, Z.; Shen, B.; Cao, X.; Ding, X. Acute Effects of Hemodiafiltration Versus Conventional Hemodialysis on Endothelial Function and Inflammation: A Randomized Crossover Study. Medicine 2016, 95, e3440. [Google Scholar] [CrossRef]
- Catar, R.; Moll, G.; Kamhieh-Milz, J.; Luecht, C.; Chen, L.; Zhao, H.; Ernst, L.; Willy, K.; Girndt, M.; Fiedler, R.; et al. Expanded Hemodialysis Therapy Ameliorates Uremia-Induced Systemic Microinflammation and Endothelial Dysfunction by Modulating VEGF, TNF-α and AP-1 Signaling. Front. Immunol. 2021, 12, 774052. [Google Scholar] [CrossRef]
- Ulrich, C.; Wildgrube, S.; Fiedler, R.; Seibert, E.; Kneser, L.; Fick, S.; Schäfer, C.; Markau, S.; Trojanowicz, B.; Girndt, M. NLRP3 Inflammasome Activation in Hemodialysis and Hypertensive Patients with Intact Kidney Function. Toxins 2020, 12, 675. [Google Scholar] [CrossRef]
- Lonnemann, G. Chronic inflammation in hemodialysis: The role of contaminated dialysate. Blood Purif. 2000, 18, 214–223. [Google Scholar] [CrossRef]
- Panichi, V.; Migliori, M.; De Pietro, S.; Taccola, D.; Andreini, B.; Metelli, M.R.; Giovannini, L.; Palla, R. The link of biocompatibility to cytokine production. Kidney Int. Suppl. 2000, 76, S96–S103. [Google Scholar] [CrossRef]
- Panichi, V.; Migliori, M.; De Pietro, S.; Taccola, D.; Bianchi, A.M.; Norpoth, M.; Giovannini, L.; Palla, R.; Tetta, C. C-reactive protein as a marker of chronic inflammation in uremic patients. Blood Purif. 2000, 18, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Wiegner, R.; Chakraborty, S.; Huber-Lang, M. Complement-coagulation crosstalk on cellular and artificial surfaces. Immunobiology 2016, 221, 1073–1079. [Google Scholar] [CrossRef] [PubMed]
- Conway, E.M. Complement-coagulation connections. Blood Coagul. Fibrinolysis. 2018, 29, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Oncul, S.; Afshar-Kharghan, V. The interaction between the complement system and hemostatic factors. Curr. Opin. Hematol. 2020, 27, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Morena, M.; Delbosc, S.; Dupuy, A.M.; Canaud, B.; Cristol, J.P. Overproduction of reactive oxygen species in end-stage renal disease patients: A potential component of hemodialysis-associated inflammation. Hemodial. Int. 2005, 9, 37–46. [Google Scholar] [CrossRef]
- Deppisch, R.M.; Beck, W.; Goehl, H.; Ritz, E. Complement components as uremic toxins and their potential role as mediators of microinflammation. Kidney Int. Suppl. 2001, 78, S271–S277. [Google Scholar] [CrossRef]
- Saha, M.; Allon, M. Diagnosis, Treatment, and Prevention of Hemodialysis Emergencies. Clin. J. Am. Soc. Nephrol. 2017, 12, 357–369. [Google Scholar] [CrossRef]
- Daugirdas, J.T.; Ing, T.S.; Roxe, D.M.; Ivanovich, P.T.; Krumlovsky, F.; Popli, S.; McLaughlin, M.M. Severe anaphylactoid reactions to cuprammonium cellulose hemodialyzers. Arch. Intern. Med. 1985, 145, 489–494. [Google Scholar] [CrossRef]
- Akhavan, B.J.; Osborn, U.A.; Mathew, R. Anaphylactic reaction to ethylene oxide in a hemodialysis patient. SAGE Open Med. Case Rep. 2019, 7, 2050313x19838744. [Google Scholar] [CrossRef]
- Butani, L.; Calogiuri, G. Hypersensitivity reactions in patients receiving hemodialysis. Ann. Allergy Asthma Immunol. 2017, 118, 680–684. [Google Scholar] [CrossRef]
- Pethő, Á.; Piecha, D.; Mészáros, T.; Urbanics, R.; Moore, C.; Canaud, B.; Rosivall, L.; Mollnes, T.E.; Steppan, S.; Szénási, G.; et al. A porcine model of hemodialyzer reactions: Roles of complement activation and rinsing back of extracorporeal blood. Ren. Fail. 2021, 43, 1609–1620. [Google Scholar] [CrossRef] [PubMed]
- Szebeni, J. Complement activation-related pseudoallergy: A stress reaction in blood triggered by nanomedicines and biologicals. Mol. Immunol. 2014, 61, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Mas, S.; Bosch-Panadero, E.; Abaigar, P.; Camarero, V.; Mahillo, I.; Civantos, E.; Sanchez-Ospina, D.; Ruiz-Priego, A.; Egido, J.; Ortiz, A.; et al. Influence of dialysis membrane composition on plasma bisphenol A levels during online hemodiafiltration. PLoS ONE. 2018, 13, e0193288. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Sanz, A.; Sánchez-Villanueva, R.; Domínguez-Ortega, J.; Álvarez, L.; Fiandor, A.; Nozal, P.; Sanz, P.; Pizarro-Sánchez, M.S.; Andrés, E.; Cabezas, A.; et al. Characterization of hypersensitivity reactions to polysulfone hemodialysis membranes. Ann. Allergy Asthma Immunol. 2022, 128, 713–720.e2. [Google Scholar] [CrossRef] [PubMed]
- Daugirdas, J.T.; Bernardo, A.A. Hemodialysis effect on platelet count and function and hemodialysis- associated thrombocytopenia. Kidney Int. 2012, 82, 147–157. [Google Scholar] [CrossRef]
- Tharmaraj, D.; Kerr, P.G. Haemolysis in haemodialysis. Nephrology 2017, 22, 838–847. [Google Scholar] [CrossRef]
- Boyer, C.J.; Swartz, R.D. Severe Clotting during Extracorporeal Dialysis Procedures. Semin. Dial. 1991, 4, 69–71. [Google Scholar] [CrossRef]
- Sudusinghe, D.; Riddell, A.; Gandhi, T.; Chowdary, P.; Davenport, A. Increased risk of dialysis circuit clotting in hemodialysis patients with COVID-19 is associated with elevated FVIII, fibrinogen and D-dimers. Hemodial. Int. 2023, 27, 38–44. [Google Scholar] [CrossRef]
- Stegmayr, B.G. Sources of Mortality on Dialysis with an Emphasis on Microemboli. Semin. Dial. 2016, 29, 442–446. [Google Scholar] [CrossRef]
- Wagner, S.; Rode, C.; Wojke, R.; Canaud, B. Observation of microbubbles during standard dialysis treatments. Clin. Kidney J. 2015, 8, 400–404. [Google Scholar] [CrossRef]
- Forsberg, U.; Jonsson, P.; Stegmayr, B. Microemboli induced by air bubbles may be deposited in organs as a consequence of contamination during medical care. Clin. Kidney J. 2023, 16, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, P.; Stegmayr, C.; Stegmayr, B.; Forsberg, U. Venous chambers in clinical use for hemodialysis have limited capacity to eliminate microbubbles from entering the return bloodline: An in vitro study. Artif. Organs. 2023, 47, 961–970. [Google Scholar] [CrossRef] [PubMed]
- Stegmayr, B.; Brännström, T.; Forsberg, U.; Jonson, P.; Stegmayr, C.; Hultdin, J. Microbubbles of air may occur in the organs of hemodialysis patients. Asaio J. 2012, 58, 177–179. [Google Scholar] [CrossRef] [PubMed]
- Canaud, B.; Aljama, P.; Tielemans, C.; Gasparovic, V.; Gutierrez, A.; Locatelli, F. Pathochemical toxicity of perfluorocarbon-5070, a liquid test performance fluid previously used in dialyzer manufacturing, confirmed in animal experiment. J. Am. Soc. Nephrol. 2005, 16, 1819–1823. [Google Scholar] [CrossRef] [PubMed]
- Canaud, B. Performance liquid test as a cause for sudden deaths of dialysis patients: Perfluorohydrocarbon, a previously unrecognized hazard for dialysis patients. Nephrol. Dial. Transplant. 2002, 17, 545–548. [Google Scholar] [CrossRef]
- Chávez-Iñiguez, J.S.; Medina-González, R.; Ron-Magaña, A.; Madero, M.; Ramírez-Ramírez, A.C.; Rifkin, B.S.; Torres-Vázquez, E.; Chávez-Alonso, G.; Gómez-Fregoso, J.A.; Rodríguez-García, G.; et al. Methemoglobinemia in Hemodialysis Patients due to Acute Chlorine Intoxication: A Case Series Calling Attention on an Old Problem. Blood Purif. 2023, 52, 835–843. [Google Scholar] [CrossRef]
- D'Haese, P.C.; De Broe, M.E. Adequacy of dialysis: Trace elements in dialysis fluids. Nephrol. Dial. Transplant. 1996, 11 (Suppl. 2), 92–97. [Google Scholar] [CrossRef]
- Humudat, Y.R.; Al-Naseri, S.K. Heavy Metals in Dialysis Fluid and Blood Samples from Hemodialysis Patients in Dialysis Centers in Baghdad, Iraq. J. Health Pollut. 2020, 10, 200901. [Google Scholar] [CrossRef]
- Hilborn, E.D.; Soares, R.M.; Servaites, J.C.; Delgado, A.G.; Magalhães, V.F.; Carmichael, W.W.; Azevedo, S.M. Sublethal microcystin exposure and biochemical outcomes among hemodialysis patients. PLoS ONE 2013, 8, e69518. [Google Scholar] [CrossRef]
- Jochimsen, E.M.; Carmichael, W.W.; An, J.; Cardo, D.M.; Cookson, S.T.; Holmes, C.E.; Antunes, M.B.; de Melo Filho, D.A.; Lyra, T.M.; Barreto, V.S.; et al. Liver failure and death after exposure to microcystins at a hemodialysis center in Brazil. N. Engl. J. Med. 1998, 338, 873–878. [Google Scholar] [CrossRef]
- Pouria, S.; de Andrade, A.; Barbosa, J.; Cavalcanti, R.L.; Barreto, V.T.; Ward, C.J.; Preiser, W.; Poon, G.K.; Neild, G.H.; Codd, G.A. Fatal microcystin intoxication in haemodialysis unit in Caruaru, Brazil. Lancet 1998, 352, 21–26. [Google Scholar] [CrossRef]
- Gejyo, F.; Homma, N.; Suzuki, Y.; Arakawa, M. Serum levels of beta 2-microglobulin as a new form of amyloid protein in patients undergoing long-term hemodialysis. N. Engl. J. Med. 1986, 314, 585–586. [Google Scholar] [PubMed]
- Gejyo, F.; Odani, S.; Yamada, T.; Honma, N.; Saito, H.; Suzuki, Y.; Nakagawa, Y.; Kobayashi, H.; Maruyama, Y.; Hirasawa, Y.; et al. Beta 2-microglobulin: A new form of amyloid protein associated with chronic hemodialysis. Kidney Int. 1986, 30, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Bardin, T.; Zingraff, J.; Kuntz, D.; Drüeke, T. Dialysis-related amyloidosis. Nephrol. Dial. Transplant. 1986, 1, 151–154. [Google Scholar] [PubMed]
- Drüeke, T.; Touam, M.; Zingraff, J. Dialysis-associated amyloidosis. Adv. Ren. Replace. Ther. 1995, 2, 24–39. [Google Scholar] [CrossRef]
- Koch, K.M. Dialysis-related amyloidosis. Kidney Int. 1992, 41, 1416–1429. [Google Scholar] [CrossRef]
- Miyata, T.; Jadoul, M.; Kurokawa, K.; Van Ypersele de Strihou, C. Beta-2 microglobulin in renal disease. J. Am. Soc. Nephrol. 1998, 9, 1723–1735. [Google Scholar] [CrossRef]
- Schwalbe, S.; Holzhauer, M.; Schaeffer, J.; Galanski, M.; Koch, K.M.; Floege, J. Beta 2-microglobulin associated amyloidosis: A vanishing complication of long-term hemodialysis? Kidney Int. 1997, 52, 1077–1083. [Google Scholar] [CrossRef]
- Portales-Castillo, I.; Yee, J.; Tanaka, H.; Fenves, A.Z. Beta-2 Microglobulin Amyloidosis: Past, Present, and Future. Kidney360 2020, 1, 1447–1455. [Google Scholar] [CrossRef]
- Lindner, A.; Charra, B.; Sherrard, D.J.; Scribner, B.H. Accelerated atherosclerosis in prolonged maintenance hemodialysis. N. Engl. J. Med. 1974, 290, 697–701. [Google Scholar] [CrossRef]
- Poznyak, A.V.; Sadykhov, N.K.; Kartuesov, A.G.; Borisov, E.E.; Sukhorukov, V.N.; Orekhov, A.N. Atherosclerosis Specific Features in Chronic Kidney Disease (CKD). Biomedicines 2022, 10, 2094. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, J.; Herrlinger, S.; Pruy, A.; Metzger, T.; Wanner, C. Inflammation enhances cardiovascular risk and mortality in hemodialysis patients. Kidney Int. 1999, 55, 648–658. [Google Scholar] [CrossRef] [PubMed]
- Pecoits-Filho, R.; Lindholm, B.; Stenvinkel, P. The malnutrition, inflammation, and atherosclerosis (MIA) syndrome—The heart of the matter. Nephrol. Dial. Transplant. 2002, 17 (Suppl. 11), 28–31. [Google Scholar] [CrossRef] [PubMed]
- Stenvinkel, P.; Heimbürger, O.; Paultre, F.; Diczfalusy, U.; Wang, T.; Berglund, L.; Jogestrand, T. Strong association between malnutrition, inflammation, and atherosclerosis in chronic renal failure. Kidney Int. 1999, 55, 1899–1911. [Google Scholar] [CrossRef]
- Valdivielso, J.M.; Rodríguez-Puyol, D.; Pascual, J.; Barrios, C.; Bermúdez-López, M.; Sánchez-Niño, M.D.; Pérez-Fernández, M.; Ortiz, A. Atherosclerosis in Chronic Kidney Disease: More, Less, or Just Different? Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1938–1966. [Google Scholar] [CrossRef]
- Davenport, A.; Peters, S.A.; Bots, M.L.; Canaud, B.; Grooteman, M.P.; Asci, G.; Locatelli, F.; Maduell, F.; Morena, M.; Nubé, M.J.; et al. Higher convection volume exchange with online hemodiafiltration is associated with survival advantage for dialysis patients: The effect of adjustment for body size. Kidney Int. 2016, 89, 193–199. [Google Scholar] [CrossRef]
- Peters, S.A.; Bots, M.L.; Canaud, B.; Davenport, A.; Grooteman, M.P.; Kircelli, F.; Locatelli, F.; Maduell, F.; Morena, M.; Nubé, M.J.; et al. Haemodiafiltration and mortality in end-stage kidney disease patients: A pooled individual participant data analysis from four randomized controlled trials. Nephrol. Dial. Transplant. 2016, 31, 978–984. [Google Scholar] [CrossRef]
- Cheung, A.K.; Sarnak, M.J.; Yan, G.; Berkoben, M.; Heyka, R.; Kaufman, A.; Lewis, J.; Rocco, M.; Toto, R.; Windus, D.; et al. Cardiac diseases in maintenance hemodialysis patients: Results of the HEMO Study. Kidney Int. 2004, 65, 2380–2389. [Google Scholar] [CrossRef]
- Echefu, G.; Stowe, I.; Burka, S.; Basu-Ray, I.; Kumbala, D. Pathophysiological concepts and screening of cardiovascular disease in dialysis patients. Front. Nephrol. 2023, 3, 1198560. [Google Scholar] [CrossRef]
- Jankowski, J.; Floege, J.; Fliser, D.; Bohm, M.; Marx, N. Cardiovascular Disease in Chronic Kidney Disease: Pathophysiological Insights and Therapeutic Options. Circulation 2021, 143, 1157–1172. [Google Scholar] [CrossRef]
- Coresh, J.; Longenecker, J.C.; Miller, E.R., 3rd; Young, H.J.; Klag, M.J. Epidemiology of cardiovascular risk factors in chronic renal disease. J. Am. Soc. Nephrol. 1998, 9 (Suppl. 12), S24–S30. [Google Scholar] [PubMed]
- Foley, R.N.; Parfrey, P.S.; Sarnak, M.J. Epidemiology of cardiovascular disease in chronic renal disease. J. Am. Soc. Nephrol. 1998, 9 (Suppl. 12), S16–S23. [Google Scholar] [CrossRef] [PubMed]
- Foley, R.N.; Parfrey, P.S.; Sarnak, M.J. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am. J. Kidney Dis. 1998, 32 (Suppl. 3), S112–S119. [Google Scholar] [CrossRef] [PubMed]
- Eknoyan, G.; Lameire, N.; Barsoum, R.; Eckardt, K.U.; Levin, A.; Levin, N.; Locatelli, F.; Macleod, A.; Vanholder, R.; Walker, R.; et al. The burden of kidney disease: Improving global outcomes. Kidney Int. 2004, 66, 1310–1314. [Google Scholar] [CrossRef] [PubMed]
- Canaud, B.; Kooman, J.P.; Selby, N.M.; Taal, M.; Maierhofer, A.; Kopperschmidt, P.; Francis, S.; Collins, A.; Kotanko, P. Hidden risks associated with conventional short intermittent hemodialysis: A call for action to mitigate cardiovascular risk and morbidity. World J. Nephrol. 2022, 11, 39–57. [Google Scholar] [CrossRef]
- Carrero, J.J.; Stenvinkel, P.; Cuppari, L.; Ikizler, T.A.; Kalantar-Zadeh, K.; Kaysen, G.; Mitch, W.E.; Price, S.R.; Wanner, C.; Wang, A.Y.; et al. Etiology of the protein- energy wasting syndrome in chronic kidney disease: A consensus statement from the International Society of Renal Nutrition and Metabolism (ISRNM). J. Ren. Nutr. 2013, 23, 77–90. [Google Scholar] [CrossRef]
- Carrero, J.J.; Thomas, F.; Nagy, K.; Arogundade, F.; Avesani, C.M.; Chan, M.; Chmielewski, M.; Cordeiro, A.C.; Espinosa-Cuevas, A.; Fiaccadori, E.; et al. Global Prevalence of Protein- Energy Wasting in Kidney Disease: A Meta-analysis of Contemporary Observational Studies from the International Society of Renal Nutrition and Metabolism. J. Ren. Nutr. 2018, 28, 380–392. [Google Scholar] [CrossRef]
- Canaud, B.; Morena-Carrere, M.; Leray-Moragues, H.; Cristol, J.P. Fluid Overload and Tissue Sodium Accumulation as Main Drivers of Protein Energy Malnutrition in Dialysis Patients. Nutrients 2022, 14, 4489. [Google Scholar] [CrossRef]
- Bergström, J. Factors causing catabolism in maintenance hemodialysis patients. Miner. Electrolyte Metab. 1992, 18, 280–283. [Google Scholar]
- Gutierrez, A.; Alvestrand, A.; Bergström, J. Membrane selection and muscle protein catabolism. Kidney Int. Suppl. 1992, 38, S86–S90. [Google Scholar]
- Löfberg, E.; Essen, P.; McNurlan, M.; Wernerman, J.; Garlick, P.; Anderstam, B.; Bergström, J.; Alvestrand, A. Effect of hemodialysis on protein synthesis. Clin. Nephrol. 2000, 54, 284–294. [Google Scholar] [PubMed]
- Gamboa, J.L.; Roshanravan, B.; Towse, T.; Keller, C.A.; Falck, A.M.; Yu, C.; Frontera, W.R.; Brown, N.J.; Ikizler, T.A. Skeletal Muscle Mitochondrial Dysfunction Is Present in Patients with CKD before Initiation of Maintenance Hemodialysis. Clin. J. Am. Soc. Nephrol. 2020, 15, 926–936. [Google Scholar] [CrossRef] [PubMed]
- Ryan, A.S. Role of Skeletal Muscle Mitochondrial Dysfunction in CKD. Clin. J. Am. Soc. Nephrol. 2020, 15, 912–913. [Google Scholar] [CrossRef] [PubMed]
- Chazot, G.; Lemoine, S.; Kocevar, G.; Kalbacher, E.; Sappey-Marinier, D.; Rouvière, O.; Juillard, L. Intracellular Phosphate and ATP Depletion Measured by Magnetic Resonance Spectroscopy in Patients Receiving Maintenance Hemodialysis. J. Am. Soc. Nephrol. 2021, 32, 229–237. [Google Scholar] [CrossRef]
- Molina, P.; Vizcaíno, B.; Molina, M.D.; Beltrán, S.; González-Moya, M.; Mora, A.; Castro-Alonso, C.; Kanter, J.; Ávila, A.I.; Górriz, J.L.; et al. The effect of high-volume online haemodiafiltration on nutritional status and body composition: The ProtEin Stores prEservaTion (PESET) study. Nephrol. Dial. Transplant. 2018, 33, 1223–1235. [Google Scholar] [CrossRef]
- Panichi, V.; Manca-Rizza, G.; Paoletti, S.; Taccola, D.; Consani, C.; Filippi, C.; Mantuano, E.; Sidoti, A.; Grazi, G.; Antonelli, A.; et al. Effects on inflammatory and nutritional markers of haemodiafiltration with online regeneration of ultrafiltrate (HFR) vs online haemodiafiltration: A cross-over randomized multicentre trial. Nephrol. Dial. Transplant. 2006, 21, 756–762. [Google Scholar] [CrossRef]
- Parker, T.F., 3rd; Wingard, R.L.; Husni, L.; Ikizler, T.A.; Parker, R.A.; Hakim, R.M. Effect of the membrane biocompatibility on nutritional parameters in chronic hemodialysis patients. Kidney Int. 1996, 49, 551–556. [Google Scholar] [CrossRef]
- Schiffl, H.; Lang, S.M.; Stratakis, D.; Fischer, R. Effects of ultrapure dialysis fluid on nutritional status and inflammatory parameters. Nephrol. Dial. Transplant. 2001, 16, 1863–1869. [Google Scholar] [CrossRef]
- Stenvinkel, P.; Larsson, T.E. Chronic Kidney Disease: A Clinical Model of Premature Aging. Am. J. Kidney Dis. 2013, 62, 339–351. [Google Scholar] [CrossRef]
- Losappio, V.; Franzin, R.; Infante, B.; Godeas, G.; Gesualdo, L.; Fersini, A.; Castellano, G.; Stallone, G. Molecular Mechanisms of Premature Aging in Hemodialysis: The Complex Interplay Between Innate and Adaptive Immune Dysfunction. Int. J. Mol. Sci. 2020, 21, 3422. [Google Scholar] [CrossRef]
- Ortiz, A.; Mattace-Raso, F.; Soler, M.J.; Fouque, D. Ageing meets kidney disease. Nephrol. Dial. Transplant. 2023, 38, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Ebert, T.; Neytchev, O.; Witasp, A.; Kublickiene, K.; Stenvinkel, P.; Shiels, P.G. Inflammation and Oxidative Stress in Chronic Kidney Disease and Dialysis Patients. Antioxid. Redox Signal. 2021, 35, 1426–1448. [Google Scholar] [CrossRef] [PubMed]
- Carracedo, J.; Merino, A.; Nogueras, S.; Carretero, D.; Berdud, I.; Rami, R.; Tetta, C.; Rodri, M.; Marti, A.; Aljama, P. On-line hemodiafiltration reduces the proinflammatory CD14+CD16+ monocyte-derived dendritic cells: A prospective, crossover study. J. Am. Soc. Nephrol. 2006, 17, 2315–2321. [Google Scholar] [CrossRef] [PubMed]
- Lang, S.M.; Becker, A.; Fischer, R.; Huber, R.M.; Schiffl, H. Acute effects of hemodialysis on lung function in patients with end-stage renal disease. Wien. Klin. Wochenschr. 2006, 118, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Zoccali, C.; Tripepi, R.; Torino, C.; Bellantoni, M.; Tripepi, G.; Mallamaci, F. Lung congestion as a risk factor in end-stage renal disease. Blood Purif. 2013, 36, 184–191. [Google Scholar] [CrossRef]
- Yigla, M.; Abassi, Z.; Reisner, S.A.; Nakhoul, F. Pulmonary hypertension in hemodialysis patients: An unrecognized threat. Semin. Dial. 2006, 19, 353–357. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, K.; Li, L.; Wang, N.; Cong, T.; Wei, Y.; Cao, S.; Wen, X.; Meng, Q.; Lin, H.; et al. Clinical influencing factors affecting pulmonary hypertension in hemodialysis patients. Kidney Res. Clin. Pract. 2023. [Google Scholar] [CrossRef]
- Campos, I.; Chan, L.; Zhang, H.; Deziel, S.; Vaughn, C.; Meyring-Wösten, A.; Kotanko, P. Intradialytic Hypoxemia in Chronic Hemodialysis Patients. Blood Purif. 2016, 41, 177–187. [Google Scholar] [CrossRef]
- Meyring-Woesten, A.; Zhang, H.; Ye, X.; Fuertinger, D.H.; Chan, L.; Kappel, F.; Artemyev, M.; Ginsberg, N.; Wang, Y.; Thijssen, S.; et al. Intradialytic Hypoxemia and Clinical Outcomes in Patients on Hemodialysis. Clin. J. Am. Soc. Nephrol. 2016, 11, 616–625. [Google Scholar] [CrossRef]
- Kooman, J.P.; Stenvinkel, P.; Shiels, P.G.; Feelisch, M.; Canaud, B.; Kotanko, P. The oxygen cascade in patients treated with hemodialysis and native high-altitude dwellers: Lessons from extreme physiology to benefit patients with end-stage renal disease. Am. J. Physiol. Renal Physiol. 2021, 320, F249–F261. [Google Scholar] [CrossRef]
- Swift, O.; Sharma, S.; Ramanarayanan, S.; Umar, H.; Laws, K.R.; Vilar, E.; Farrington, K. Prevalence and outcomes of chronic liver disease in patients receiving dialysis: Systematic review and meta-analysis. Clin. Kidney J. 2022, 15, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Leong, A.S.; Disney, A.P.; Gove, D.W. Spallation and migration of silicone from blood-pump tubing in patients on hemodialysis. N. Engl. J. Med. 1982, 306, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Gagnon, A.L.; Desai, T. Dermatological diseases in patients with chronic kidney disease. J. Nephropathol. 2013, 2, 104–109. [Google Scholar] [CrossRef]
- Escamilla, D.A.; Lakhani, A.; Antony, S.; Villegas, K.N.; Gupta, M.; Ramnath, P.; Pineda, M.I.; Bedor, A.; Banegas, D.; Martinez, E.C. Dermatological Manifestations in Patients with Chronic Kidney Disease: A Review. Cureus 2024, 16, e52253. [Google Scholar] [CrossRef] [PubMed]
- Gafter, U.; Mamet, R.; Korzets, A.; Malachi, T.; Schoenfeld, N. Bullous dermatosis of end-stage renal disease: A possible association between abnormal porphyrin metabolism and aluminium. Nephrol. Dial. Transplant. 1996, 11, 1787–1791. [Google Scholar] [CrossRef]
- Yang, L.Y.; Wang, Y.L.; Zuo, Y.G. Pemphigoid diseases in patients with end-stage kidney diseases: Pathogenesis and treatment. Front. Immunol. 2024, 15, 1427943. [Google Scholar] [CrossRef]
- Steiger, S.; Rossaint, J.; Zarbock, A.; Anders, H.J. Secondary Immunodeficiency Related to Kidney Disease (SIDKD)-Definition, Unmet Need, and Mechanisms. J. Am. Soc. Nephrol. 2022, 33, 259–278. [Google Scholar] [CrossRef]
- Kato, S.; Chmielewski, M.; Honda, H.; Pecoits-Filho, R.; Matsuo, S.; Yuzawa, Y.; Tranaeus, A.; Stenvinkel, P.; Lindholm, B. Aspects of immune dysfunction in end-stage renal disease. Clin. J. Am. Soc. Nephrol. 2008, 3, 1526–1533. [Google Scholar] [CrossRef]
- Carracedo, J.; Ramírez, R.; Madueño, J.A.; Soriano, S.; Rodríguez-Benot, A.; Rodríguez, M.; Martín-Malo, A.; Aljama, P. Cell apoptosis and hemodialysis-induced inflammation. Kidney Int. Suppl. 2002, 61, 89–93. [Google Scholar] [CrossRef]
- Franzin, R.; Stasi, A.; Caggiano, G.; Squiccimarro, E.; Losappio, V.; Fiorentino, M.; Alfieri, C.; Stallone, G.; Gesualdo, L.; Castellano, G. Enhancing Immune Protection in Hemodialysis Patients: Role of the Polymethyl Methacrylate Membrane. Blood Purif. 2023, 52 (Suppl. 1), 1–13. [Google Scholar] [CrossRef]
- Vilar, E.; Farrington, K. Emerging importance of residual renal function in end-stage renal failure. Semin. Dial. 2011, 24, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Shinkman, R. The Big Business of Dialysis Care. Catal. Carryover. 2016, 2. [Google Scholar]
- Vanholder, R.; Annemans, L.; Brown, E.; Gansevoort, R.; Gout-Zwart, J.J.; Lameire, N.; Morton, R.L.; Oberbauer, R.; Postma, M.J.; Tonelli, M.; et al. Reducing the costs of chronic kidney disease while delivering quality health care: A call to action. Nat. Rev. Nephrol. 2017, 13, 393–409. [Google Scholar] [CrossRef] [PubMed]
- Vanholder, R.; Van Biesen, W.; Lameire, N. Renal replacement therapy: How can we contain the costs? Lancet 2014, 383, 1783–1785. [Google Scholar] [CrossRef] [PubMed]
- Abdelrasoul, A.; Westphalen, H.; Saadati, S.; Shoker, A. Hemodialysis biocompatibility mathematical models to predict the inflammatory biomarkers released in dialysis patients based on hemodialysis membrane characteristics and clinical practices. Sci. Rep. 2021, 11, 23080. [Google Scholar] [CrossRef]
- Busink, E.; Kendzia, D.; Kircelli, F.; Boeger, S.; Petrovic, J.; Smethurst, H.; Mitchell, S.; Apel, C. A systematic review of the cost-effectiveness of renal replacement therapies, and consequences for decision-making in the end-stage renal disease treatment pathway. Eur. J. Health Econ. 2023, 24, 377–392. [Google Scholar] [CrossRef]
- Chu, H.; Yang, C.; Lin, Y.; Wu, J.; Kong, G.; Li, P.; Zhang, L.; Zhao, M.; China Kidney Disease Network Work Group. Hospitalizations of Chronic Dialysis Patients: A National Study in China. Kidney Dis. 2023, 9, 298–305. [Google Scholar] [CrossRef]
- Usvyat, L.A.; Kooman, J.P.; van der Sande, F.M.; Wang, Y.; Maddux, F.W.; Levin, N.W.; Kotanko, P. Dynamics of hospitalizations in hemodialysis patients: Results from a large US provider. Nephrol. Dial. Transplant. 2014, 29, 442–448. [Google Scholar] [CrossRef]
- Murphy, S.W.; Foley, R.N.; Barrett, B.J.; Kent, G.M.; Morgan, J.; Barré, P.; Campbell, P.; Fine, A.; Goldstein, M.B.; Handa, S.P.; et al. Comparative hospitalization of hemodialysis and peritoneal dialysis patients in Canada. Kidney Int. 2000, 57, 2557–2563. [Google Scholar] [CrossRef]
- Plantinga, L.C.; Jaar, B.G. Preventing repeat hospitalizations in dialysis patients: A call for action. Kidney Int. 2009, 76, 249–251. [Google Scholar] [CrossRef]
- Chan, K.E.; Lazarus, J.M.; Wingard, R.L.; Hakim, R.M. Association between repeat hospitalization and early intervention in dialysis patients following hospital discharge. Kidney Int. 2009, 76, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Walker, R.C.; Howard, K.; Tong, A.; Palmer, S.C.; Marshall, M.R.; Morton, R.L. The economic considerations of patients and caregivers in choice of dialysis modality. Hemodial. Int. 2016, 20, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Walker, R.; Marshall, M.R.; Morton, R.L.; McFarlane, P.; Howard, K. The cost-effectiveness of contemporary home haemodialysis modalities compared with facility haemodialysis: A systematic review of full economic evaluations. Nephrology 2014, 19, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Treharne, C.; Liu, F.X.; Arici, M.; Crowe, L.; Farooqui, U. Peritoneal dialysis and in-centre haemodialysis: A cost- utility analysis from a UK payer perspective. Appl. Health Econ. Health Policy 2014, 12, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Kodierleitfaden. Nephrologie 2021 of the German Society for Nephrology. 2021. Available online: https://wwwmydrgde/kodierleitfaden/kodierleitfaden2021html#kodierleitfaden2021 (accessed on 13 October 2024).
- England, N. National Tariff Payment System. 2021. Available online: https://wwwenglandnhsuk/pay-syst/national-tariff/national-tariff-payment-system/ (accessed on 13 October 2024).
- Marcelli, D.; Bayh, I.; Merello, J.I.; Ponce, P.; Heaton, A.; Kircelli, F.; Chazot, C.; Di Benedetto, A.; Marelli, C.; Ladanyi, E.; et al. Dynamics of the erythropoiesis stimulating agent resistance index in incident hemodiafiltration and high-flux hemodialysis patients. Kidney Int. 2016, 90, 192–202. [Google Scholar] [CrossRef]
- Stefansson, B.V.; Abramson, M.; Nilsson, U.; Haraldsson, B. Hemodiafiltration improves plasma 25-hepcidin levels: A prospective, randomized, blinded, cross-over study comparing hemodialysis and hemodiafiltration. Nephron Extra 2012, 2, 55–65. [Google Scholar] [CrossRef]
- Pedrini, L.A.; Zawada, A.M.; Winter, A.C.; Pham, J.; Klein, G.; Wolf, M.; Feuersenger, A.; Ruggiero, P.; Feliciani, A.; Barbieri, C.; et al. Effects of high-volume online mixed- hemodiafiltration on anemia management in dialysis patients. PLoS ONE 2019, 14, e0212795. [Google Scholar] [CrossRef]
- Nesrallah, G.E.; Lindsay, R.M.; Cuerden, M.S.; Garg, A.X.; Port, F.; Austin, P.C.; Moist, L.M.; Pierratos, A.; Chan, C.T.; Zimmerman, D.; et al. Intensive hemodialysis associates with improved survival compared with conventional hemodialysis. J. Am. Soc. Nephrol. 2012, 23, 696–705. [Google Scholar] [CrossRef]
- Farzi, S.; Farzi, S.; Moladoost, A.; Ehsani, M.; Shahriari, M.; Moieni, M. Caring Burden and Quality of Life of Family Caregivers in Patients Undergoing Hemodialysis: A Descriptive-Analytic Study. Int. J. Community Based Nurs. Midwifery 2019, 7, 88–96. [Google Scholar]
- Jafari, H.; Ebrahimi, A.; Aghaei, A.; Khatony, A. The relationship between care burden and quality of life in caregivers of hemodialysis patients. BMC Nephrol. 2018, 19, 321. [Google Scholar] [CrossRef]
- van Oevelen, M.; Bonenkamp, A.A.; van Eck van der Sluijs, A.; Bos, W.J.; Douma, C.E.; van Buren, M.; Meuleman, Y.; Dekker, F.W.; van Jaarsveld, B.C.; Abrahams, A.C. Health-related quality of life and symptom burden in patients on haemodialysis. Nephrol. Dial. Transplant. 2024, 39, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, A.; Teixeira-Pinto, A.; Lim, W.H.; Howard, K.; Chapman, J.R.; Castells, A.; Roger, S.D.; Bourke, M.J.; Macaskill, P.; Williams, G.; et al. Health-Related Quality of Life in People across the Spectrum of, C.K.D. Kidney Int. Rep. 2020, 5, 2264–2274. [Google Scholar] [CrossRef] [PubMed]
- Guedes, M.; Guetter, C.R.; Erbano, L.H.; Palone, A.G.; Zee, J.; Robinson, B.M.; Pisoni, R.; de Moraes, T.P.; Pecoits-Filho, R.; Baena, C.P. Physical health-related quality of life at higher achieved hemoglobin levels among chronic kidney disease patients: A systematic review and meta-analysis. BMC Nephrol. 2020, 21, 259. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, F.O.; Finkelstein, S.H.; Wuerth, D.; Shirani, S.; Troidle, L. Effects of home hemodialysis on health- related quality of life measures. Semin. Dial. 2007, 20, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Kraus, M.A.; Fluck, R.J.; Weinhandl, E.D.; Kansal, S.; Copland, M.; Komenda, P.; Finkelstein, F.O. Intensive Hemodialysis and Health-Related Quality of Life. Am. J. Kidney Dis. 2016, 68, S33–S42. [Google Scholar] [CrossRef] [PubMed]
- Ethier, I.; Nevis, I.; Suri, R.S. Quality of Life and Hemodynamic Effects of Switching From Hemodialysis to Hemodiafiltration: A Canadian Controlled Cohort Study. Can. J. Kidney Health Dis. 2021, 8, 20543581211057717. [Google Scholar] [CrossRef]
- Kawanishi, H.; Koremoto, M.; Franssen, C.F.M.; van Londen, M. Clotting Propensity of Surface-Treated Membranes in a Hemodialysis Set-up That Avoids Systemic Anticoagulation. Semin. Nephrol. 2023, 43, 151482. [Google Scholar] [CrossRef]
- Ehlerding, G.; Erlenkötter, A.; Gauly, A.; Griesshaber, B.; Kennedy, J.; Rauber, L.; Ries, W.; Schmidt-Gürtler, H.; Stauss-Grabo, M.; Wagner, S.; et al. Performance and Hemocompatibility of a Novel Polysulfone Dialyzer: A Randomized Controlled Trial. Kidney360 2021, 2, 937–947. [Google Scholar] [CrossRef]
- Melchior, P.; Erlenkötter, A.; Zawada, A.M.; Delinski, D.; Schall, C.; Stauss-Grabo, M.; Kennedy, J.P. Complement activation by dialysis membranes and its association with secondary membrane formation and surface charge. Artif. Organs. 2021, 45, 770–778. [Google Scholar] [CrossRef]
- Zawada, A.M.; Lang, T.; Ottillinger, B.; Kircelli, F.; Stauss-Grabo, M.; Kennedy, J.P. Impact of Hydrophilic Modification of Synthetic Dialysis Membranes on Hemocompatibility and Performance. Membranes 2022, 12, 932. [Google Scholar] [CrossRef]
- Zawada, A.M.; Melchior, P.; Erlenkötter, A.; Delinski, D.; Stauss-Grabo, M.; Kennedy, J.P. Polyvinylpyrrolidone in hemodialysis membranes: Impact on platelet loss during hemodialysis. Hemodial. Int. 2021, 25, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Gauly, A.; Fleck, N.; Kircelli, F. Advanced hemodialysis equipment for more eco-friendly dialysis. Int. Urol. Nephrol. 2022, 54, 1059–1065. [Google Scholar] [CrossRef] [PubMed]
- Canaud, B.; Lucena, R.; Ward, R. Water and dialysis fluid purity for contemporary hemodialysis. Semin. Dial. 2023, 983. [Google Scholar] [CrossRef] [PubMed]
- Canaud, B.; Lertdumrongluk, P. Ultrapure dialysis fluid: A new standard for contemporary hemodialysis. Nephrourol. Mon. 2012, 4, 519–523. [Google Scholar] [CrossRef]
- Blankestijn, P.J.; Vernooij, R.W.; Hockham, C.; Strippoli, G.F.; Canaud, B.; Hegbrant, J.; Barth, C.; Covic, A.; Cromm, K.; Cucui, A.; et al. Effect of Hemodiafiltration or Hemodialysis on Mortality in Kidney Failure. N. Engl. J. Med. 2023, 389, 700–709. [Google Scholar] [CrossRef]
- Vernooij, R.W.; Bots, M.L.; Strippoli, G.F.; Canaud, B.; Cromm, K.; Woodward, M.; Blankestijn, P.J.; CONVINCE scientific committee. CONVINCE in the context of existing evidence on haemodiafiltration. Nephrol. Dial. Transplant. 2022, 37, 1006–1013. [Google Scholar] [CrossRef]
- O'Hare, A.M. Patient-Centered Care in Renal Medicine: Five Strategies to Meet the Challenge. Am. J. Kidney Dis. 2018, 71, 732–736. [Google Scholar] [CrossRef]
- Masakane, I.; Ito, M.; Tanida, H.; Nawano, T. Patient-Centered Care Could Improve Quality of Life and Survival of Dialysis Patients: Dialysis Prescription and Daily Practice. Blood Purif. 2023, 52 (Suppl. 1), 1–12. [Google Scholar] [CrossRef]
- Canaud, B.; Davenport, A.; Leray-Moragues, H.; Morena-Carrere, M.; Cristol, J.P.; Kooman, J.; Kotanko, P. Digital Health Support: Current Status and Future Development for Enhancing Dialysis Patient Care and Empowering Patients. Toxins 2024, 16, 211. [Google Scholar] [CrossRef]
- Shinkman, R. Is “Empowered Dialysis” the Key to Better Outcomes? Catal. Carryover. 2018, 4. [Google Scholar]
- Nygårdh, A.; Malm, D.; Wikby, K.; Ahlström, G. The complexity in the implementation process of empowerment-based chronic kidney care: A case study. BMC Nurs. 2014, 13, 22. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hornig, C.; Bowry, S.K.; Kircelli, F.; Kendzia, D.; Apel, C.; Canaud, B. Hemoincompatibility in Hemodialysis-Related Therapies and Their Health Economic Perspectives. J. Clin. Med. 2024, 13, 6165. https://doi.org/10.3390/jcm13206165
Hornig C, Bowry SK, Kircelli F, Kendzia D, Apel C, Canaud B. Hemoincompatibility in Hemodialysis-Related Therapies and Their Health Economic Perspectives. Journal of Clinical Medicine. 2024; 13(20):6165. https://doi.org/10.3390/jcm13206165
Chicago/Turabian StyleHornig, Carsten, Sudhir K. Bowry, Fatih Kircelli, Dana Kendzia, Christian Apel, and Bernard Canaud. 2024. "Hemoincompatibility in Hemodialysis-Related Therapies and Their Health Economic Perspectives" Journal of Clinical Medicine 13, no. 20: 6165. https://doi.org/10.3390/jcm13206165
APA StyleHornig, C., Bowry, S. K., Kircelli, F., Kendzia, D., Apel, C., & Canaud, B. (2024). Hemoincompatibility in Hemodialysis-Related Therapies and Their Health Economic Perspectives. Journal of Clinical Medicine, 13(20), 6165. https://doi.org/10.3390/jcm13206165





